Shear Stress Enhances the Paracrine-Mediated Immunoregulatory Function of Human Periodontal Ligament Stem Cells via the ERK Signalling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of hPDLSCs
2.2. Flow Cytometry Analysis
2.3. Induction of Osteogenic Differentiation
2.4. Induction of Adipogenic Differentiation
2.5. Alizarin Red S and Von Kossa Staining
2.6. Oil Red O Staining
2.7. Isolation and Activation of CD4 T Cells from PBMCs
2.8. Shear Stress Stimulation
2.9. Cell Viability Assay
2.10. Immunofluorescent Staining
2.11. Conditioned Medium Preparation and Treatment
2.12. T Cell Proliferation Analysis Using Resazurin Assay
2.13. Development of Regulatory T Cell (Treg) Cells
2.14. RNA Isolation and Real-Time RT-PCR Analysis
2.15. Enzyme-Linked Immunosorbent Assay (ELISA)
2.16. IDO Activity Assay and Kynurenine Measurement
2.17. Western Blot Assay
2.18. Statistical Analysis
3. Results
3.1. Shear Stress Enhanced the Expression of Immunosuppressive Regulators
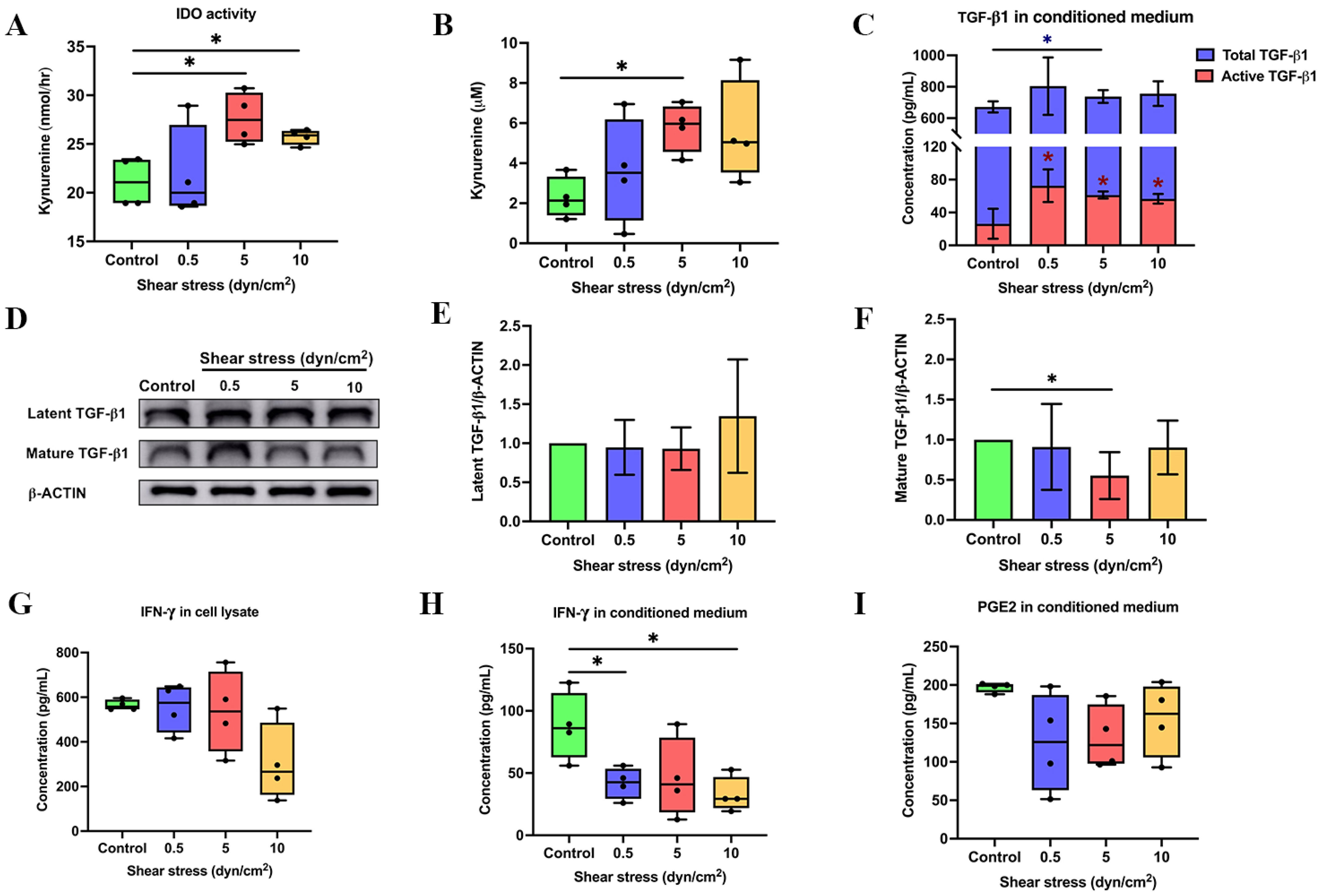
3.2. Shear Stress Enhanced the Product of IDO Activity via ERK1/2 Signalling Pathway
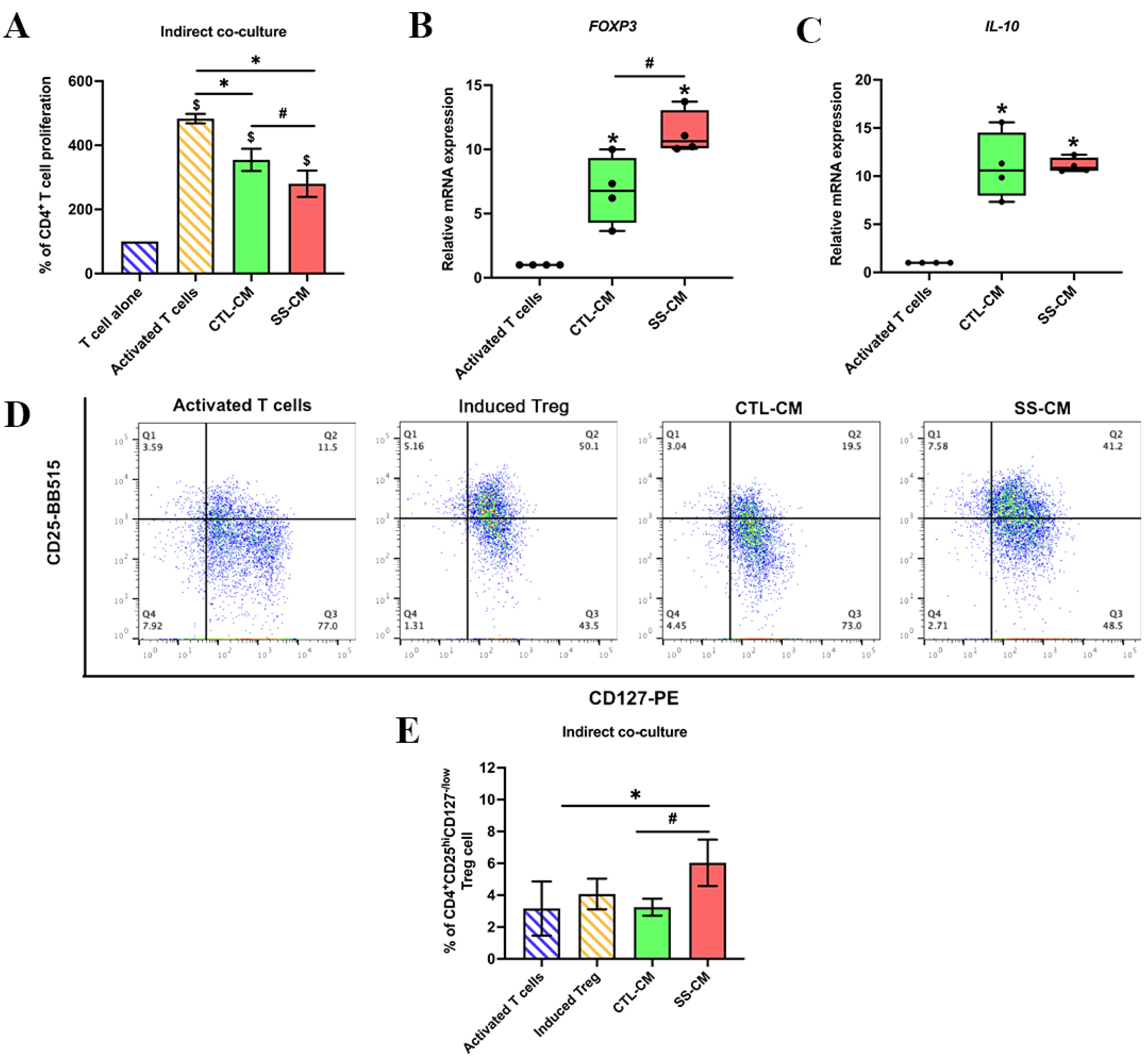
3.3. Shear Stress-Derived Conditioned Medium (SS-CM) Suppressed T Cell Proliferation
3.4. Shear Stress-Derived Conditioned Medium (SS-CM) Induced Regulatory T Cell Differentiation
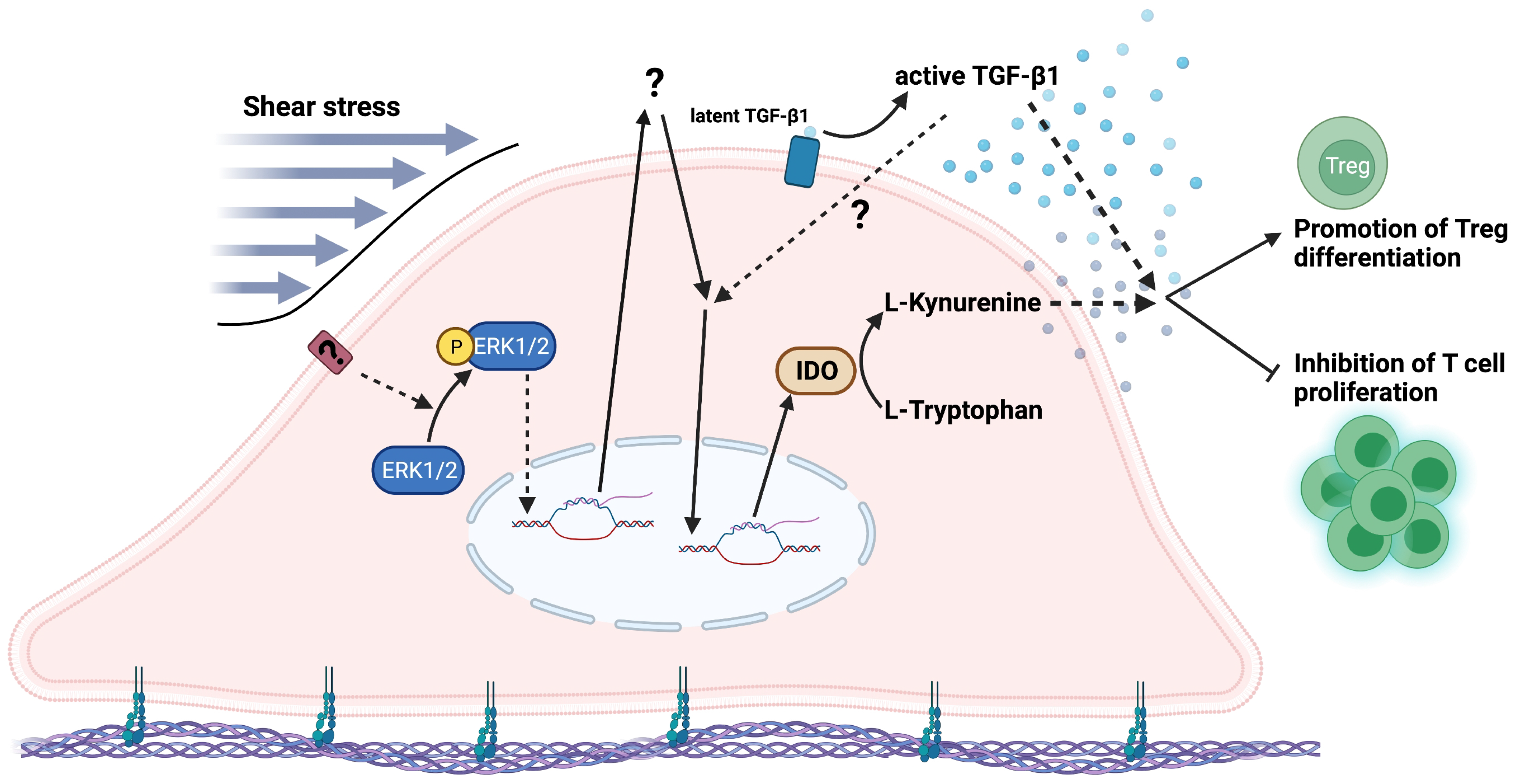
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHR | Aryl hydrocarbon receptor |
| BCA | Bicinchoninic acid |
| CHX | Cycloheximide |
| COX2 | Cyclooxygenase 2 |
| CTL-CM | Non-shear stress-induced hPDLSC-derived conditioned medium |
| DI | Deionised water |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK1/2 | Extracellular signalling-regulated kinase1/2 |
| FOXP3 | Forkhead box P3 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| hPDLSCs | Human periodontal ligament stem cells |
| IDO | Indoleamine 2,3dioxygenase |
| IFN- | Interferon gamma |
| IL-1 | Interleukin-1 beta |
| IL-2 | Interleukin-2 |
| IL-12 | Interleukin-12 |
| IL-10 | Interleukin-10 |
| Kyn | Kynurenine |
| MSCs | Mesenchymal stem cells |
| PBMCs | Peripheral blood mononucleated cells |
| P-ERK1/2 | Phosphorylated extracellular signalling-regulated kinase ½ |
| PGE2 | Prostaglandin E2 |
| PI | Protease inhibitor |
| P(I)3K | Phosphoinositide 3-kinase |
| RIPA | Radioimmunoprecipitation assay |
| RT | Room temperature |
| SS-CM | Shear stress-induced hPDLSC-derived conditioned medium |
| TCA | Trichloroacetic acid |
| TLRs | Toll-like receptors |
| Treg | Regulatory T cell |
| TGF-1 | Transforming growth factor beta 1 |
References
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef]
- Huang, C.-Y.C.; Pelaez, D.; Bendala, J.D.; Garcia-Godoy, F.; Cheung, H.S. Plasticity of stem cells derived from adult periodontal ligament. Regen. Med. 2009, 4, 809–821. [Google Scholar] [CrossRef]
- Ding, G.; Liu, Y.; Wang, W.; Wei, F.; Liu, D.; Fan, Z.; An, Y.; Zhang, C.; Wang, S. Allogeneic Periodontal Ligament Stem Cell Therapy for Periodontitis in Swine. Stem Cells 2010, 28, 1829–1838. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal Ligament Stem Cell-Mediated Treatment for Periodontitis in Miniature Swine. Stem Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Mrozik, K.M.; Wada, N.; Marino, V.; Richter, W.; Shi, S.; Wheeler, D.L.; Gronthos, S.; Bartold, P.M. Regeneration of periodontal tissues using allogeneic periodontal ligament stem cells in an ovine model. Regen. Med. 2013, 8, 711–723. [Google Scholar] [CrossRef]
- Isaka, J.; Ohazama, A.; Kobayashi, M.; Nagashima, C.; Takiguchi, T.; Kawasaki, H.; Tachikawa, T.; Hasegawa, K. Participation of periodontal ligament cells with regeneration of alveolar bone. J. Periodontol. 2001, 72, 314–323. [Google Scholar] [CrossRef]
- Chaikeawkaew, D.; Everts, V.; Pavasant, P. TLR3 activation modulates immunomodulatory properties of human periodontal ligament cells. J. Periodontol. 2020, 91, 1225–1236. [Google Scholar] [CrossRef]
- Liu, O.; Xu, J.; Ding, G.; Liu, D.; Fan, Z.; Zhang, C.; Chen, W.; Ding, Y.; Tang, Z.; Wang, S. Periodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1. Stem Cells 2013, 31, 1371–1382. [Google Scholar] [CrossRef]
- Konermann, A.; Stabenow, D.; Knolle, P.A.; Held, S.A.E.; Deschner, J.; Jäger, A. Regulatory role of periodontal ligament fibroblasts for innate immune cell function and differentiation. Innate Immun. 2012, 18, 745–752. [Google Scholar] [CrossRef]
- Konermann, A.; Beyer, M.; Deschner, J.; Allam, J.P.; Novak, N.; Winter, J.; Jepsen, S.; Jäger, A. Human periodontal ligament cells facilitate leukocyte recruitment and are influenced in their immunomodulatory function by Th17 cytokine release. Cell. Immunol. 2012, 272, 137–143. [Google Scholar] [CrossRef]
- Shin, C.; Kim, M.; Han, J.A.; Choi, B.; Hwang, D.; Do, Y.; Yun, J.H. Human periodontal ligament stem cells suppress T-cell proliferation via down-regulation of non-classical major histocompatibility complex-like glycoprotein CD1b on dendritic cells. J. Periodontal Res. 2017, 52, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, B.; Bao, J.; Zhang, Y.; Lei, L.; Yan, F. Macrophage polarization in periodontal ligament stem cells enhanced periodontal regeneration. Stem Cell Res. Ther. 2019, 10, 320. [Google Scholar] [CrossRef]
- Wada, N.; Tomokiyo, A.; Gronthos, S.; Bartold, P.M. Immunomodulatory Properties of PDLSC and Relevance to Periodontal Regeneration. Curr. Oral Health Rep. 2015, 2, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shen, S.; Fu, H.; Wang, Z.; Li, X.; Sui, X.; Yuan, M.; Liu, S.; Wang, G.; Guo, Q. Immunomodulatory Functions of Mesenchymal Stem Cells in Tissue Engineering. Stem Cells Int. 2019, 2019, 967–1206. [Google Scholar] [CrossRef] [Green Version]
- Kode, J.A.; Mukherjee, S.; Joglekar, M.V.; Hardikar, A.A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009, 11, 377–391. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Volarevic, V. The Cross-Talk between Mesenchymal Stem Cells and Immune Cells in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 2472. [Google Scholar] [CrossRef]
- English, K.; Barry, F.P.; Field-Corbett, C.P.; Mahon, B.P. IFN-γ and TNF-α differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 2007, 110, 91–100. [Google Scholar] [CrossRef]
- Clark, D.A.; Coker, R. Molecules in focus Transforming growth factor-beta (TGF-β). Int. J. Biochem. Cell Biol. 1998, 30, 293–298. [Google Scholar] [CrossRef]
- De Araújo Farias, V.; Carrillo-Gálvez, A.B.; Martín, F.; Anderson, P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018, 43, 25–37. [Google Scholar] [CrossRef]
- Groh, M.E.; Maitra, B.; Szekely, E.; Koç, O.N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 2005, 33, 928–934. [Google Scholar] [CrossRef]
- Kehrl, J.H.; Wakefield, L.M.; Roberts, A.B.; Jakowlew, S.; Alvarez-Mon, M.; Derynck, R.; Sporn, M.B.; Fauci, A.S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 1986, 163, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.-J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Konkel, J.E.; Zhang, D.; Zanvit, P.; Chia, C.; Zangarle-Murray, T.; Jin, W.; Wang, S.; Chen, W. Transforming Growth Factor-β Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity 2017, 46, 660–674. [Google Scholar] [CrossRef] [Green Version]
- Mellor, A.L.; Munn, D.; Chandler, P.; Keskin, D.; Johnson, T.; Marshall, B.; Jhaver, K.; Baban, B. Tryptophan catabolism and T cell responses. Adv. Exp. Med. Biol. 2003, 527, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Keskin, D.B.; Johnson, T.; Chandler, P.; Munn, D.H. Cells Expressing Indoleamine 2,3-Dioxygenase Inhibit T Cell Responses. J. Immunol. 2002, 168, 3771. [Google Scholar] [CrossRef] [Green Version]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; You, S.; McGrath, B.C.; Cavener, D.R.; Vacca, C.; Orabona, C.; Bianchi, R.; Belladonna, M.L.; Volpi, C.; et al. The Combined Effects of Tryptophan Starvation and Tryptophan Catabolites Down-Regulate T Cell Receptor ζ-Chain and Induce a Regulatory Phenotype in Naive T Cells. J. Immunol. 2006, 176, 6752. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Choudhury, S.; Musaelyan, K.; Myint, A.M.; Thuret, S.; Price, J.; Pariante, C.M. Interleukin-1β: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 2012, 37, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Issaranggun Na Ayuthaya, B.; Satravaha, P.; Pavasant, P. Interleukin-12 modulates the immunomodulatory properties of human periodontal ligament cells. J. Periodontal Res. 2017, 52, 546–555. [Google Scholar] [CrossRef]
- Andrukhov, O.; Hong, J.S.-A.; Andrukhova, O.; Blufstein, A.; Moritz, A.; Rausch-Fan, X. Response of human periodontal ligament stem cells to IFN-γ and TLR-agonists. Sci. Rep. 2017, 7, 12856. [Google Scholar] [CrossRef] [Green Version]
- Jung, I.D.; Jeong, Y.-I.; Lee, C.-M.; Noh, K.T.; Jeong, S.K.; Chun, S.H.; Choi, O.H.; Park, W.S.; Han, J.; Shin, Y.K.; et al. COX-2 and PGE2 signaling is essential for the regulation of IDO expression by curcumin in murine bone marrow-derived dendritic cells. Int. Immunopharmacol. 2010, 10, 760–768. [Google Scholar] [CrossRef]
- Braun, D.; Longman, R.S.; Albert, M.L. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood 2005, 106, 2375–2381. [Google Scholar] [CrossRef] [Green Version]
- Carlin, J.M.; Borden, E.C.; Sondel, P.M.; Byrne, G.I. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J. Immunol. 1987, 139, 2414–2418. [Google Scholar]
- Chen, W. IDO: More than an enzyme. Nat. Immunol. 2011, 12, 809–811. [Google Scholar] [CrossRef]
- Jiang, N.; He, D.; Ma, Y.; Su, J.; Wu, X.; Cui, S.; Li, Z.; Zhou, Y.; Yu, H.; Liu, Y. Force-Induced Autophagy in Periodontal Ligament Stem Cells Modulates M1 Macrophage Polarization via AKT Signaling. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Wang, Z.; Maruyama, K.; Sakisaka, Y.; Suzuki, S.; Tada, H.; Suto, M.; Saito, M.; Yamada, S.; Nemoto, E. Cyclic Stretch Force Induces Periodontal Ligament Cells to Secrete Exosomes That Suppress IL-1β Production Through the Inhibition of the NF-κB Signaling Pathway in Macrophages. Front. Immunol. 2019, 10, 1310. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.F.; Vaidya, A.B.; Evans, S.M.; Lee, H.J.; Aertker, B.M.; Alexander, A.J.; Price, K.M.; Ozuna, J.A.; Liao, G.P.; Aroom, K.R.; et al. Biomechanical Forces Promote Immune Regulatory Function of Bone Marrow Mesenchymal Stromal Cells. Stem Cells 2017, 35, 1259–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukkapalli, S.S.; Lele, T.P. Periodontal cell mechanotransduction. Open Biol. 2018, 8, 180053. [Google Scholar] [CrossRef] [Green Version]
- Aveic, S.; Craveiro, R.B.; Wolf, M.; Fischer, H. Current Trends in vitro Modeling to Mimic Cellular Crosstalk in Periodontal Tissue. Adv. Healthc. Mater. 2020, 10, 2001269. [Google Scholar] [CrossRef]
- McCulloch, C.A.; Lekic, P.; McKee, M.D. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontology 2000, 24, 56–72. [Google Scholar] [CrossRef]
- Afanador, E.; Yokozeki, M.; Oba, Y.; Kitase, Y.; Takahashi, T.; Kudo, A.; Moriyama, K. Messenger RNA expression of periostin and Twist transiently decrease by occlusal hypofunction in mouse periodontal ligament. Arch. Oral Biol. 2005, 50, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Manokawinchoke, J.; Limjeerajarus, N.; Limjeerajarus, C.; Sastravaha, P.; Everts, V.; Pavasant, P. Mechanical Force–induced TGF-beta1 Increases Expression of SOST/POSTN by hPDL Cells. J. Dent. Res. 2015, 94, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, S.G.; Viechnicki, B.; Kim, S.; Nah, H.D. Engineering of a periodontal ligament construct: Cell and fibre alignment induced by shear stress. J. Clin. Periodontol. 2011, 38, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, J.M.; Dillon, E.M. The water binding capacity of the periodontal ligament and its role in mechanical function. J. Periodontal Res. 1983, 18, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.C.; Day, B.; Harper, L.; Yee, J.; Hsu, C.Y.; Larijani, L.; Rohani, L.; Duan, N.; Kallos, M.S.; Rancourt, D.E. Fluid shear stress promotes embryonic stem cell pluripotency via interplay between β-catenin and vinculin in bioreactor culture. Stem Cells 2021, 39, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Russo, T.A.; Banuth, A.M.M.; Nader, H.B.; Dreyfuss, J.L. Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis. PLoS ONE 2020, 15, e0241040. [Google Scholar] [CrossRef]
- Arora, S.; Srinivasan, A.; Leung, A.; Toh, Y.-C. Bio-Mimicking Shear Stress Environments for Enhancing Mesenchymal Stem Cell Differentiation. Curr. Stem Cell Res. Ther. 2020, 15, 414–427. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, Y. The microRNA 132 Regulates Fluid Shear Stress-Induced Differentiation in Periodontal Ligament Cells through mTOR Signaling Pathway. Cell. Physiol. Biochem. 2014, 33, 433–445. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, L.; Chen, Y.; Gui, J.; Li, Q.; Huang, Y.; Liu, M.; Jia, X.; Song, W.; Ji, J.; et al. The effects of fluid shear stress on proliferation and osteogenesis of human periodontal ligament cells. J. Biomech. 2016, 49, 572–579. [Google Scholar] [CrossRef]
- Tang, M.; Peng, Z.; Mai, Z.; Chen, L.; Mao, Q.; Chen, Z.; Chen, Q.; Liu, L.; Wang, Y.; Ai, H. Fluid shear stress stimulates osteogenic differentiation of human periodontal ligament cells via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling pathways. J. Periodontol. 2014, 85, 1806–1813. [Google Scholar] [CrossRef]
- Zheng, L.; Huang, Y.; Song, W.; Gong, X.; Liu, M.; Jia, X.; Zhou, G.; Chen, L.; Li, A.; Fan, Y. Fluid shear stress regulates metalloproteinase-1 and 2 in human periodontal ligament cells: Involvement of extracellular signal-regulated kinase (ERK) and P38 signaling pathways. J. Biomech. 2012, 45, 2368–2375. [Google Scholar] [CrossRef]
- Van Der Pauw, M.T.M.; Klein-Nulend, J.; Van Den Bos, T.; Burger, E.H.; Everts, V.; Beertsen, W. Response of periodontal ligament fibroblasts and gingival fibroblasts to pulsating fluid flow: Nitric oxide and prostaglandin E2 release and expression of tissue non-specific alkaline phosphatase activity. J. Periodontal Res. 2000, 35, 335–343. [Google Scholar] [CrossRef]
- Limjeerajarus, N.; Keawprachum, B.; Pliankum, M.; Pavasant, P.; Limjeerajarus, C.N. Numerical data on the shear stress distribution generated by a rotating rod within a stationary ring over a 35-mm cell culture dish. Data Brief 2018, 21, 2253–2258. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D.J.M. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT. Method 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Arce, F.; Breckpot, K.; Stephenson, H.; Karwacz, K.; Ehrenstein, M.; Collins, M.; Escors, D. Selective ERK Activation Differentiates Mouse and Human Tolerogenic Dendritic Cells, Expands Antigen-Specific Regulatory T Cells, and Suppresses Experimental Inflammatory Arthritis. Arthritis Rheum. 2011, 63, 84–95. [Google Scholar] [CrossRef]
- Martkamchan, S.; Onlamoon, N.; Wang, S.; Pattanapanyasat, K.; Ammaranond, P. The Effects of Anti-CD3/CD28 Coated Beads and IL-2 on Expanded T Cell for Immunotherapy. Adv. Clin. Exp. Med. 2016, 25, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Kouzbari, K.; Hossan, M.R.; Arrizabalaga, J.H.; Varshney, R.; Simmons, A.D.; Gostynska, S.; Nollert, M.U.; Ahamed, J. Oscillatory shear potentiates latent TGF-β1 activation more than steady shear as demonstrated by a novel force generator. Sci. Rep. 2019, 9, 6065. [Google Scholar] [CrossRef] [Green Version]
- Forteza, M.J.; Polyzos, K.A.; Baumgartner, R.; Suur, B.E.; Mussbacher, M.; Johansson, D.K.; Hermansson, A.; Hansson, G.K.; Ketelhuth, D.F.J. Activation of the Regulatory T-Cell/Indoleamine 2,3-Dioxygenase Axis Reduces Vascular Inflammation and Atherosclerosis in Hyperlipidemic Mice. Front. Immunol. 2018, 9, 950. [Google Scholar] [CrossRef] [Green Version]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.W.; Feng, G. Relationship between interferon-γ, indoleamine 2, 3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef]
- Pallotta, M.T.; Orabona, C.; Volpi, C.; Vacca, C.; Belladonna, M.L.; Bianchi, R.; Servillo, G.; Brunacci, C.; Calvitti, M.; Bicciato, S. Indoleamine 2, 3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011, 12, 870–878. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Sipos, K.; Go, Y.-M.; Law, R.; Rong, J.; McDonald, J. Differential Effect of Shear Stress on Extracellular Signal-regulated Kinase and N-terminal Jun Kinase in Endothelial Cells Gi2- and Gβ/gamma-Dependent Signaling Pathways. J. Biol. Chem. 1997, 272, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.L.; Lin, Y.H.; Xiao, H.; Xing, S.; Chen, H.; Chi, P.D.; Zhang, G. Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-κB pathways: Impairment in T cell functions. J. Virol. 2014, 88, 6660–6671. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4(+) T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Li, X.; Song, W.; Li, D.; Yu, D.; Zeng, X.; Li, M.; Leng, X.; Li, X. CD4(+)CD25 (+)CD127 (low/-) T cells: A more specific Treg population in human peripheral blood. Inflammation 2012, 35, 1773–1780. [Google Scholar] [CrossRef]
- Seddiki, N.; Santner-Nanan, B.; Martinson, J.; Zaunders, J.; Sasson, S.; Landay, A.; Solomon, M.; Selby, W.; Alexander, S.I.; Nanan, R.; et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006, 203, 1693–1700. [Google Scholar] [CrossRef]
- De Zoeten, E.F.; Lee, I.; Wang, L.; Chen, C.; Ge, G.; Wells, A.D.; Hancock, W.W.; Ozkaynak, E. Foxp3 processing by proprotein convertases and control of regulatory T cell function. J. Biol. Chem. 2009, 284, 5709–5716. [Google Scholar] [CrossRef] [Green Version]
- Kondĕlková, K.; Vokurková, D.; Krejsek, J.; Borská, L.; Fiala, Z.; Ctirad, A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med. 2010, 53, 73–77. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwittayarak, R.; Klincumhom, N.; Ngaokrajang, U.; Namangkalakul, W.; Ferreira, J.N.; Pavasant, P.; Osathanon, T. Shear Stress Enhances the Paracrine-Mediated Immunoregulatory Function of Human Periodontal Ligament Stem Cells via the ERK Signalling Pathway. Int. J. Mol. Sci. 2022, 23, 7119. https://doi.org/10.3390/ijms23137119
Suwittayarak R, Klincumhom N, Ngaokrajang U, Namangkalakul W, Ferreira JN, Pavasant P, Osathanon T. Shear Stress Enhances the Paracrine-Mediated Immunoregulatory Function of Human Periodontal Ligament Stem Cells via the ERK Signalling Pathway. International Journal of Molecular Sciences. 2022; 23(13):7119. https://doi.org/10.3390/ijms23137119
Chicago/Turabian StyleSuwittayarak, Ravipha, Nuttha Klincumhom, Utapin Ngaokrajang, Worachat Namangkalakul, João N. Ferreira, Prasit Pavasant, and Thanaphum Osathanon. 2022. "Shear Stress Enhances the Paracrine-Mediated Immunoregulatory Function of Human Periodontal Ligament Stem Cells via the ERK Signalling Pathway" International Journal of Molecular Sciences 23, no. 13: 7119. https://doi.org/10.3390/ijms23137119
APA StyleSuwittayarak, R., Klincumhom, N., Ngaokrajang, U., Namangkalakul, W., Ferreira, J. N., Pavasant, P., & Osathanon, T. (2022). Shear Stress Enhances the Paracrine-Mediated Immunoregulatory Function of Human Periodontal Ligament Stem Cells via the ERK Signalling Pathway. International Journal of Molecular Sciences, 23(13), 7119. https://doi.org/10.3390/ijms23137119







