A Combination of the Natural Molecules Gallic Acid and Carvacrol Eradicates P. aeruginosa and S. aureus Mature Biofilms
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Properties of Solutions of G, K and Q on Planktonic Bacteria
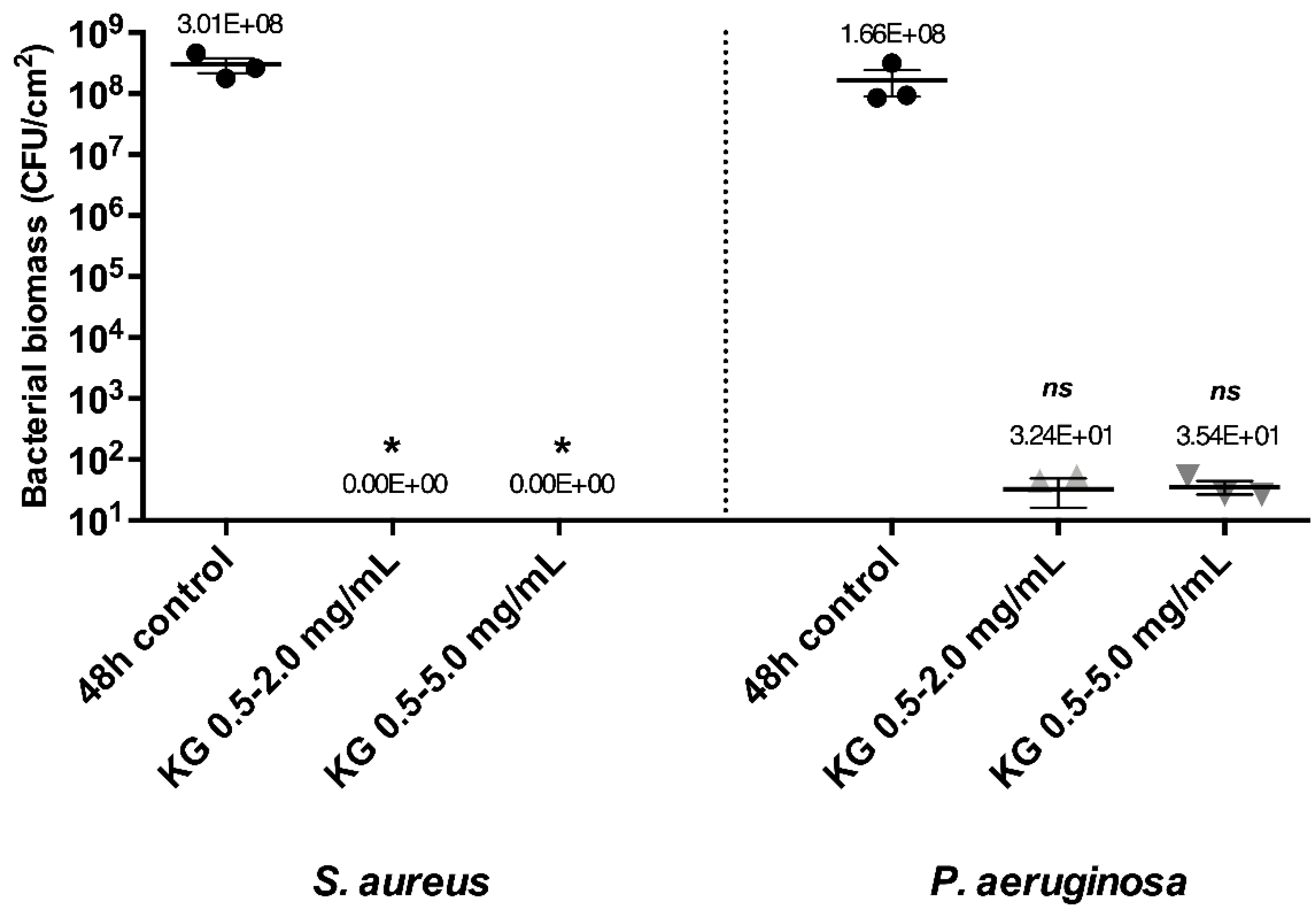
2.2. Eradicating Efficiency of Active Molecules on Mono-Species Mature Biofilms
2.3. Eradicating Efficiency of KG Combination on Dual-Species Mature Biofilms
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strains and Culture Conditions
4.3. Active Molecules Solubility Assays
4.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination
4.5. Mono- and Dual-Species Mature Biofilm Formation
4.6. Biofilm Eradicating Treatments
4.7. Enumeration of Viable Bacteria in Mono- or Dual-Species Biofilms after Eradicating Treatments
4.8. Bacterial Biomass Viability after Treatments
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Z.; Tseng, C.H.; Pei, Z.; Blaser, M.J. Molecular Analysis of Human Forearm Superficial Skin Bacterial Biota. Proc. Natl. Acad. Sci. USA 2007, 104, 2927–2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowler, P.G. Antibiotic Resistance and Biofilm Tolerance: A Combined Threat in the Treatment of Chronic Infections. J. Wound Care 2018, 27, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Fromantin, I.; Seyer, D.; Rollot, F.; Meaume, S.; Chautty, A.; Diallo, A.; Escande Marie, C.; Teot, L.; Larreta-Garde, V. Occurrence and Persistence of Biofilms on Cared Chronic Wounds: A Large Multicentric Clinical Study. Wound Med. 2018, 23, 28–34. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the Chronic Wound Microbiota of 2,963 Patients by 16S RDNA Pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Azevedo, M.-M.; Lisboa, C.; Cobrado, L.; Pina-Vaz, C.; Rodrigues, A. Hard-to-Heal Wounds, Biofilm and Wound Healing: An Intricate Interrelationship. Br. J. Nurs. 2020, 29, S6–S13. [Google Scholar] [CrossRef]
- Ashrafi, M.; Novak-Frazer, L.; Bates, M.; Baguneid, M.; Alonso-Rasgado, T.; Xia, G.; Rautemaa-Richardson, R.; Bayat, A. Validation of Biofilm Formation on Human Skin Wound Models and Demonstration of Clinically Translatable Bacteria-Specific Volatile Signatures. Sci. Rep. 2018, 8, 9431. [Google Scholar] [CrossRef]
- Vetrivel, A.; Ramasamy, M.; Vetrivel, P.; Natchimuthu, S.; Arunachalam, S.; Kim, G.-S.; Murugesan, R. Pseudomonas aeruginosa Biofilm Formation and Its Control. Biologics 2021, 1, 312–336. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Okshevsky, M.; Meyer, R.L. Big Bad Biofilms: How Communities of Bacteria Cause Long-Term Infections. Front. Young Minds 2016, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Xia, G.; Shi, C.; Wan, J.; Liu, L.; Chen, Y.; Wu, Y.; Zhang, W.; Zhou, M.; He, H.; et al. Therapeutic strategies against bacterial biofilms. Fundam. Res. 2021, 1, 193–212. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination Strategies to Enhance the Efficacy of Antimicrobial Peptides against Bacterial Biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, E.; Cheng, Y.; Mahmood, T.; Ge, F.; Zhou, K.; Bao, M.; Lv, L.; Li, L.; Yi, J.; et al. Is Combined Medication with Natural Medicine a Promising Therapy for Bacterial Biofilm Infection? Biomed. Pharmacother. 2020, 128, 110184. [Google Scholar] [CrossRef]
- Estrela, A.B.; Abraham, W.-R. Combining Biofilm-Controlling Compounds and Antibiotics as a Promising New Way to Control Biofilm Infections. Pharmaceuticals 2010, 3, 1374–1393. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Monte, J.; Abreu, A.; Borges, A.; Simões, L.; Simões, M. Antimicrobial Activity of Selected Phytochemicals against Escherichia coli and Staphylococcus aureus and Their Biofilms. Pathogens 2014, 3, 473–498. [Google Scholar] [CrossRef] [Green Version]
- Sarabhai, S.; Sharma, P.; Capalash, N. Ellagic Acid Derivatives from Terminalia Chebula Retz. Downregulate the Expression of Quorum Sensing Genes to Attenuate Pseudomonas aeruginosa PAO1 Virulence. PLoS ONE 2013, 8, e53441. [Google Scholar] [CrossRef] [Green Version]
- Özkalp, B.; Sevgi, F.; Özcan, M.; Özcan, M.M. The Antibacterial Activity of Essential Oil of Oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 88, 272–274. [Google Scholar]
- Nostro, A.; Procopio, F.; Pizzimenti, F.C.; Cannatelli, M.A.; Bisignano, G.; Marino, A.; Blanco, A.R.; Cioni, P.L.; Roccaro, A.S. Effects of Oregano, Carvacrol and Thymol on Staphylococcus aureus and Staphylococcus epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; et al. Synergistic Antibacterial Effect of Curcumin against Methicillin-Resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Myszka, K.; Schmidt, M.T.; Majcher, M.; Juzwa, W.; Olkowicz, M.; Czaczyk, K. Inhibition of Quorum Sensing-Related Biofilm of Pseudomonas fluorescens KM121 by Thymus Vulgare Essential Oil and Its Major Bioactive Compounds. Int. Biodeterior. Biodegrad. 2016, 114, 252–259. [Google Scholar] [CrossRef]
- Rudrappa, T.; Bais, H.P. Curcumin, a Known Phenolic from Curcuma Longa, Attenuates the Virulence of Pseudomonas aeruginosa PAO1 in Whole Plant and Animal Pathogenicity Models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Moshe, M.; Lellouche, J.; Banin, E. Curcumin: A Natural Antibiofilm Agent; World Scientific Publishing Co., Pte Ltd.: Singapore, 2011; pp. 89–93. [Google Scholar]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Ibberson, C.B.; Whiteley, M. The Social Life of Microbes in Chronic Infection. Curr. Opin. Microbiol. 2020, 53, 44–50. [Google Scholar] [CrossRef]
- Alves, P.M.; Al-Badi, E.; Withycombe, C.; Jones, P.M.; Purdy, K.J.; Maddocks, S.E. Interaction between Staphylococcus aureus and Pseudomonas aeruginosa Is Beneficial for Colonisation and Pathogenicity in a Mixed Biofilm. Pathog. Dis. 2018, 76, fty003. [Google Scholar] [CrossRef]
- Fu, L.; Lu, W.; Zhou, X. Phenolic Compounds and In Vitro Antibacterial and Antioxidant Activities of Three Tropic Fruits: Persimmon, Guava, and Sweetsop. Biomed Res. Int. 2016, 2016, 4287461. [Google Scholar] [CrossRef] [Green Version]
- Marinelli, L.; Fornasari, E.; Eusepi, P.; Ciulla, M.; Genovese, S.; Epifano, F.; Fiorito, S.; Turkez, H.; Örtücü, S.; Mingoia, M.; et al. Carvacrol Prodrugs as Novel Antimicrobial Agents. Eur. J. Med. Chem. 2019, 178, 515–529. [Google Scholar] [CrossRef]
- Shariati, A.; Asadian, E.; Fallah, F.; Azimi, T.; Hashemi, A.; Yasbolaghi Sharahi, J.; Taati Moghadam, M. Evaluation of Nano-Curcumin Effects on Expression Levels of Virulence Genes and Biofilm Production of Multidrug-Resistant Pseudomonas aeruginosa Isolated from Burn Wound Infection in Tehran, Iran. Infect. Drug Resist. 2019, 12, 2223–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of Gallic Acid on the Growth and Biofilm Formation of Escherichia coli and Streptococcus mutans. J. Food Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, A.T.W. Epigallocatechin Gallate and Gallic Acid Affect Colonization of Abiotic Surfaces by Oral Bacteria. Arch. Oral Biol. 2020, 120, 2020. [Google Scholar] [CrossRef] [PubMed]
- Sarjit, A.; Wang, Y.; Dykes, G.A. Antimicrobial Activity of Gallic Acid Against Thermophilic campylobacter is Strain Specific and Associated with a Loss of Calcium Ions. Food Microbiol. 2015, 46, 227–233. [Google Scholar] [CrossRef]
- Banin, E.; Vasil, M.L.; Greenberg, E.P. Iron and Pseudomonas aeruginosa Biofilm Formation. Proc. Natl. Acad. Sci. USA 2005, 102, 11076–11081. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.; Cockayne, A.; Williams, P.H.; Morrissey, J.A. Iron-Responsive Regulation of Biofilm Formation in Staphylococcus aureus Involves Fur-Dependent and Fur-Independent Mechanisms. J. Bacteriol. 2005, 187, 8211–8215. [Google Scholar] [CrossRef] [Green Version]
- Burt, S.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic Wound Infections: The Role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Trizna, E.Y.; Yarullina, M.N.; Baidamshina, D.R.; Mironova, A.V.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Khabibrakhmanova, A.M.; Kurbangalieva, A.R.; Bogachev, M.I.; et al. Bidirectional Alterations in Antibiotics Susceptibility in Staphylococcus aureus—Pseudomonas aeruginosa Dual-Species Biofilm. Sci. Rep. 2020, 10, 14849. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Kumar-Singh, S.; Goossens, H.; Malhotra-Kumar, S. In Vivo and In Vitro Interactions between Pseudomonas aeruginosa and Staphylococcus spp. Front. Cell. Infect. Microbiol. 2017, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.; Isenberg, H. Antimicrobial Susceptibility Testing. In Clinical Microbiology Procedures Handbook; ASM Press: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Lefebvre, E.; Lembré, P.; Picard, J.; El-Guermah, L.; Seyer, D.; Larreta-Garde, V. Ephemeral Biogels to Control An-ti-biofilm Agent Delivery: From Conception to the Construction of an Active Dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Agrappi, S.; Bortolin, M.; Toscano, M.; Romanò, C.L.; De Vecchi, E. How to Study Biofilms after Microbial Colonization of Materials Used in Orthopaedic Implants. Int. J. Mol. Sci. 2016, 17, 293. [Google Scholar] [CrossRef] [PubMed]




| Active Molecules | G | K | Q | ||
|---|---|---|---|---|---|
| Antibacterial effect | MIC (mg/mL) | S. aureus CIP 4.83 | 2.5 | 0.128 | 0.064 |
| P. aeruginosa CIP 103 467 | 2.5 | 2.0 | 0.128 | ||
| MBC (mg/mL) | S. aureus CIP 4.83 | 5.0 | 0.512 | ND | |
| P. aeruginosa CIP 103 467 | 5.0 | 2.0 | ND | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobin, M.; Proust, R.; Lack, S.; Duciel, L.; Des Courtils, C.; Pauthe, E.; Gand, A.; Seyer, D. A Combination of the Natural Molecules Gallic Acid and Carvacrol Eradicates P. aeruginosa and S. aureus Mature Biofilms. Int. J. Mol. Sci. 2022, 23, 7118. https://doi.org/10.3390/ijms23137118
Gobin M, Proust R, Lack S, Duciel L, Des Courtils C, Pauthe E, Gand A, Seyer D. A Combination of the Natural Molecules Gallic Acid and Carvacrol Eradicates P. aeruginosa and S. aureus Mature Biofilms. International Journal of Molecular Sciences. 2022; 23(13):7118. https://doi.org/10.3390/ijms23137118
Chicago/Turabian StyleGobin, Maxime, Richard Proust, Stéphane Lack, Laura Duciel, Céline Des Courtils, Emmanuel Pauthe, Adeline Gand, and Damien Seyer. 2022. "A Combination of the Natural Molecules Gallic Acid and Carvacrol Eradicates P. aeruginosa and S. aureus Mature Biofilms" International Journal of Molecular Sciences 23, no. 13: 7118. https://doi.org/10.3390/ijms23137118
APA StyleGobin, M., Proust, R., Lack, S., Duciel, L., Des Courtils, C., Pauthe, E., Gand, A., & Seyer, D. (2022). A Combination of the Natural Molecules Gallic Acid and Carvacrol Eradicates P. aeruginosa and S. aureus Mature Biofilms. International Journal of Molecular Sciences, 23(13), 7118. https://doi.org/10.3390/ijms23137118






