Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts
Abstract
:1. Introduction
2. Results
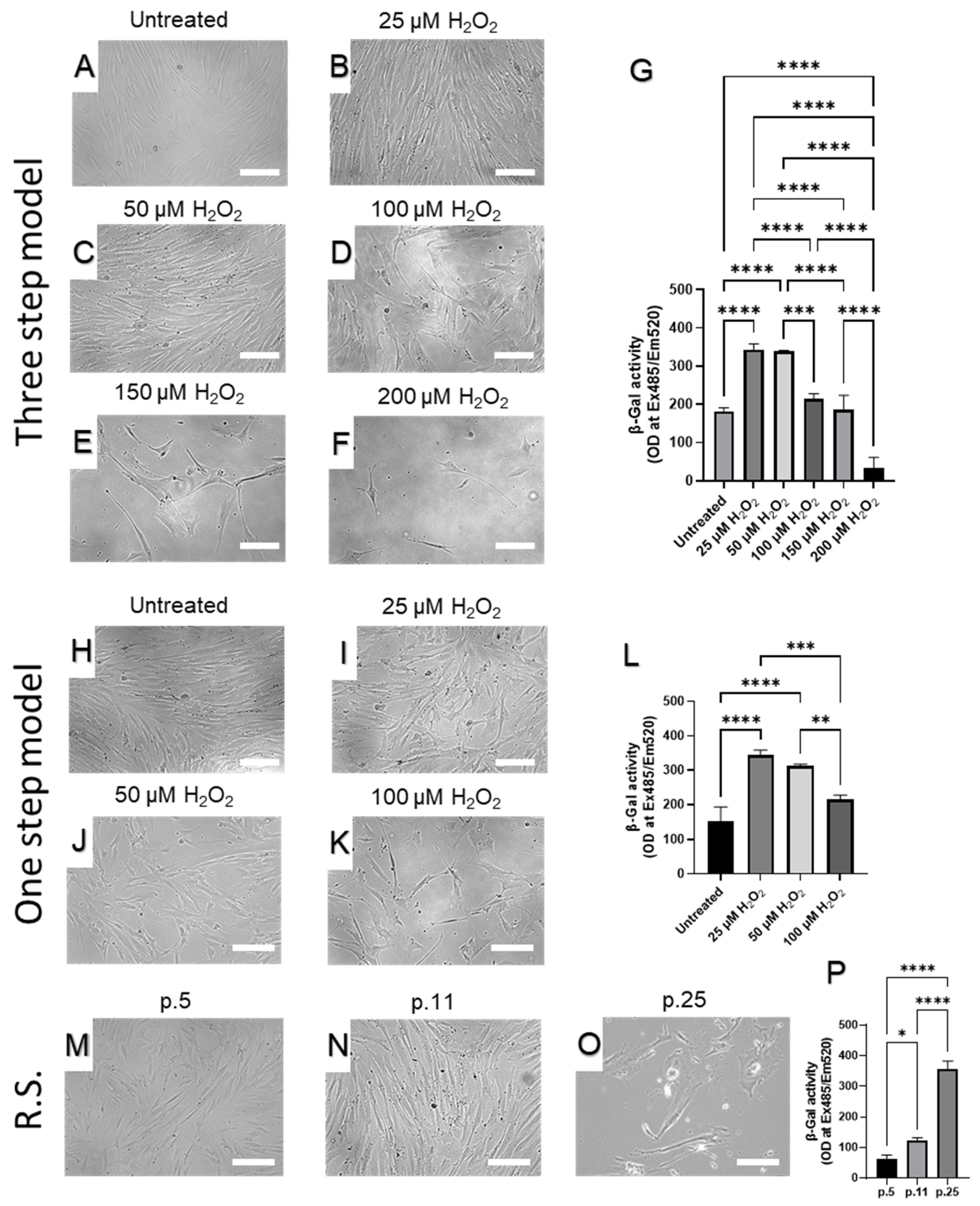
2.1. Setup of the Senescence Model System
2.2. Senescence-Induced Morphological Transition
2.3. β-Galactosidase as a Biomarker for Senescence-Associated Phenotype
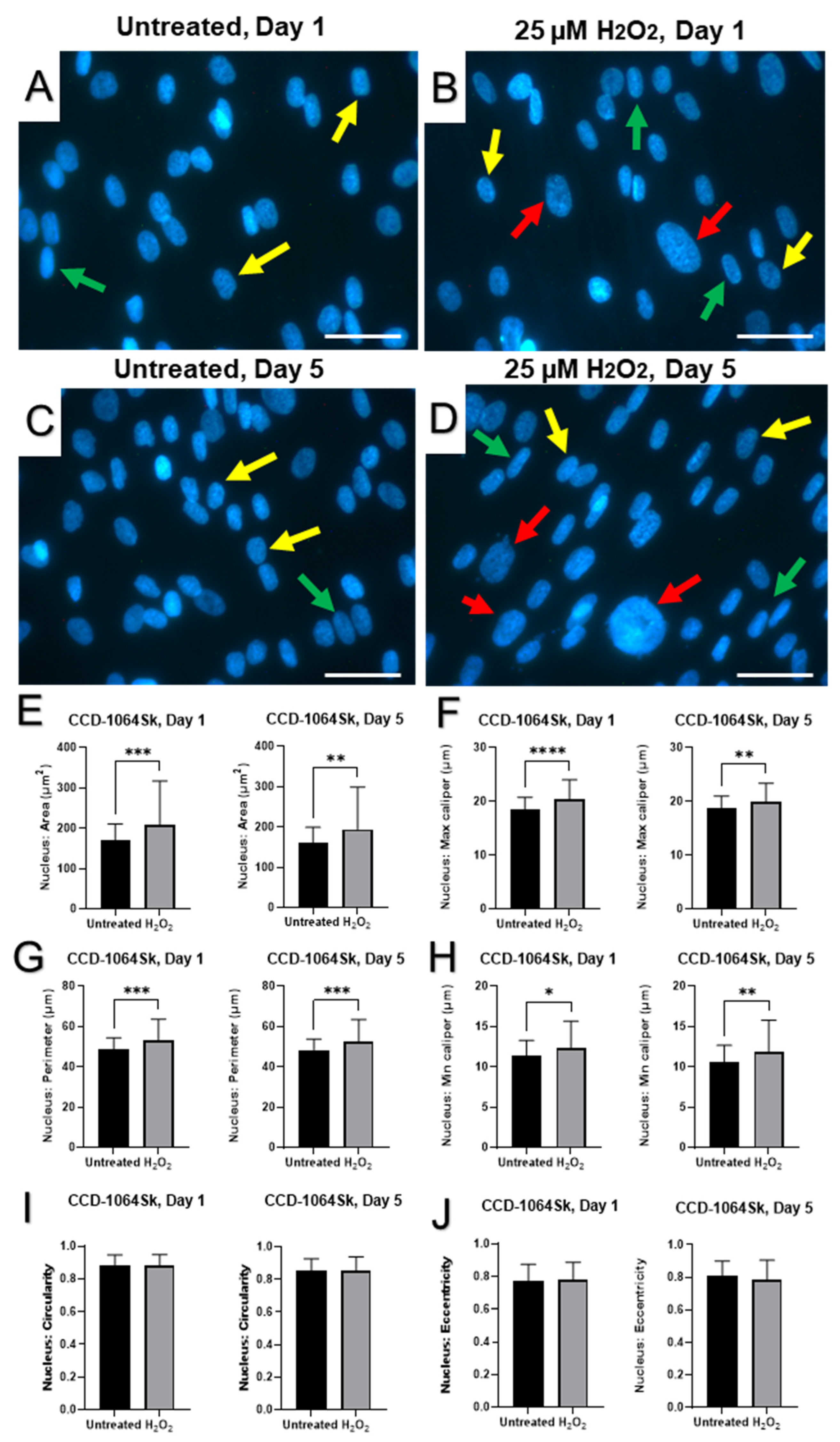
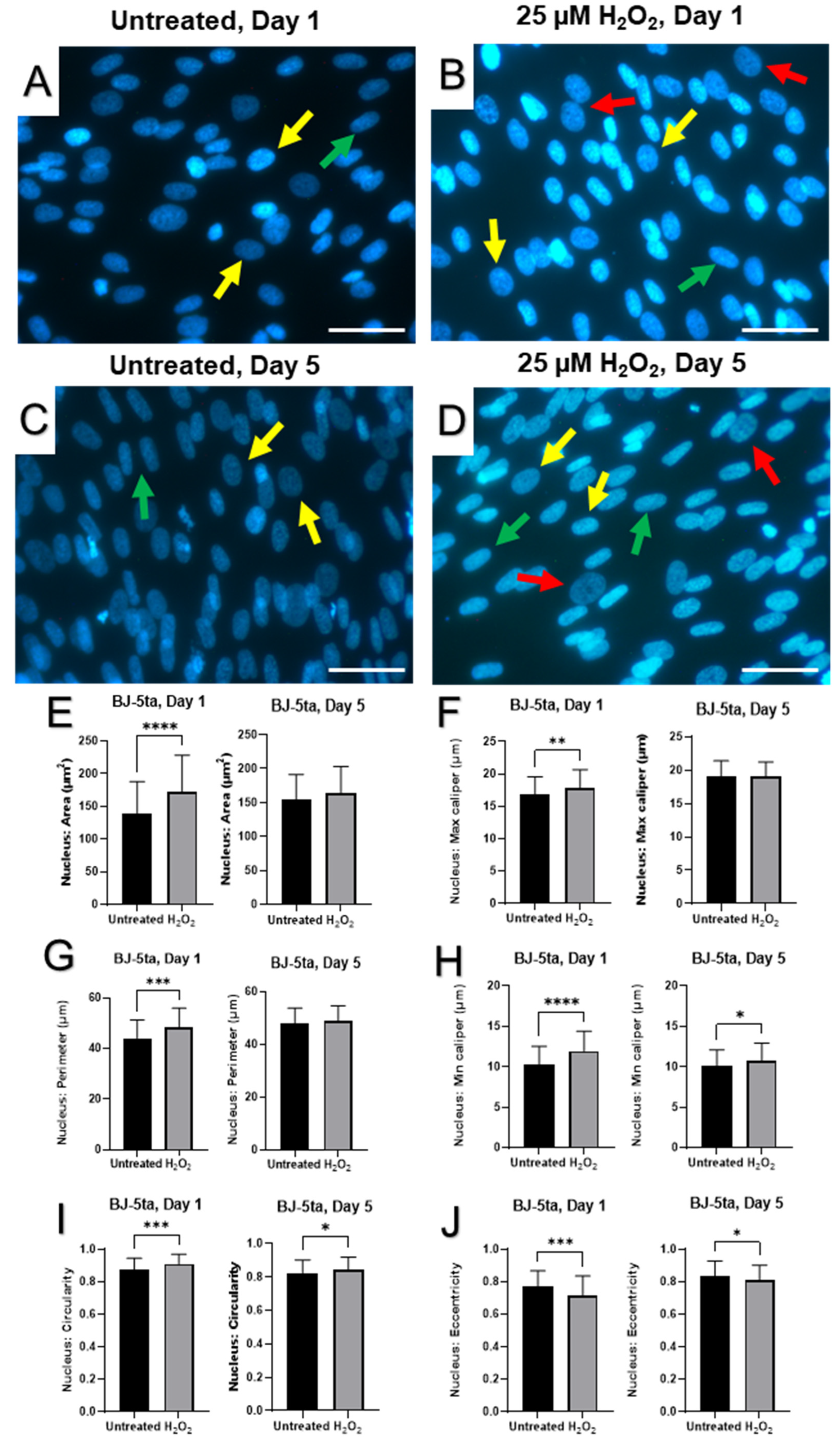
2.4. Senescence-Associated Changes in Nuclear Morphology
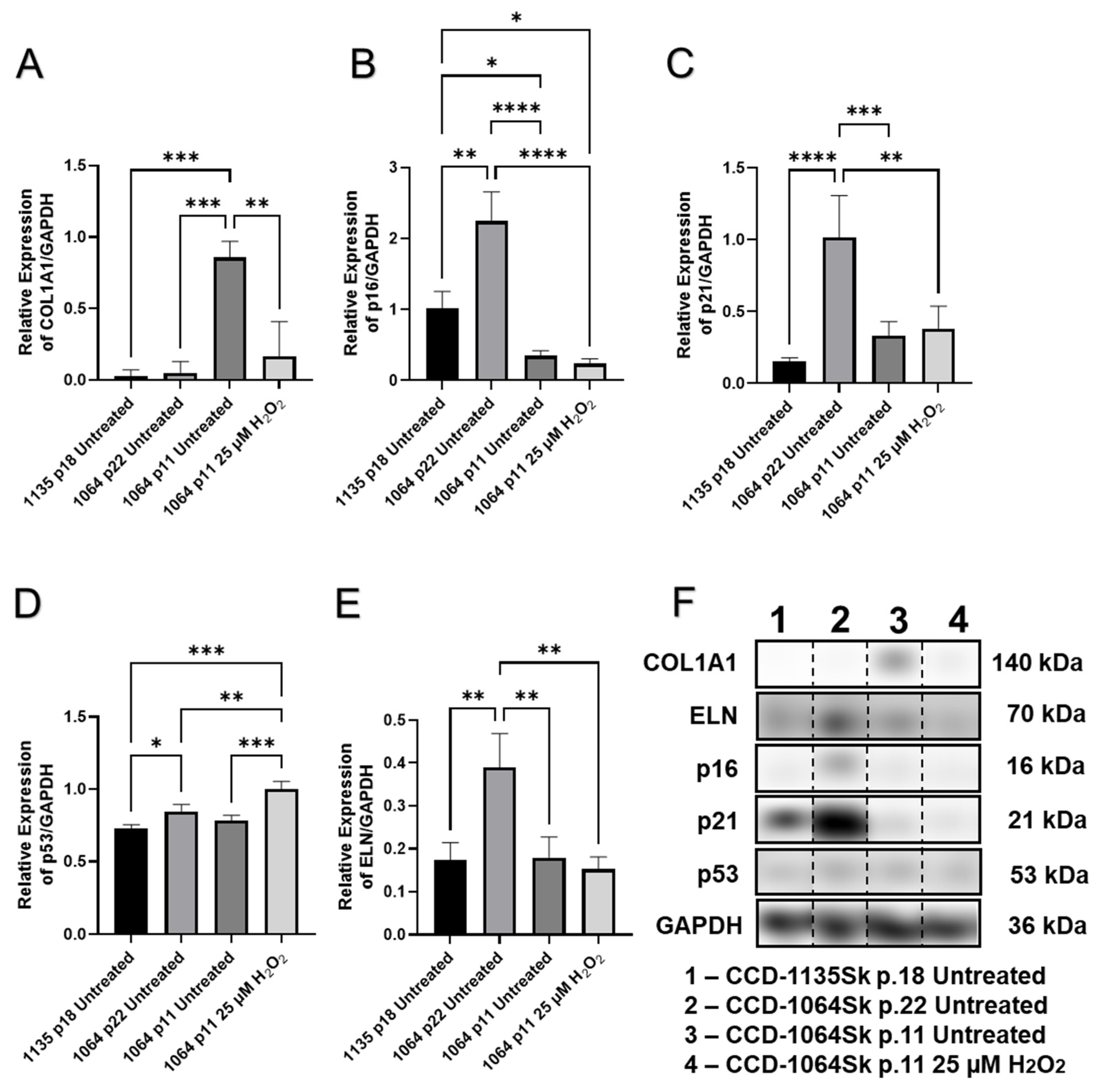
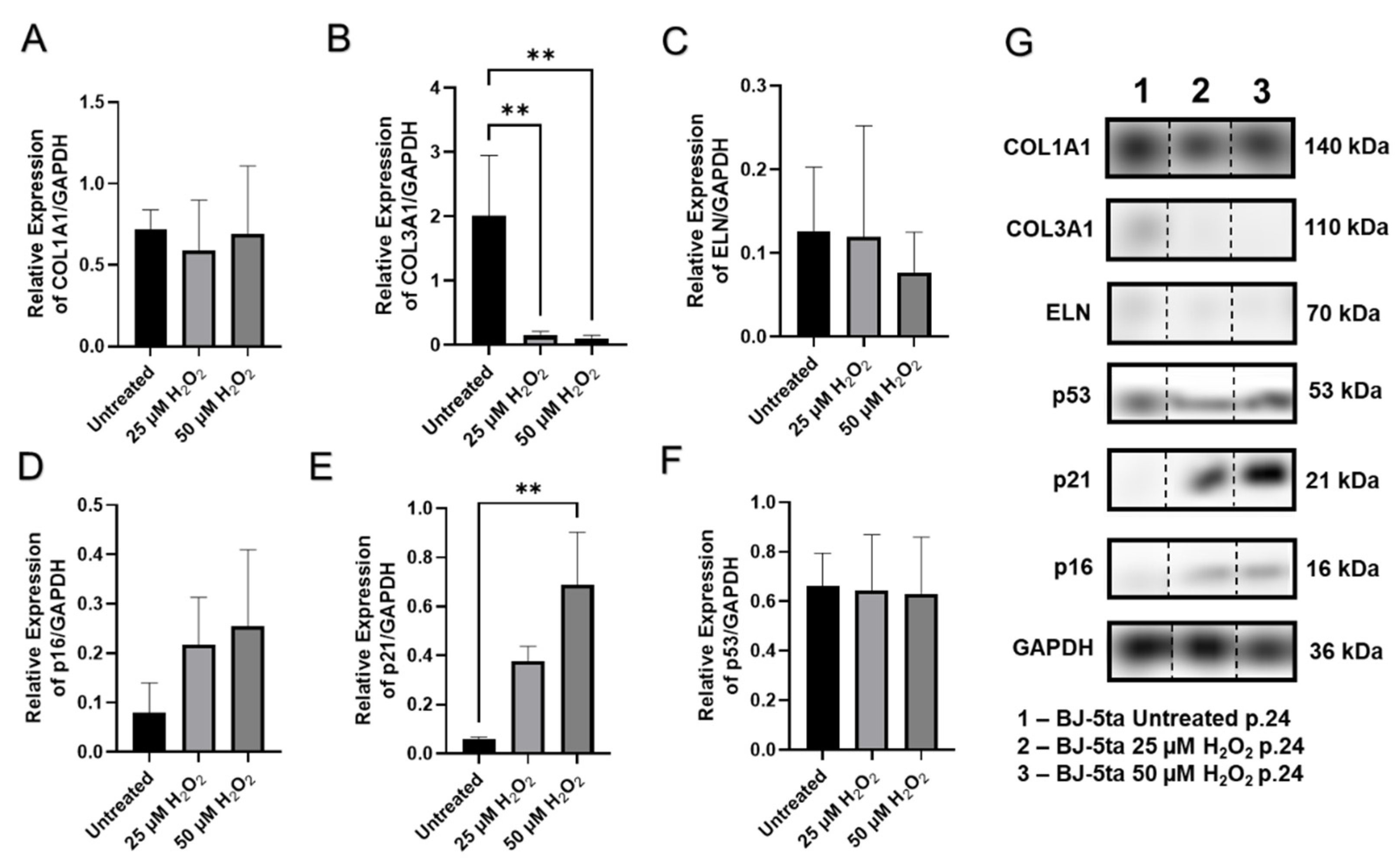
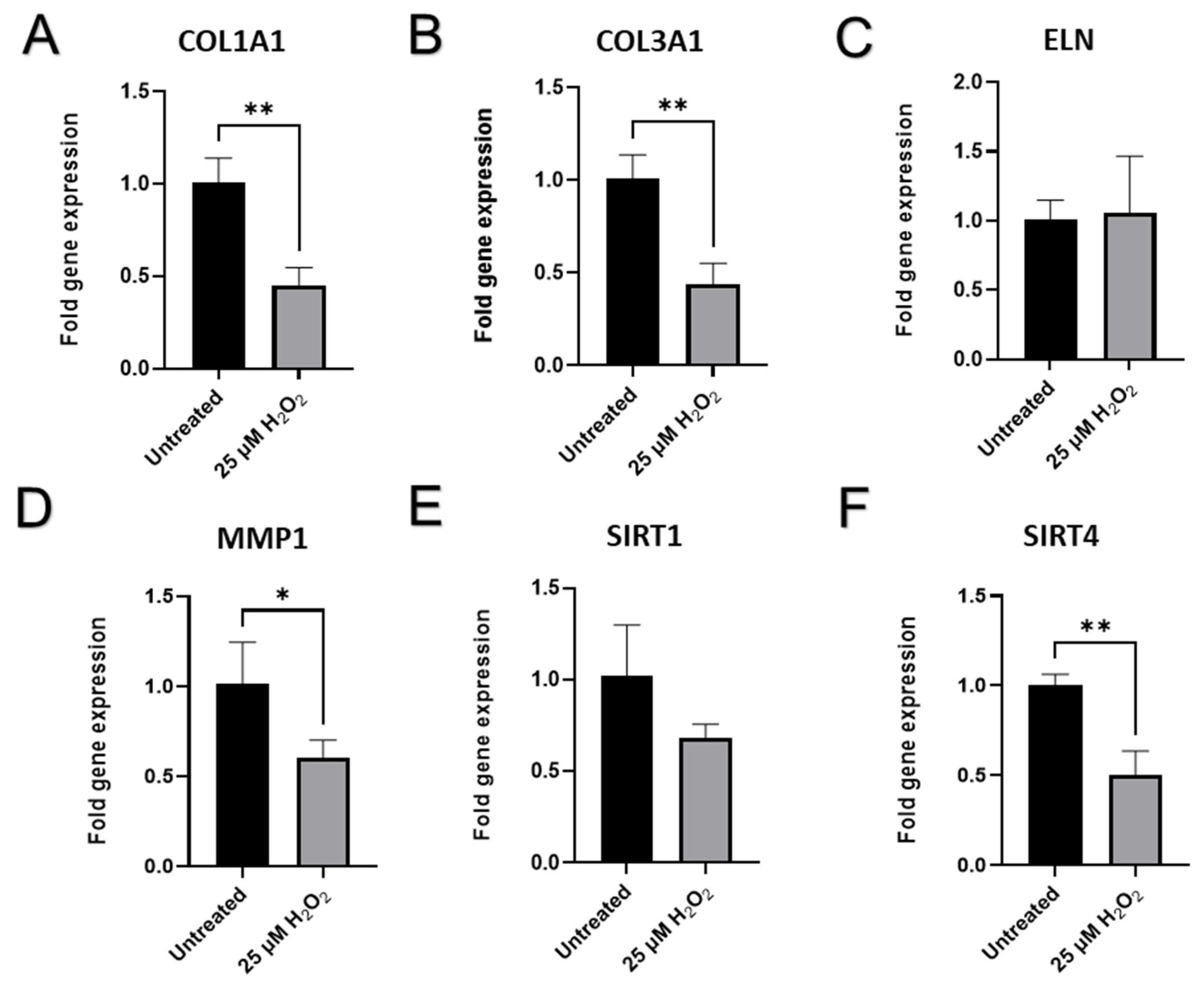
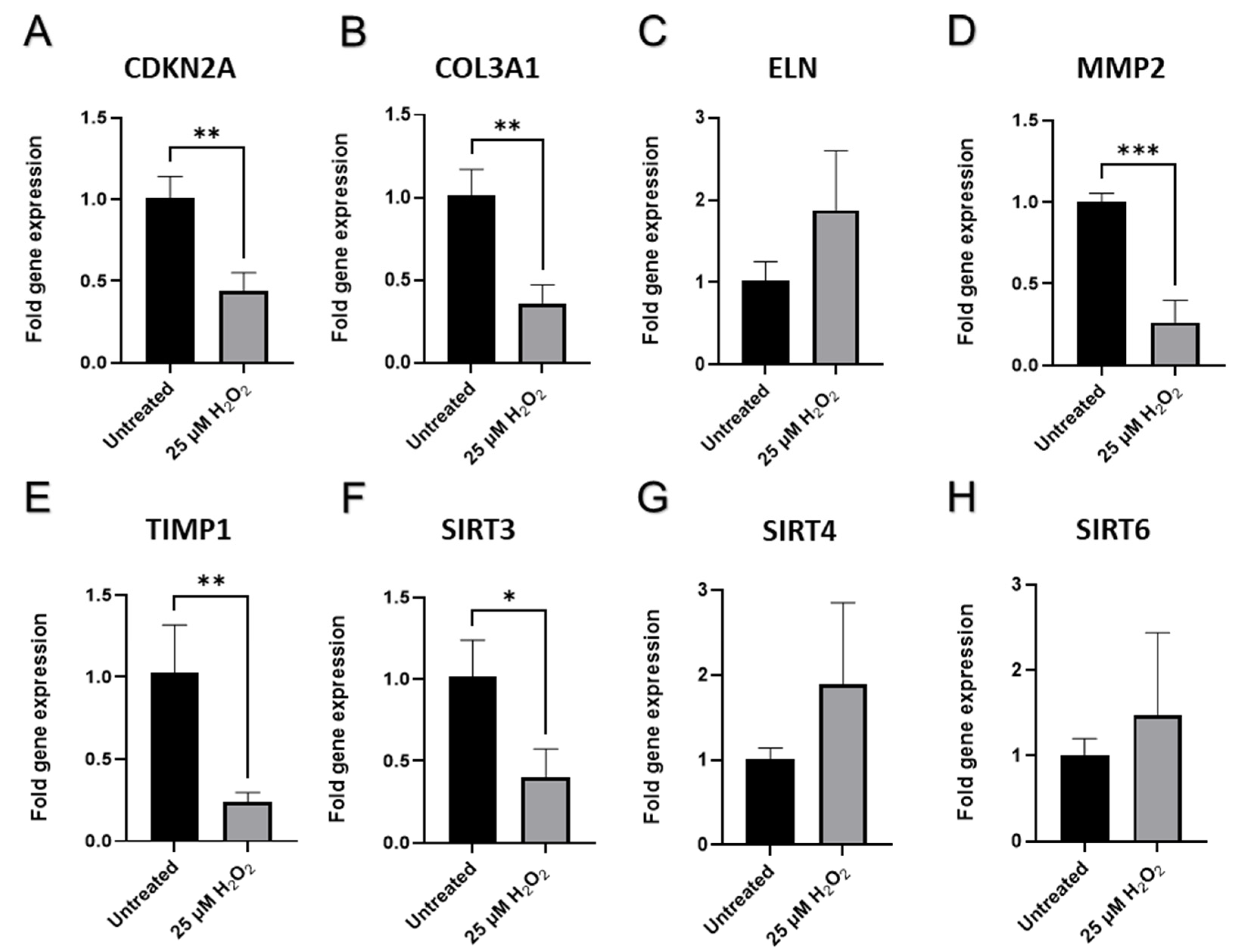
2.5. Senescence-Related Changes to Gene Expression Profiles Are Accompanied by Elevation in the Expression of Cell-Cycle Regulators
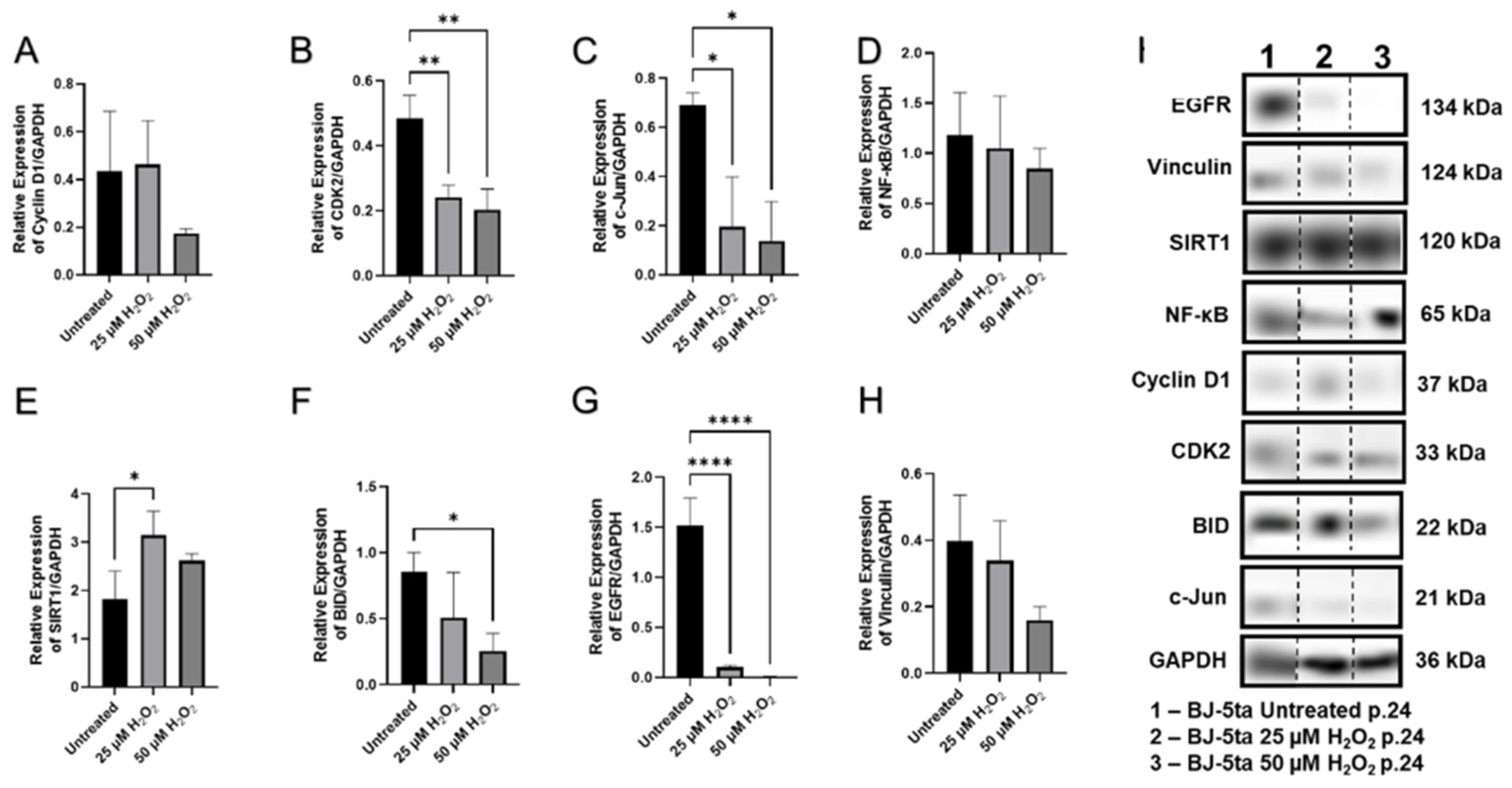
2.6. Senescence-Related Changes in the Expression of Genes and Proteins Involved in Cell Cycle Regulation, Cellular Replication, and Metabolic Responses
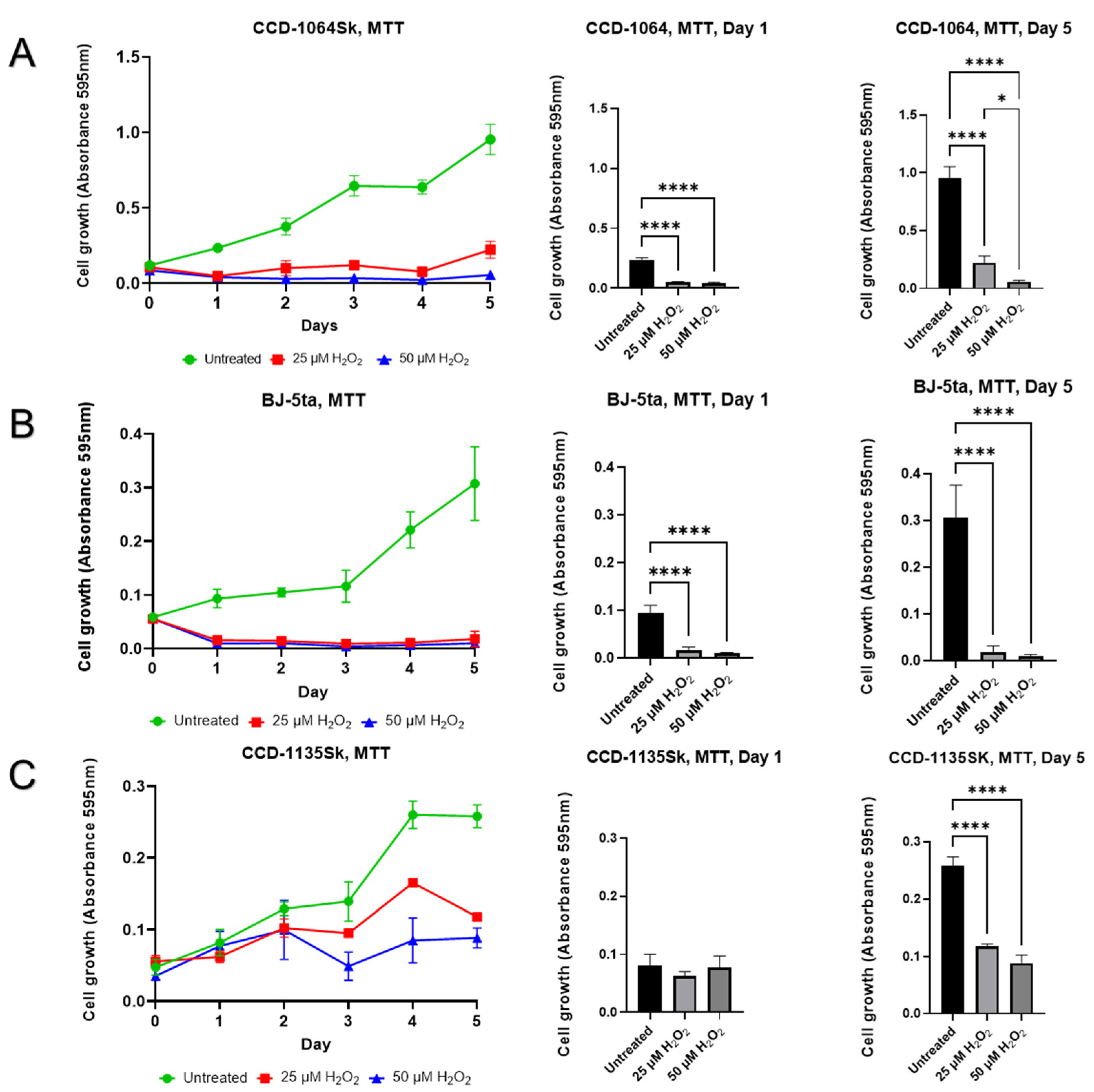
2.7. Aspects of Senescence-Associated Cellular Viability
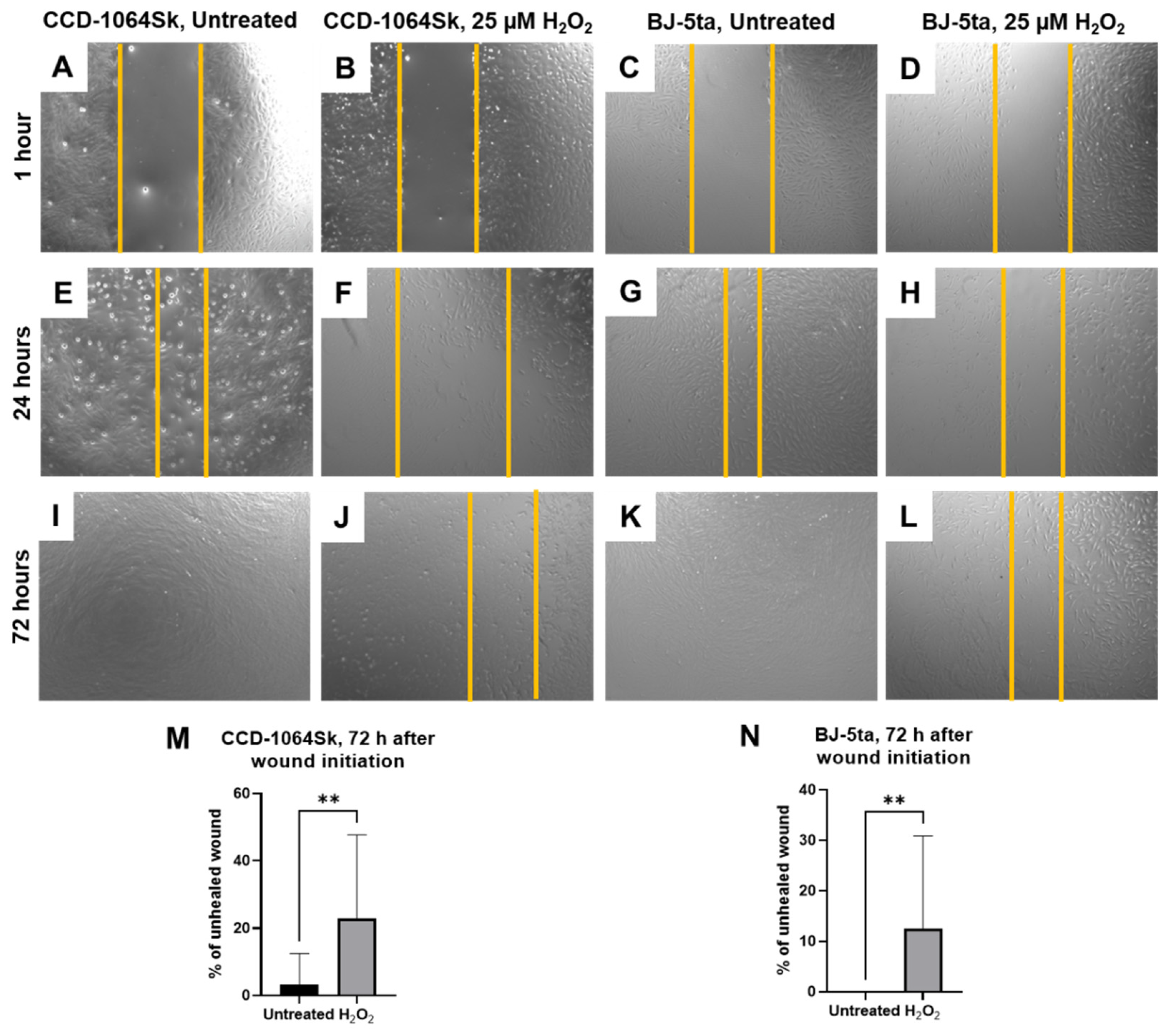
2.8. Senescent Fibroblasts Showed Reduced Ability in the Healing Process
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture and Maintenance
5.2. Senescence-Associated Phenotype Modelling
5.2.1. Three-Step Model of Skin Fibroblast Senescence
5.2.2. One-step Hydrogen Peroxide Skin Fibroblast Senescence Model
5.3. β-Galactosidase Analysis
5.4. Cell Viability/Cytotoxicity
5.4.1. The Micro-Culture Tetrazolium Assay (MTT)
5.4.2. Neutral Red Stain
5.4.3. Crystal Violet Stain
5.5. Protein Extraction and Quantification
5.6. Western Immunoblotting
5.7. RNA Isolation
5.8. Quantitative Real-Time PCR (RT-qPCR)
5.9. Wound-Healing Assay
5.10. Immunocytochemistry
5.11. QuPath Analysis
5.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boraldi, F.; Annovi, G.; Tiozzo, R.; Sommer, P.; Quaglino, D. Comparison of ex vivo and In Vitro human fibroblast ageing models. Mech. Ageing Dev. 2010, 131, 625–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalfalah, F.; Sobek, S.; Bornholz, B.; Götz-Rösch, C.; Tigges, J.; Fritsche, E.; Krutmann, J.; Köhrer, K.; Deenen, R.; Ohse, S.; et al. Inadequate mito-biogenesis in primary dermal fibroblasts from old humans is associated with impairment of PGC1A-independent stimulation. Exp. Gerontol. 2014, 56, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Coutière, P.; Woillez, M.; Mérillon, J.-M.; Vitrac, X. DNA macroarray study of skin aging-related genes expression modulation by antioxidant plant extracts on a replicative senescence model of human dermal fibroblasts. Phytother. Res. 2011, 25, 686–693. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berneburg, M.; Plettenberg, H.; Medve-König, K.; Pfahlberg, A.; Gers-Barlag, H.; Gefeller, O.; Krutmann, J. Induction of the photoaging-associated mitochondrial common deletion In Vivo in normal human skin. J. Investig. Dermatol. 2004, 122, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.M.; Han, S.; Oh, J.-H.; Lee, Y.M.; Park, C.-H.; Shin, C.-Y.; Lee, D.H.; Chung, J.H. A synthetic peptide blocking TRPV1 activation inhibits UV-induced skin responses. J. Dermatol. Sci. 2017, 88, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Toutfaire, M.; Bauwens, E.; Debacq-Chainiaux, F. The impact of cellular senescence in skin ageing: A notion of mosaic and therapeutic strategies. Biochem. Pharmacol. 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The role of microRNAs in organismal and skin aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef]
- Kemp, M.G.; Spandau, D.F.; Travers, J.B. Impact of age and insulin-like growth factor-1 on DNA damage responses in UV-irradiated human skin. Molecules 2017, 22, 356. [Google Scholar] [CrossRef] [Green Version]
- Lin’Kova, N.S.; Drobintseva, A.O.; Orlova, O.A.; Kuznetsova, E.P.; Polyakova, V.O.; Kvetnoy, I.M.; Khavinson, V.K. Peptide regulation of skin fibroblast functions during their aging In Vitro. Bull. Exp. Biol. Med. 2016, 161, 175–178. [Google Scholar] [CrossRef]
- Bertschmann, J.; Thalappilly, S.; Riabowol, K. The ING1a model of rapid cell senescence. Mech. Ageing Dev. 2019, 177, 109–117. [Google Scholar] [CrossRef] [PubMed]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence In Vitro and In Vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Horan, M.A.; Herrick, S.E.; Tarnuzzer, R.W.; Schultz, G.S.; Ferguson, M.W.J. Age-related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res. 1997, 290, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Maibach, H.I. Degenerative changes in aging skin. In Textbook of Aging Skin, 2nd ed.; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 15–30. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [Green Version]
- Rattan, S.I.S. Origins of the Hayflick system, the phenomenon and the limit. In Cellular Ageing and Replicative Senescence. Healthy Ageing and Longevity; Rattan, S.I.S., Hayflick, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 3–14. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Ott, C.; Jung, T.; Grune, T.; Höhn, A. SIPS as a model to study age-related changes in proteolysis and aggregate formation. Mech. Ageing Dev. 2017, 170, 72–81. [Google Scholar] [CrossRef]
- Brack, C.; Lithgow, G.J.; Osiewacz, H.D.; Toussaint, O. EMBO WORKSHOP REPORT: Molecular and cellular gerontology Serpiano, Switzerland, September 18-22, 1999. EMBO J. 2000, 19, 1929–1934. [Google Scholar] [CrossRef] [Green Version]
- Toussaint, O.; Michiels, C.; Raes, M.; Remacle, J. Cellular aging and the importance of energetic factors. Exp. Gerontol. 1995, 30, 1–22. [Google Scholar] [CrossRef]
- Toussaint, O.; Medrano, E.; von Zglinicki, T. Cellular and Molecular Mechanisms of Stress-Induced Premature Senescence (SIPS) of Human Diploid Fibroblasts and Melanocytes. 2000. Available online: www.elsevier.nl/locate/expgero (accessed on 1 May 2022).
- Chen, Q.; Fischer, A.; Reagan, J.D.; Yan, L.-J.; Ames, B.N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl. Acad. Sci. USA 1995, 92, 4337–4341. [Google Scholar] [CrossRef] [Green Version]
- Marionnet, C.; Pierrard, C.; Lejeune, F.; Sok, J.; Thomas, M.; Bernerd, F. Different Oxidative Stress Response in Keratinocytes and Fibroblasts of Reconstructed Skin Exposed to Non Extreme Daily-Ultraviolet Radiation. PLoS ONE 2010, 5, e12059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhard, D.; Matt, K.; Burger, K.; Bergemann, J. Shortwave UV-Induced Damage as Part of the Solar Damage Spectrum Is Not a Major Contributor to Mitochondrial Dysfunction. J. Biochem. Mol. Toxicol. 2014, 28, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Derm.-Endocrinol. 2012, 4, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Douki, T.; Reynaud-Angelin, A.; Cadet, J.; Sage, E. Bipyrimidine Photoproducts Rather than Oxidative Lesions Are the Main Type of DNA Damage Involved in the Genotoxic Effect of Solar UVA Radiation. Biochemistry 2003, 42, 9221–9226. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Chang, T.-M.; Chang, Y.-J.; Wen, H.-Y. UVB Irradiation Regulates ERK1/2- and p53-Dependent Thrombomodulin Expression in Human Keratinocytes. PLoS ONE 2013, 8, e67632. [Google Scholar] [CrossRef] [Green Version]
- Greussing, R.; Hackl, M.; Charoentong, P.; Pauck, A.; Monteforte, R.; Cavinato, M.; Hofer, E.; Scheideler, M.; Neuhaus, M.; Micutkova, L.; et al. Identification of microRNA-mRNA functional interactions in UVB-induced senescence of human diploid fibroblasts. BMC Genom. 2013, 14, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilnytskyy, Y.; Koturbash, I.; Kovalchuk, O. Radiation-induced bystander effects In Vivo are epigenetically regulated in a tissue-specific manner. Environ. Mol. Mutagen. 2008, 50, 105–113. [Google Scholar] [CrossRef]
- Dickey, J.S.; Zemp, F.J.; Martin, O.A.; Kovalchuk, O. The role of miRNA in the direct and indirect effects of ionizing radiation. Radiat. Environ. Biophys. 2011, 50, 491–499. [Google Scholar] [CrossRef]
- Sidler, C.; Li, D.; Wang, B.; Kovalchuk, I.; Kovalchuk, O. SUV39H1 downregulation induces deheterochromatinization of satellite regions and senescence after exposure to ionizing radiation. Front. Genet. 2014, 5, 411. [Google Scholar] [CrossRef] [Green Version]
- Kovalchuk, O.; Burke, P.; Besplug, J.; Slovack, M.; Filkowski, J.; Pogribny, I. Methylation changes in muscle and liver tissues of male and female mice exposed to acute and chronic low-dose X-ray-irradiation. Mutat. Res. Mol. Mech. Mutagen. 2004, 548, 75–84. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.E.; Choi, K.J.; Bae, S.; Kim, D.H. Characterization of DNA damage-induced cellular senescence by ionizing radiation in endothelial cells. Int. J. Radiat. Biol. 2014, 90, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Rodemann, H.P. Differential degradation of intracellular proteins in human skin fibroblasts of mitotic and mitomycin-C (MMC)-induced postmitotic differentiation states In Vitro. Differentiation 1989, 42, 37–43. [Google Scholar] [CrossRef] [PubMed]
- De Magalhães, J.P. From cells to ageing: A review of models and mechanisms of cellular senescence and their impact on human ageing. Exp. Cell Res. 2004, 300, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Robles, S.J.; Adami, G.R. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 1998, 16, 1113–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, Y.Y.; Liu, H.; Zhang, X.; Hecker, L.; Bernard, K.; Desai, L.; Liu, G.; Thannickal, V.J. Histone Modifications in Senescence-Associated Resistance to Apoptosis by Oxidative Stress. Redox Biol. 2013, 1, 8–16. [Google Scholar] [CrossRef] [Green Version]
- I Shlush, L.; Itzkovitz, S.; Cohen, A.; Rutenberg, A.; Berkovitz, R.; Yehezkel, S.; Shahar, H.; Selig, S.; Skorecki, K. Quantitative digital in situ senescence-associated β-galactosidase assay. BMC Cell Biol. 2011, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Caldini, R.; Chevanne, M.; Mocali, A.; Tombaccini, D.; Paoletti, F. Premature induction of aging in sublethally H2O2-treated young MRC5 fibroblasts correlates with increased glutathione peroxidase levels and resistance to DNA breakage. Mech. Ageing Dev. 1998, 105, 137–150. [Google Scholar] [CrossRef]
- Chen, Q.; Ames, B.N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc. Natl. Acad. Sci. USA 1994, 91, 4130–4134. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Kajiya, H.; Ozeki, S.; Okabe, K.; Ikebe, T. Reactive oxygen species promotes cellular senescence in normal human epidermal keratinocytes through epigenetic regulation of p16INK4a. Biochem. Biophys. Res. Commun. 2014, 452, 622–628. [Google Scholar] [CrossRef]
- Kirkland, J.B. Lipid peroxidation, protein thiol oxidation and DNA damage in hydrogen peroxide-induced injury to endothelial cells: Role of activation of poly(ADP-ribose)polymerase. Biochim. Biophys. Acta 1991, 1092, 319–325. [Google Scholar] [CrossRef]
- Gerasymchuk, M. Genomic instability and aging: Causes and consequences. Genome Stab. 2021, 26, 533–553. [Google Scholar] [CrossRef]
- Hayflick, L. The limited In Vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin In Vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, R.U.; Soujanya, M.; Mishra, R.K. Deterioration of nuclear morphology and architecture: A hallmark of senescence and aging. Ageing Res. Rev. 2021, 67, 101264. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Hughes, A.L.; Madeo, F.; Ruckenstuhl, C. The crucial impact of lysosomes in aging and longevity. Ageing Res. Rev. 2016, 32, 2–12. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and In Vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Skandalis, S.S.; Karalis, T.; Heldin, P. Intracellular hyaluronan: Importance for cellular functions. Semin. Cancer Biol. 2019, 62, 20–30. [Google Scholar] [CrossRef]
- Propato, A.; Cutrona, G.; Francavilla, V.; Ulivi, M.; Schiaffella, E.; Landt, O.; Dunbar, R.; Cerundolo, V.; Ferrarini, M.; Barnaba, V. Apoptotic cells overexpress vinculin and induce vinculin-specific cytotoxic T-cell cross-priming. Nat. Med. 2001, 7, 807–813. [Google Scholar] [CrossRef]
- Meng, Q.; Xia, Y. c-Jun, at the crossroad of the signaling network. Protein Cell 2011, 2, 889–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisdom, R.; Johnson, R.S.; Moore, C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999, 18, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochette, L.; Mazini, L.; Meloux, A.; Zeller, M.; Cottin, Y.; Vergely, C.; Malka, G. Anti-Aging Effects of GDF11 on Skin. Int. J. Mol. Sci. 2020, 21, 2598. [Google Scholar] [CrossRef] [PubMed]
- Bonkowski, M.; Sinclair, M.S.B.D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 253–295. [Google Scholar] [CrossRef] [Green Version]
- Serrano, L.; Martínez-Redondo, P.; Marazuela-Duque, A.; Vazquez, B.N.; Dooley, S.J.; Voigt, P.; Beck, D.B.; Kane-Goldsmith, N.; Tong, Q.; Rabanal, R.M.; et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev. 2013, 27, 639–653. [Google Scholar] [CrossRef] [Green Version]
- Dryden, S.C.; Nahhas, F.A.; Nowak, J.E.; Goustin, A.-S.; Tainsky, M.A. Role for Human SIRT2 NAD-Dependent Deacetylase Activity in Control of Mitotic Exit in the Cell Cycle. Mol. Cell. Biol. 2003, 23, 3173–3185. [Google Scholar] [CrossRef] [Green Version]
- Toiber, D.; Sebastian, C.; Mostoslavsky, R. Characterization of Nuclear Sirtuins: Molecular Mechanisms and Physiological Relevance. Handb. Exp. Pharmacol. 2011, 206, 189–224. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Tian, R.; Huang, J.; Yang, Y.; Dai, J.; Jiang, R.; Zhang, L. Metformin alleviated endotoxemia-induced acute lung injury via restoring AMPK-dependent suppression of mTOR. Chem. Interact. 2018, 291, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Karnewar, S.; Neeli, P.K.; Panuganti, D.; Kotagiri, S.; Mallappa, S.; Jain, N.; Jerald, M.K.; Kotamraju, S. Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: Relevance in age-associated vascular dysfunction. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Hu, B.; Feng, G.; Xiang, M.; Deng, Y.; Tan, M.; Li, J.; Song, J. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology 2019, 21, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J. Shedding Light on the Effects of Calorie Restriction and Its Mimetics on Skin Biology. Nutrients 2020, 12, 1529. [Google Scholar] [CrossRef]
- Frippiat, C.; Chen, Q.M.; Zdanov, S.; de Magalhaes, J.P.; Remacle, J.; Toussaint, O. Subcytotoxic H2O2 Stress Triggers a Release of Transforming Growth Factor-β1, Which Induces Biomarkers of Cellular Senescence of Human Diploid Fibroblasts. J. Biol. Chem. 2001, 276, 2531–2537. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.M.; Bartholomew, J.C.; Campisi, J.; Acosta, M.; Reagan, J.D.; Ames, B.N. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem. J. 1998, 332, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Sidler, C.; Woycicki, R.; Kovalchuk, I.; Kovalchuk, O. WI-38 senescence is associated with global and site-specific hypomethylation. Aging 2014, 6, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Chhunchha, B.; Singh, P.; Stamer, W.D.; Singh, D.P. Prdx6 retards senescence and restores trabecular meshwork cell health by regulating reactive oxygen species. Cell Death Discov. 2017, 3, 17060. [Google Scholar] [CrossRef] [Green Version]
- Robbins, E.; Levine, E.M.; Eagle, H. Morphologic changes accompanying senescence of cultured human diploid cells. J. Exp. Med. 1970, 131, 1211–1222. [Google Scholar] [CrossRef]
- Burova, E.; Borodkina, A.; Shatrova, A.; Nikolsky, N. Sublethal Oxidative Stress Induces the Premature Senescence of Human Mesenchymal Stem Cells Derived from Endometrium. Oxidative Med. Cell. Longev. 2013, 2013, 474931. [Google Scholar] [CrossRef]
- Ho, C.; Ng, S.; Chuang, H.; Wen, S.; Kuo, C.; Mahalakshmi, B.; Kuo, W. Extracts of Jasminum sambac flowers fermented by Lactobacillus rhamnosus inhibit H 2 O 2 - and UVB-induced aging in human dermal fibroblasts. Environ. Toxicol. 2020, 36, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Ng, S.-C.; Hsu, J.-Y.; Liu, H.; Chen, C.-J.; Huang, C.-Y.; Kuo, W.-W. Galangin Reverses H2O2-Induced Dermal Fibroblast Senescence via SIRT1-PGC-1α/Nrf2 Signaling. Int. J. Mol. Sci. 2022, 23, 1387. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.; Witkin, K.L.; Cohen-Fix, O. Sizing up the nucleus: Nuclear shape, size and nuclear-envelope assembly. J. Cell Sci. 2009, 122, 1477–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aifuwa, I.; Giri, A.; Longe, N.; Lee, S.H.; An, S.S.; Wirtz, D. Senescent stromal cells induce cancer cell migration via inhibition of RhoA/ROCK/myosin-based cell contractility. Oncotarget 2015, 6, 30516–30531. Available online: www.impactjournals.com/oncotarget/ (accessed on 1 May 2022). [CrossRef] [PubMed] [Green Version]
- Morris, T.A.; DeLorenzo, R.J.; Tombes, R.M. CaMK-II inhibition reduces Cyclin D1 levels and enhances the association of p27 kip1 with Cdk2 to cause G1 arrest in NIH 3T3 cells. Exp. Cell Res. 1998, 240, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Dulić, V.; Drullinger, L.F.; Lees, E.; I Reed, S.; Stein, G.H. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: Accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc. Natl. Acad. Sci. USA 1993, 90, 11034–11038. [Google Scholar] [CrossRef] [Green Version]
- Soydas, T.; Sayitoglu, M.; Sarac, E.Y.; Cınar, S.; Solakoglu, S.; Tiryaki, T.; Sultuybek, G.K. Metformin reverses the effects of high glucose on human dermal fibroblasts of aged skin via downregulating RELA/p65 expression. J. Physiol. Biochem. 2021, 77, 443–450. [Google Scholar] [CrossRef]
- Soydas, T.; Sarac, E.Y.; Cinar, S.; Dogan, S.; Solakoglu, S.; Tuncdemir, M.; Sultuybek, G.K. The protective effects of metformin in an in vitro model of aging 3T3 fibroblast under the high glucose conditions. J. Physiol. Biochem. 2018, 74, 273–281. [Google Scholar] [CrossRef]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.-M.; Chen, C.-W.; Lin, T.-Y.; Kuo, Y.-H. N-phenethyl caffeamide and photodamage: Protecting skin by inhibiting type I procollagen degradation and stimulating collagen synthesis. Food Chem. Toxicol. 2014, 72, 154–161. [Google Scholar] [CrossRef]
- Lämmermann, I.; Terlecki-Zaniewicz, L.; Weinmüllner, R.; Schosserer, M.; Dellago, H.; Branco, A.D.D.M.; Autheried, D.; Sevcnikar, B.; Kleissl, L.; Berlin, I.; et al. Blocking negative effects of senescence in human skin fibroblasts with a plant extract. Npj Aging Mech. Dis. 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Martino, O.; Tito, A.; De Lucia, A.; Cimmino, A.; Cicotti, F.; Apone, F.; Colucci, G.; Calabrò, V. Hibiscus syriacus Extract from an Established Cell Culture Stimulates Skin Wound Healing. BioMed Res. Int. 2017, 2017, 7932019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Quan, T. Molecular Mechanisms of Skin Aging and Age-Related Diseases, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Kang, S. Fitzpatrick’s Dermatology, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Toussaint, O.; Dumont, P.; Dierick, J.-F.; Pascal, T.; Frippiat, C.; Chainiaux, F.; Sluse, F.; Eliaers, F.; Remacle, J. Stress-Induced Premature Senescence: Essence of Life, Evolution, Stress, and Aging. Ann. N. Y. Acad. Sci. 2010, 908, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; Mccurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature pell penescence pssociated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016, 343–346. [Google Scholar] [CrossRef]
- Silasi, G.; Diaz-Heijtz, R.; Besplug, J.; Rodriguez-Juarez, R.; Titov, V.; Kolb, B.; Kovalchuk, O. Selective brain responses to acute and chronic low-dose X-ray irradiation in males and females. Biochem. Biophys. Res. Commun. 2004, 325, 1223–1235. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034. [Google Scholar] [CrossRef] [Green Version]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]


| Effective H2O2 Dose(s) | Lethal Dose | Time of Exposure | H2O2 Solvent | Type of Cells | Senescence Markers | Additional Findings in Senescent Cells | Reference |
|---|---|---|---|---|---|---|---|
| 25 μM | >50 μM | N/A | PBS | FSE cells, and BJ fibroblasts | ↑ SA-β-Gal | No differences with varying cell density | [39] |
| 50 μM | 1 mM | 30 min, every 2 days within 8 days | PBS | Human MRC5 fibroblasts | ↑ SA-β-Gal | ↓ c-fos; H2O2-induced DNA damage inversely correlated with GPx | [40] |
| 100 µM and 200 µM | N/A | 2 h | Cell culture medium | IMR-90 lung fibroblasts | Senescence morphology, ↑ SA-β-Gal, ↑ p16, p21, & caveolin-1 | ↑ Bcl-2 gene with H4K16Ac, and ↓ with H4K20Me3, contributing to its apoptosis-resistant phenotype | [38] |
| 200 µM | >300 μM | 2 h | Cell culture medium | F65 diploid foreskin fibroblasts | Senescence morphology, ↓ ODC; ↓ TK | ↓ number of PD by 35.3 +/- 10.3%; Catalase & Deferoxamine protected cells from H2O2-induced replicative cessation | [41] |
| 800 µM | N/A | 0–72 h | Cell culture medium with 10% FBS and supplemented with 5-AzazC (10 μM) or ROS inducer menadione (10 μM) | normal human epidermal keratinocytes | ↑ SA-β-Gal; ↑ p16; p21; p53 was not affected. | ↓ expression of phosphorylated Rb and CDK4, resulting in arrest in G0/G1 phase; menadione ↑ the expression of mRNA and protein of p16INK4a, when antioxidant drug N-acetylcysteine ↓ it | [42] |
| Characteristic | H2O2-Induced SIPS Fibroblasts |
|---|---|
| Cell shape & size | Enlarged, flattened, irregular |
| Nuclear circularity | Increased |
| β-Gal level | High |
| BID | Decreased |
| CDK2 | Low |
| c-Jun | Low |
| Collagens (type I, III) | Low |
| Cyclin D1 | No change detected |
| EGFR | Decreased |
| Elastin | Increased |
| Hyaluronan | Moderately increased |
| MMP 1, 2 | Low |
| MKI67 | High |
| NF-kB | No change detected |
| P16 | Moderate |
| P21 | High |
| P53 | No change detected |
| SIRT 1 | No change detected |
| SIRT 3, 4 | Low |
| SIRT 6 | No change detected |
| TIMP 1 | Low |
| Vinculin | Low |
| Cell viability | Decreased |
| Wound healing | Decreased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts. Int. J. Mol. Sci. 2022, 23, 7124. https://doi.org/10.3390/ijms23137124
Gerasymchuk M, Robinson GI, Kovalchuk O, Kovalchuk I. Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts. International Journal of Molecular Sciences. 2022; 23(13):7124. https://doi.org/10.3390/ijms23137124
Chicago/Turabian StyleGerasymchuk, Marta, Gregory Ian Robinson, Olga Kovalchuk, and Igor Kovalchuk. 2022. "Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts" International Journal of Molecular Sciences 23, no. 13: 7124. https://doi.org/10.3390/ijms23137124








