Establishment of In Vitro and In Vivo Anticolorectal Cancer Efficacy of Lithocholic Acid-Based Imidazolium Salts
Abstract
:1. Introduction
2. Results and Discussion
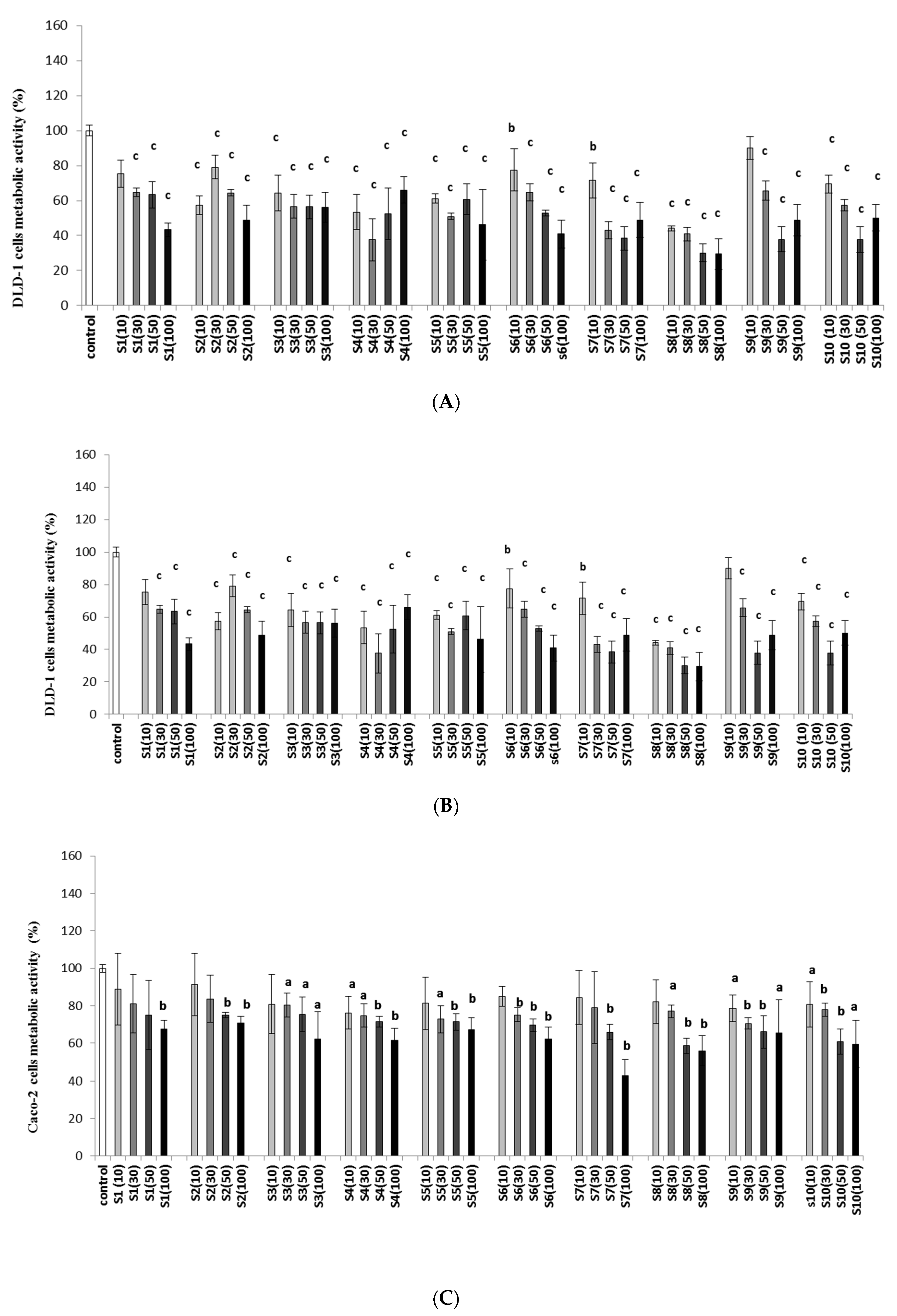
2.1. Imidazolium Salts Decrease Colon Cancer Viability and Metabolic Activity
2.2. Effect of IMSs in In Vivo Model
2.3. Effect of IMSs on Biochemical Parameters
2.4. Effect of IMSs on Histopathological Analysis
3. Materials and Methods
3.1. Synthesis of Imidazolium Salts
3.2. Cell Culture
3.3. Cytotoxicity Assay (IC50 Values)
3.4. Metabolic Activity of Cells
3.5. In Vivo Evaluation of Salt S6
3.6. DLD-1 Xenograft Model
3.7. Biochemical Analysis
3.8. Histopathological and Immunohistochemical Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alyabsi, M.; Algarni, M.; Alshammari, K. Trends in Colorectal Cancer Incidence Rates in Saudi Arabia (2001–2016) Using Saudi National Registry: Early- versus Late-Onset Disease. Front. Oncol. 2021, 11, 730689. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Francies, F.Z.; Oyomno, M.; Dlamini, Z. Colorectal Cancer Genetics, Incidence and Risk Factors: In Search for Targeted Therapies. Cancer Manag. Res. 2020, 12, 9869–9882. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’Callaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C.; et al. Effect of the Plane of Surgery Achieved on Local Recurrence in Patients with Operable Rectal Cancer: A Prospective Study Using Data from the MRC CR07 and NCIC-CTG CO16 Randomised Clinical Trial. Lancet 2009, 373, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Banfill, K.; Aznar, M.C.; Whitehurst, P.; Faivre Finn, C. The Evolving Role of Radiotherapy in Non-Small Cell Lung Cancer. Br. J. Radiol. 2019, 92, 20190524. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Glimelius, B.; Tiret, E.; Cervantes, A.; Arnold, D. Rectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2013, 24, 81–88. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y. Imidazolium Salts and Their Polymeric Materials for Biological Applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, J.Y.G. Sustainable Chemistry: Imidazolium Salts in Biomass Conversion and CO2 Fixation. Energy Environ. Sci. 2010, 3, 408–417. [Google Scholar] [CrossRef]
- Borowiecki, P.; Milner-Krawczyk, M.; Brzezińska, D.; Wielechowska, M.; Plenkiewicz, J. Synthesis and Antimicrobial Activity of Imidazolium and Triazolium Chiral Ionic Liquids: Synthesis and Antimicrobial Activity of Chiral Ionic Liquids. Eur. J. Org. Chem. 2013, 2013, 712–720. [Google Scholar] [CrossRef]
- Coleman, D.; Špulák, M.; Garcia, M.T.; Gathergood, N. Antimicrobial Toxicity Studies of Ionic Liquids Leading to a ‘Hit’ MRSA Selective Antibacterial Imidazolium Salt. Green Chem. 2012, 14, 1350–1356. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Chen, H.-B.; Cao, Z.-X.; Zhou, Z.-H. Synthesis, Spectral, and Structural Characterizations of Imidazole Oxalato Molybdenum(IV/V/VI) Complexes. Dalton Trans. 2013, 42, 1627–1636. [Google Scholar] [CrossRef]
- Liu, L.; Wu, H.; Riduan, S.N.; Ying, J.Y.; Zhang, Y. Short Imidazolium Chains Effectively Clear Fungal Biofilm in Keratitis Treatment. Biomaterials 2013, 34, 1018–1023. [Google Scholar] [CrossRef]
- Raghavan, S.; Manogaran, P.; Gadepalli Narasimha, K.K.; Kalpattu Kuppusami, B.; Mariyappan, P.; Gopalakrishnan, A.; Venkatraman, G. Synthesis and Anticancer Activity of Novel Curcumin–Quinolone Hybrids. Bioorg. Med. Chem. Lett. 2015, 25, 3601–3605. [Google Scholar] [CrossRef]
- Coa, J.C.; Castrillón, W.; Cardona, W.; Carda, M.; Ospina, V.; Muñoz, J.A.; Vélez, I.D.; Robledo, S.M. Synthesis, Leishmanicidal, Trypanocidal and Cytotoxic Activity of Quinoline-Hydrazone Hybrids. Eur. J. Med. Chem. 2015, 101, 746–753. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, X.; Zhang, Y.; Qing, C.; Zhang, H. Synthesis and Antitumor Activity of 1-Mesityl-3-(2-Naphthoylmethano)-1H-Imidazolium Bromide. Bioorg. Med. Chem. Lett. 2010, 20, 1844–1847. [Google Scholar] [CrossRef]
- Song, W.-J.; Yang, X.-D.; Zeng, X.-H.; Xu, X.-L.; Zhang, G.-L.; Zhang, H.-B. Synthesis and Cytotoxic Activities of Novel Hybrid Compounds of Imidazole Scaffold-Based 2-Substituted Benzofurans. RSC Adv. 2012, 2, 4612. [Google Scholar] [CrossRef]
- Kaushik, N.; Attri, P.; Kaushik, N.; Choi, E. Synthesis and Antiproliferative Activity of Ammonium and Imidazolium Ionic Liquids against T98G Brain Cancer Cells. Molecules 2012, 17, 13727–13739. [Google Scholar] [CrossRef]
- Chang, K.-H.; Lee, L.; Chen, J.; Li, W.-S. Lithocholic Acid Analogues, New and Potent α-2,3-Sialyltransferase Inhibitors. Chem. Commun. 2006, 6, 629–631. [Google Scholar] [CrossRef]
- Benis, K.A.; Schneider, G.B. The Effects of Vitamin D Binding Protein-Macrophage Activating Factor and Colony-Stimulating Factor-1 on Hematopoietic Cells in Normal and Osteopetrotic Rats. Blood 1996, 88, 2898–2905. [Google Scholar] [CrossRef]
- Trah, J.; Arand, J.; Oh, J.; Pagerols-Raluy, L.; Trochimiuk, M.; Appl, B.; Heidelbach, H.; Vincent, D.; Saleem, M.A.; Reinshagen, K.; et al. Lithocholic Bile Acid Induces Apoptosis in Human Nephroblastoma Cells: A Non-Selective Treatment Option. Sci. Rep. 2020, 10, 20349. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.A.; Beach, A.; Davies, G.F.; Harkness, T.A.A.; LeBlanc, A.; Titorenko, V.I. Lithocholic Bile Acid Selectively Kills Neuroblastoma Cells, While Sparing Normal Neuronal Cells. Oncotarget 2011, 2, 761–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luu, T.H.; Bard, J.-M.; Carbonnelle, D.; Chaillou, C.; Huvelin, J.-M.; Bobin-Dubigeon, C.; Nazih, H. Lithocholic Bile Acid Inhibits Lipogenesis and Induces Apoptosis in Breast Cancer Cells. Cell. Oncol. 2018, 41, 13–24. [Google Scholar] [CrossRef]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1α,25-Dihydroxyvitamin D3 Inhibits Angiogenesis In Vitro and In Vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Peters, U.; McGlynn, K.A.; Chatterjee, N.; Gunter, E.; Garcia-Closas, M.; Rothman, N.; Sinha, R. Vitamin D, Calcium, and Vitamin D Receptor Polymorphism in Colorectal Adenomas. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1267–1274. [Google Scholar]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- Lajczak-McGinley, N.K.; Porru, E.; Fallon, C.M.; Smyth, J.; Curley, C.; McCarron, P.A.; Tambuwala, M.M.; Roda, A.; Keely, S.J. The Secondary Bile Acids, Ursodeoxycholic Acid and Lithocholic Acid, Protect against Intestinal Inflammation by Inhibition of Epithelial Apoptosis. Physiol. Rep. 2020, 8, e14456. [Google Scholar] [CrossRef]
- Hryniewicka, A.; Malinowska, M.; Hauschild, T.; Pieczul, K.; Morzycki, J.W. Synthesis and Antimicrobial Properties of Steroid-Based Imidazolium Salts. J. Steroid Biochem. Mol. 2019, 189, 65–72. [Google Scholar] [CrossRef]
- Hryniewicka, A.; Niemirowicz-Laskowska, K.; Wielgat, P.; Car, H.; Hauschild, T.; Morzycki, J.W. Dehydroepiandrosterone Derived Imidazolium Salts and Their Antimicrobial Efficacy. Bioorg. Chem. 2021, 108, 104550. [Google Scholar] [CrossRef]
- Malinowska, M.; Sawicka, D.; Niemirowicz-Laskowska, K.; Wielgat, P.; Car, H.; Hauschild, T.; Hryniewicka, A. Steroid-Functionalized Imidazolium Salts with an Extended Spectrum of Antifungal and Antibacterial Activity. Int. J. Mol. Sci. 2021, 22, 12180. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-D.; Zeng, X.-H.; Zhang, Y.-L.; Qing, C.; Song, W.-J.; Li, L.; Zhang, H.-B. Synthesis and Cytotoxic Activities of Novel Phenacylimidazolium Bromides. Bioorg. Med. Chem. Lett. 2009, 19, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, B.; Ke, Z.; Zhang, C.; Kng, Y.; Suhaimi, N.-A.M.; Riduan, S.N.; Zhang, Y.; Zhuo, L. Metal-Free Imidazolium Salts Inhibit the Growth of Hepatocellular Carcinoma in a Mouse Model. Lab. Investig. 2011, 91, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-X.; Wang, X.-Q.; Zhou, B.; Yang, L.-J.; Li, Y.; Zhang, H.-B.; Yang, X.-D. Synthesis and Antitumor Activity of Novel N-Substituted Carbazole Imidazolium Salt Derivatives. Sci. Rep. 2015, 5, 13101. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-L.; Wang, J.; Yu, C.-L.; Chen, W.; Li, Y.-C.; Li, Y.; Zhang, H.-B.; Yang, X.-D. Synthesis and Cytotoxic Activity of Novel 1-((Indol-3-Yyl)Methyl)–1H-Imidazolium Salts. Bioorg. Med. Chem. Lett. 2014, 24, 4926–4930. [Google Scholar] [CrossRef]
- Shelton, K.L.; DeBord, M.A.; Wagers, P.O.; Southerland, M.R.; Taraboletti, A.; Robishaw, N.K.; Jackson, D.P.; Tosanovic, R.; Kofron, W.G.; Tessier, C.A.; et al. Synthesis, Anti-Proliferative Activity, and Toxicity of C4(C5) Substituted N,N′-Bis(Arylmethyl)Imidazolium Salts. Tetrahedron 2016, 72, 5729–5743. [Google Scholar] [CrossRef] [Green Version]
- DeBord, M.A.; Southerland, M.R.; Wagers, P.O.; Tiemann, K.M.; Robishaw, N.K.; Whiddon, K.T.; Konopka, M.C.; Tessier, C.A.; Shriver, L.P.; Paruchuri, S.; et al. Synthesis, Characterization, in Vitro SAR and in Vivo Evaluation of N,N′-Bisnaphthylmethyl 2-Alkyl Substituted Imidazolium Salts against NSCLC. Bioorg. Med. Chem. Lett. 2017, 27, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-B.; Liu, Y.; Liu, Z.-F.; Duan, S.-Z.; Li, M.-Y.; Chen, W.; Li, Y.; Zhang, H.-B.; Yang, X.-D. Synthesis and Cytotoxic Activity of Novel Tetrahydrobenzodifuran–Imidazolium Salt Derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 1808–1814. [Google Scholar] [CrossRef]
- Deng, G.; Zhou, B.; Wang, J.; Chen, Z.; Gong, L.; Gong, Y.; Wu, D.; Li, Y.; Zhang, H.; Yang, X. Synthesis and Antitumor Activity of Novel Steroidal Imidazolium Salt Derivatives. Eur. J. Med. Chem. 2019, 168, 232–252. [Google Scholar] [CrossRef]
- Stromyer, M.L.; Southerland, M.R.; Satyal, U.; Sikder, R.K.; Weader, D.J.; Baughman, J.A.; Youngs, W.J.; Abbosh, P.H. Synthesis, Characterization, and Biological Activity of a Triphenylphosphonium-Containing Imidazolium Salt against Select Bladder Cancer Cell Lines. Eur. J. Med. Chem. 2020, 185, 111832. [Google Scholar] [CrossRef]
- Southerland, M.R.; DeBord, M.A.; Johnson, N.A.; Crabtree, S.R.; Alexander, N.E.; Stromyer, M.L.; Wagers, P.O.; Panzner, M.J.; Wesdemiotis, C.; Shriver, L.P.; et al. Synthesis, Characterization, In Vitro SAR Study, and Preliminary In Vivo Toxicity Evaluation of Naphthylmethyl Substituted Bis-Imidazolium Salts. Bioorg. Med. Chem. 2021, 30, 115893. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Hsieh, Y.-Y.; Lo, H.-L.; Li, A.; Chou, C.-J.; Yang, P.-M. In Vitro and In Silico Mechanistic Insights into MiR-21-5p-Mediated Topoisomerase Drug Resistance in Human Colorectal Cancer Cells. Biomolecules 2019, 9, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.X.; Amidon, G.L.; Polli, J.E.; Zhao, H.; Mehta, M.U.; Conner, D.P.; Shah, V.P.; Lesko, L.J.; Chen, M.; Lee, V.H.L.; et al. Biopharmaceutics Classification System: The Scientific Basis for Biowaiver Extensions. Pharm. Res. 2002, 19, 921–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artursson, P.; Ungell, A.; Löfroth, J. Selective Paracellular Permeability in Two Models of Intestinal Absorption: Cultured Monolayers of Human Intestinal Epithelial Cells and Rat Intestinal Segments. Pharm. Res. 1993, 10, 1123–1129. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kondo, H.; Yasuda, T.; Watanabe, T.; Kobayashi, S.-I.; Yokohama, S. Common Solubilizers to Estimate the Caco-2 Transport of Poorly Water-Soluble Drugs. Int. J. Pharm. 2002, 246, 85–94. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole Alkaloids from Lepidium meyenii. J. Nat. Prod. 2003, 66, 1101–1103. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic Liquids as Active Pharmaceutical Ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef]
- Balk, A.; Holzgrabe, U.; Meinel, L. ‘Pro et Contra’ Ionic Liquid Drugs—Challenges and Opportunities for Pharmaceutical Translation. Eur. J. Pharm. Biopharm. 2015, 94, 291–304. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V. A Profile of the In Vitro Anti-Tumor Activity of Imidazolium-Based Ionic Liquids. Bioorg. Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ai, F.; Tian, L.; Liu, S.; Zhao, L.; Wang, X. Infliximab Enhances the Therapeutic Effects of 5-Fluorouracil Resulting in Tumor Regression in Colon Cancer. Oncotargets Ther. 2016, 9, 5999–6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkwill, F. Tumour Necrosis Factor and Cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, C.L.; Mahajan, A. Biological Agents Targeting beyond TNF-Alpha. Indian J. Crit. Care Med. 2008, 12, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Bertazza, L.; Mocellin, S. The Dual Role of Tumor Necrosis Factor (TNF) in Cancer Biology. Curr. Med. Chem. 2010, 17, 3337–3352. [Google Scholar] [CrossRef]
- Grimm, M.; Lazariotou, M.; Kircher, S.; Höfelmayr, A.; Germer, C.T.; von Rahden, B.H.A.; Waaga-Gasser, A.M.; Gasser, M. Tumor Necrosis Factor-α Is Associated with Positive Lymph Node Status in Patients with Recurrence of Colorectal Cancer—Indications for Anti-TNF-α Agents in Cancer Treatment. Anal. Cell. Pathol. 2010, 33, 151–163. [Google Scholar] [CrossRef]
- Szlosarek, P.; Charles, K.A.; Balkwill, F.R. Tumour Necrosis Factor-α as a Tumour Promoter. Eur. J. Cancer 2006, 42, 745–750. [Google Scholar] [CrossRef]
- Melling, N.; Kowitz, C.M.; Simon, R.; Bokemeyer, C.; Terracciano, L.; Sauter, G.; Izbicki, J.R.; Marx, A.H. High Ki67 Expression Is an Independent Good Prognostic Marker in Colorectal Cancer. J. Clin. Pathol. 2016, 69, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Edin, S.; Wikberg, M.L.; Dahlin, A.M.; Rutegård, J.; Öberg, Å.; Oldenborg, P.-A.; Palmqvist, R. The Distribution of Macrophages with a M1 or M2 Phenotype in Relation to Prognosis and the Molecular Characteristics of Colorectal Cancer. PLoS ONE 2012, 7, e47045. [Google Scholar] [CrossRef] [Green Version]
- Forones, N.M.; Oshima, C.; Nanogaki, S.; Tanaka, M.; Barbosa, V. Determination of proliferative activity using Ki67 and expression of p53 in colorectal cancer. Arq. Gastroenterol. 1999, 36, 122–126. [Google Scholar] [PubMed]
- Ates, G.; Vanhaecke, T.; Rogiers, V.; Rodrigues, R.M. Assaying Cellular Viability Using the Neutral Red Uptake Assay. In Cell Viability Assays; Methods in Molecular Biology; Gilbert, D.F., Friedrich, O., Eds.; Springer: New York, NY, USA, 2017; Volume 1601, pp. 19–26. ISBN 978-1-4939-6959-3. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-Based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]






| Compound (IMS) | N-Substituent | Colon Cancer Cell Line | Fibroblast Cells CRL-1475 [31] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DLD-1 | HT-29 | Caco-2 | |||||||
| µg/mL | µM/mL | µg/mL | µM/mL | µg/mL | µM/mL | µg/mL | µM/mL | ||
| S1 | methyl | 113.4 ± 0.10 | 0.20 ± 0.05 | 130.3 ± 0.12 | 0.23 ± 0.02 | 348.5± 0.21 | 0.63 ± 0.04 | 107.4 ± 2.34 | 0.19 ± 0.01 |
| S2 | ethyl | 116.4 ± 0.17 | 0.20± 0.01 | 146.1 ± 0.34 | 0.26 ± 0.04 | 380.3± 1.03 | 0.67 ± 0.02 | 113.9 ± 1.78 | 0.20 ± 0.02 |
| S3 | propyl | 109.5 ± 0.19 | 0.19 ± 0.03 | 88.4 ± 0.59 | 0.15 ± 0.03 | 401.6± 1.34 | 0.69 ± 0.01 | 36.5 ± 0.76 | 0.06 ± 0.01 |
| S4 | butyl | 154.3 ± 0.43 | 0.26 ± 0.03 | 66.6 ± 0.75 | 0.11 ± 0.02 | 661.4± 1.95 | 1.11 ± 0.03 | 99.2 ± 1.12 | 0.17 ± 0.02 |
| S5 | pentyl | 142.6 ± 0.24 | 0.23 ± 0.01 | 103.6 ± 1.34 | 0.17 ± 0.01 | 959.0± 1.56 | 1.57 ± 0.04 | 100.8 ± 2.76 | 0.17 ± 0.01 |
| S6 | hexyl | 78.9 ± 0.21 | 0.13 ± 0.04 | 101.3 ± 0.95 | 0.16 ± 0.02 | 250.1± 0.31 | 0.40 ± 0.06 | 149.6 ± 3.01 | 0.24 ± 0.03 |
| S7 | heptyl | 98.3 ± 0.22 | 0.15 ± 0.01 | 92.5 ± 1.13 | 0.14 ± 0.01 | 84.5± 0.89 | 0.13 ± 0.01 | 134.4 ± 4.17 | 0.21 ± 0.02 |
| S8 | octyl | 79.8 ± 0.24 | 0.12 ± 0.03 | 73.9 ± 0.47 | 0.11 ± 0.02 | 131.2± 1.54 | 0.20 ± 0.01 | 53.6 ± 0.57 | 0.08 ± 0.01 |
| S9 | dodecyl | 132.2 ± 0.64 | 0.19 ± 0.02 | 76.5 ± 0.67 | 0.11 ± 0.01 | 723.6 ± 0.76 | 1.02 ± 0.02 | 631.0 ± 4.78 | 0.89 ± 0.06 |
| S10 | hexadecyl | 151.9 ± 0.98 | 0.20 ± 0.02 | 80.6 ± 0.34 | 0.11± 0.01 | 188.0± 0.72 | 0.25 ± 0.01 | 841.1 ± 4.76 | 1.10 ± 0.03 |
| Parameters | Control | S6 500 mg/kg | DLD-1 | DLD-1 + S6 | DLD-1 + 5-FU 30 mg/kg | DLD-1 + 5-FU + S6 300 mg/kg | ||
|---|---|---|---|---|---|---|---|---|
| 100 mg/kg | 300 mg/kg | 500 mg/kg | ||||||
| WBC (103/mm3) | 2.53 ± 0.45 | 2.33 ± 0.31 | 1.83 ± 0.55 | 2.60 ± 0.57 | 2.15 ± 0.59 | 1.93 ± 0.31 | 1.28 ± 0.76 a,b | 1.76 ± 0.34 e |
| RBC (106/mm3) | 10.01 ± 0.03 | 9.91 ± 0.45 | 8.0 ± 2.40 | 9.95 ± 0.20 | 10.38 ± 0.38 | 10.07 ± 0.69 | 10.67 ± 0.66 b | 10.48 ± 0.38 c |
| HGB (g/dl) | 14.53 ± 0.12 | 15.05 ± 0.65 | 13.63 ± 2.16 | 14.60 ± 0.42 | 15.36 ± 0.57 | 14.53 ± 1.08 | 15.43 ± 1.07 | 15.36 ± 0.69 |
| HCT (%) | 53.43 ± 0.67 | 53.72 ± 2.38 | 50.43 ± 9.24 | 54.55 ± 1.77 | 52.29 ± 9.42 | 53.98 ± 4.97 | 57.18 ± 3.97 | 57.04 ± 2.71 |
| MCV (µm3) | 53.33 ± 0.58 | 54.17 ± 0.75 | 54.25 ± 0.50 | 54.50 ± 0.71 | 53.82 ± 1.74 | 53.75 ± 1.50 | 53.25 ± 0.5 | 54.40 ± 0.89 |
| MCH (pg) | 14.50 ± 0.17 | 15.20 ± 0.25 | 14.80 ± 0.43 | 14.65 ± 0.07 | 18.81 ± 2.71 | 14.40 ± 0.14 | 14.48 ± 0.10 | 14.68 ± 0.23 |
| MCHC (g/Dl) | 27.37 ± 0.12 | 28.02 ± 0.25 | 27.18 ± 0.92 | 26.75 ± 0.21 | 26.35 ± 4.12 | 26.88 ± 0.55 | 26.98 ± 0.29 | 26.92 ± 0.20 |
| PLT (103/mm3) | 731.33 ± 98.43 | 598.83 ± 201.32 | 668.50 ± 346.91 | 497.50 ± 89.80 | 674.67 ± 123.57 | 275.25 ± 77.62 a,b,c | 704.50 ± 141.06 d | 681.00 ± 231.92 e |
| Expression | ||
|---|---|---|
| CD68 | Ki67 | |
| DLD-1 | + | +++ |
| DLD-1 + 100 mg/kg | ++ | ++ |
| DLD-1 + 300 mg/kg | ++ | ++ |
| DLD-1 + 500 mg/kg | +++ | ++ |
| DLD-1 + 5FU | +++ | ++ |
| DLD-1+ 5FU + 500 mg/kg | +++ | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicka, D.; Hryniewicka, A.; Gohal, S.; Sadowska, A.; Pryczynicz, A.; Guzińska-Ustymowicz, K.; Sokołowska, E.; Morzycki, J.W.; Car, H. Establishment of In Vitro and In Vivo Anticolorectal Cancer Efficacy of Lithocholic Acid-Based Imidazolium Salts. Int. J. Mol. Sci. 2022, 23, 7019. https://doi.org/10.3390/ijms23137019
Sawicka D, Hryniewicka A, Gohal S, Sadowska A, Pryczynicz A, Guzińska-Ustymowicz K, Sokołowska E, Morzycki JW, Car H. Establishment of In Vitro and In Vivo Anticolorectal Cancer Efficacy of Lithocholic Acid-Based Imidazolium Salts. International Journal of Molecular Sciences. 2022; 23(13):7019. https://doi.org/10.3390/ijms23137019
Chicago/Turabian StyleSawicka, Diana, Agnieszka Hryniewicka, Sylwia Gohal, Anna Sadowska, Anna Pryczynicz, Katarzyna Guzińska-Ustymowicz, Emilia Sokołowska, Jacek W. Morzycki, and Halina Car. 2022. "Establishment of In Vitro and In Vivo Anticolorectal Cancer Efficacy of Lithocholic Acid-Based Imidazolium Salts" International Journal of Molecular Sciences 23, no. 13: 7019. https://doi.org/10.3390/ijms23137019






