Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-α-Necroptosis Pathway
Abstract
:1. Introduction
2. Inflammatory Response, Apoptosis and Necroptosis Induced by TNF-α
2.1. Cell Survival and Inflammatory Response
2.2. Apoptosis
2.3. Necroptosis
3. Role of TNF-α and Its Mechanism Underlying Neuropathic Pain
3.1. TNF-α Regulates Voltage-Gated Sodium Channels in the Peripheral Nervous System
3.2. TNF-α Induces Spinal Neuronal Excitation and Inhibition Imbalance Andneuroinflammation
3.3. Supraspinal TNF-α Mediates Neuropathic Pain, Pain-Associated Aversion, Anxiety, Depression and Memory Deficits
4. TNF-α/TNFR1-Necroptosis Pathway in Neuropathic Pain
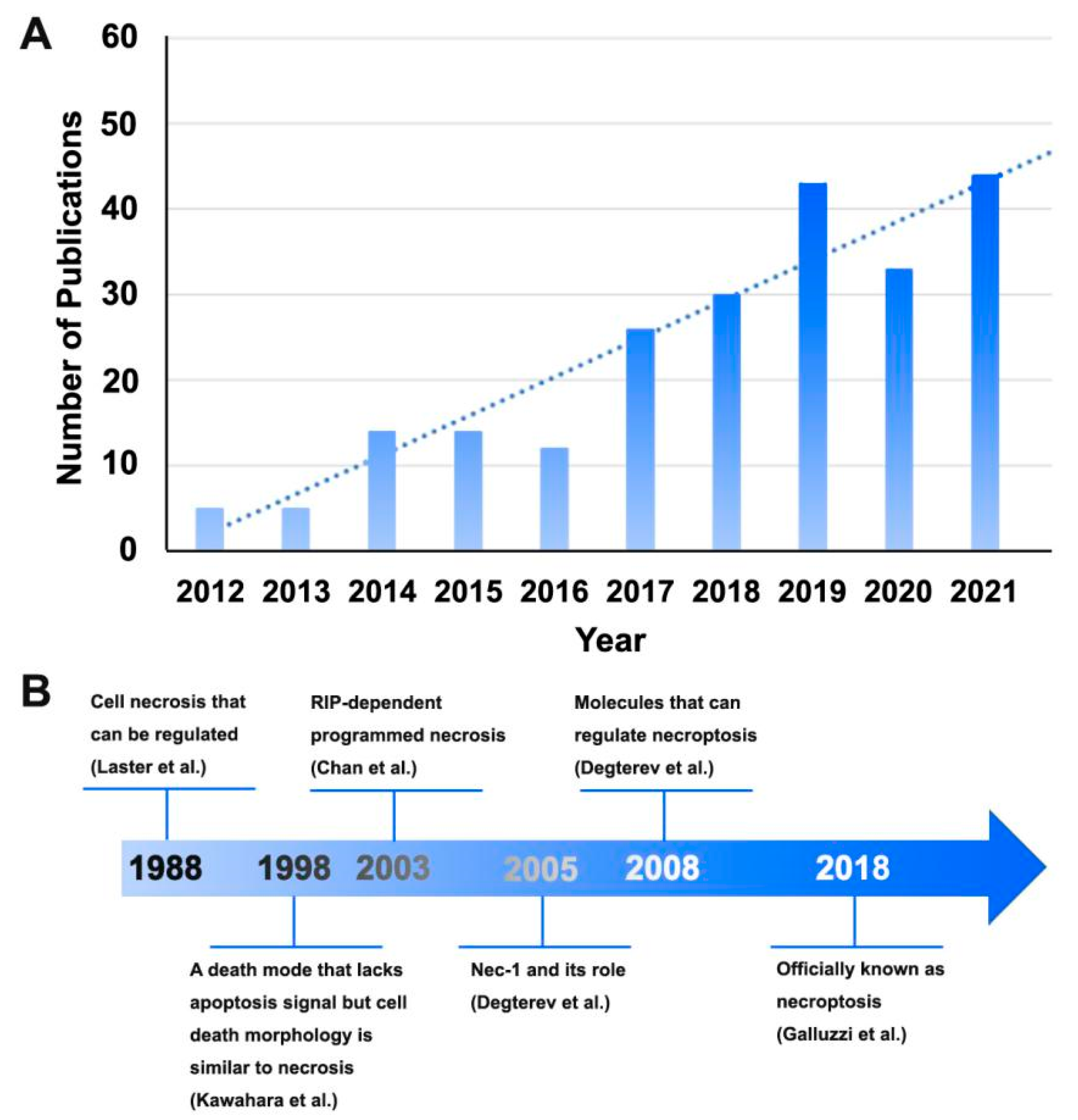
4.1. Necroptosis and Bibliometric Analysis
4.2. TNF-α/TNFR1–Necroptosis Pathway Contributes to Neurological Diseases
4.3. Role of Necroptosis in Chronic Pain
4.4. TNF-α/Necroptosis in Pain-Associated Anxiety and Depression
4.5. TNF-α/Necroptosis in Pain-Associated Memory Deficits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, K.K.; Khan, M.A.; Singh, S.K. Constitutive Inflammatory Cytokine Storm: A Major Threat to Human Health. J. Interferon Cytokine Res. 2020, 40, 19–23. [Google Scholar] [CrossRef]
- Gilron, I.; Watson, C.P.; Cahill, C.M.; Moulin, D.E. Neuropathic pain: A practical guide for the clinician. Cmaj 2006, 175, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Sommer, C. Painful neuropathies. Curr. Opin. Neurol. 2003, 16, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Basbaum, A.I. Distinct neurochemical features of acute and persistent pain. Proc. Natl. Acad. Sci. USA 1999, 96, 7739–7743. [Google Scholar] [CrossRef] [Green Version]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Gilron, I.; Baron, R.; Jensen, T. Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin. Proc. 2015, 90, 532–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuffler, D.P. Mechanisms for Reducing Neuropathic Pain. Mol. Neurobiol. 2020, 57, 67–87. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Rezq, S.; Alsemeh, A.E.; Sabry, O.M.; Mostafa, I.; Abdelfattah, M.A.O.; El-Allem, K.A.; El-Shazly, A.M.; Yasri, A.; et al. Salix tetrasperma Roxb. Extract Alleviates Neuropathic Pain in Rats via Modulation of the NF-kappaB/TNF-alpha/NOX/iNOS Pathway. Antioxidants 2019, 8, 482. [Google Scholar] [CrossRef] [Green Version]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Zimmermann, M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001, 429, 23–37. [Google Scholar] [CrossRef]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [Green Version]
- Dieleman, J.P.; Kerklaan, J.; Huygen, F.; Bouma, P.A.D.; Sturkenboom, M. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008, 137, 681–688. [Google Scholar] [CrossRef]
- Jensen, T.S.; Baron, R.; Haanpaa, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Blyth, F.M. Global burden of neuropathic pain. Pain 2018, 159, 614–617. [Google Scholar] [CrossRef]
- McDermott, A.M.; Toelle, T.R.; Rowbotham, D.J.; Schaefer, C.P.; Dukes, E.M. The burden of neuropathic pain: Results from a cross-sectional survey. Eur. J. Pain 2006, 10, 127–135. [Google Scholar] [CrossRef]
- O’Connor, A.B. Neuropathic pain: Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009, 27, 95–112. [Google Scholar] [CrossRef]
- Rosberg, H.E.; Carlsson, K.S.; Cederlund, R.I.; Ramel, E.; Dahlin, L.B. Costs and outcome for serious hand and arm injuries during the first year after trauma—A prospective study. BMC Public Health 2013, 13, 501. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.W.; Wang, S.K.; Chen, S.X.; Xin, W.J.; Liu, X.G.; Zang, Y. Upregulation of tumor necrosis factor-alpha in the anterior cingulate cortex contributes to neuropathic pain and pain-associated aversion. Neurobiol. Dis. 2019, 130, 104456. [Google Scholar] [CrossRef]
- Chen, S.X.; Liao, G.J.; Yao, P.W.; Wang, S.K.; Li, Y.Y.; Zeng, W.A.; Liu, X.G.; Zang, Y. Calpain-2 Regulates TNF-alpha Expression Associated with Neuropathic Pain Following Motor Nerve Injury. Neuroscience 2018, 376, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Wang, S.K.; Yao, P.W.; Liao, G.J.; Na, X.D.; Li, Y.Y.; Zeng, W.A.; Liu, X.G.; Zang, Y. Early CALP2 expression and microglial activation are potential inducers of spinal IL-6 up-regulation and bilateral pain following motor nerve injury. J. Neurochem. 2018, 145, 154–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.H.; Zang, Y.; Chen, X.; Pang, R.P.; Xu, J.T.; Zhou, X.; Wei, X.H.; Li, Y.Y.; Xin, W.J.; Qin, Z.H.; et al. TNF-alpha contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain 2010, 151, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Chen, S.X.; Liao, G.J.; Zhu, H.Q.; Wei, X.H.; Cui, Y.; Na, X.D.; Pang, R.P.; Xin, W.J.; Zhou, L.J.; et al. Calpain-2 contributes to neuropathic pain following motor nerve injury via up-regulating interleukin-6 in DRG neurons. Brain Behav. Immun. 2015, 44, 37–47. [Google Scholar] [CrossRef]
- Zang, Y.; He, X.H.; Xin, W.J.; Pang, R.P.; Wei, X.H.; Zhou, L.J.; Li, Y.Y.; Liu, X.G. Inhibition of NF-kappaB prevents mechanical allodynia induced by spinal ventral root transection and suppresses the re-expression of Nav1.3 in DRG neurons in vivo and in vitro. Brain Res. 2010, 1363, 151–158. [Google Scholar] [CrossRef]
- Sweitzer, S.M.; Hickey, W.F.; Rutkowski, M.D.; Pahl, J.L.; DeLeo, J.A. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: Potential relationship to neuropathic pain. Pain 2002, 100, 163–170. [Google Scholar] [CrossRef]
- Grace, P.M.; Tawfik, V.L.; Svensson, C.I.; Burton, M.D.; Loggia, M.L.; Hutchinson, M.R. The Neuroimmunology of Chronic Pain: From Rodents to Humans. J. Neurosci. 2021, 41, 855–865. [Google Scholar] [CrossRef]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef]
- Kuner, R. Central mechanisms of pathological pain. Nat. Med. 2010, 16, 1258–1266. [Google Scholar] [CrossRef]
- Li, L.; Xian, C.J.; Zhong, J.H.; Zhou, X.F. Lumbar 5 ventral root transection-induced upregulation of nerve growth factor in sensory neurons and their target tissues: A mechanism in neuropathic pain. Mol. Cell. Neurosci. 2003, 23, 232–250. [Google Scholar] [CrossRef]
- Li, L.; Xian, C.J.; Zhong, J.H.; Zhou, X.F. Effect of lumbar 5 ventral root transection on pain behaviors: A novel rat model for neuropathic pain without axotomy of primary sensory neurons. Exp. Neurol. 2002, 175, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Mizushima, T.; Katsura, H.; Fukuoka, T.; Tokunaga, A.; Noguchi, K. The effect of site and type of nerve injury on the expression of brain-derived neurotrophic factor in the dorsal root ganglion and on neuropathic pain behavior. Neuroscience 2006, 137, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.N.; Dorsi, M.J.; Li, Y.; Murinson, B.B.; Belzberg, A.J.; Griffin, J.W.; Meyer, R.A. Mechanical hyperalgesia after an L5 ventral rhizotomy or an L5 ganglionectomy in the rat. Pain 2002, 96, 63–72. [Google Scholar] [CrossRef]
- Wu, G.; Ringkamp, M.; Murinson, B.B.; Pogatzki, E.M.; Hartke, T.V.; Weerahandi, H.M.; Campbell, J.N.; Griffin, J.W.; Meyer, R.A. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J. Neurosci. 2002, 22, 7746–7753. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Doom, C.M. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J. Peripher. Nerv. Syst. 2000, 5, 96–100. [Google Scholar] [CrossRef]
- Zelenka, M.; Schafers, M.; Sommer, C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 2005, 116, 257–263. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [Green Version]
- Ransohoff, R.M.; Cardona, A.E. The myeloid cells of the central nervous system parenchyma. Nature 2010, 468, 253–262. [Google Scholar] [CrossRef]
- Sanchez-Molina, P.; Almolda, B.; Benseny-Cases, N.; Gonzalez, B.; Peralvarez-Marin, A.; Castellano, B. Specific microglial phagocytic phenotype and decrease of lipid oxidation in white matter areas during aging: Implications of different microenvironments. Neurobiol. Aging 2021, 105, 280–295. [Google Scholar] [CrossRef]
- Shields, D.C.; Haque, A.; Banik, N.L. Neuroinflammatory responses of microglia in central nervous system trauma. J. Cereb. Blood Flow Metab. 2020, 40, S25–S33. [Google Scholar] [CrossRef]
- Veremeyko, T.; Yung, A.W.Y.; Dukhinova, M.; Strekalova, T.; Ponomarev, E.D. The Role of Neuronal Factors in the Epigenetic Reprogramming of Microglia in the Normal and Diseased Central Nervous System. Front. Cell. Neurosci. 2019, 13, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, M.M. Location, Location, Location: Microglia Are Where They Live. Neuron 2017, 95, 233–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Zhao, Y.Q.; Ribeiro-da-Silva, A.; Zhang, J. Distinctive response of CNS glial cells in oro-facial pain associated with injury, infection and inflammation. Mol. Pain 2010, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Mallard, C.; Tremblay, M.E.; Vexler, Z.S. Microglia and Neonatal Brain Injury. Neuroscience 2019, 405, 68–76. [Google Scholar] [CrossRef]
- Aldskogius, H.; Kozlova, E.N. Central neuron-glial and glial-glial interactions following axon injury. Prog. Neurobiol. 1998, 55, 1–26. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, H.; Lee, S.J. Distinct roles of GT1b and CSF-1 in microglia activation in nerve injury-induced neuropathic pain. Mol. Pain 2021, 17, 17448069211020918. [Google Scholar] [CrossRef]
- Aldskogius, H. Mechanisms and consequences of microglial responses to peripheral axotomy. Front. Biosci. 2011, 3, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, J.A.; Vainchtein, I.D.; Braz, J.; Hamel, K.; Bernstein, M.; Craik, V.; Dahlgren, M.W.; Ortiz-Carpena, J.; Molofsky, A.B.; Molofsky, A.V.; et al. Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice. Elife 2021, 10, e69056. [Google Scholar] [CrossRef]
- Reischer, G.; Heinke, B.; Sandkuhler, J. Interferon-gamma facilitates the synaptic transmission between primary afferent C-fibres and lamina I neurons in the rat spinal dorsal horn via microglia activation. Mol. Pain 2020, 16, 1744806920917249. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Terayama, R.; Kishimoto, N.; Maruhama, K.; Mizutani, M.; Iida, S.; Sugimoto, T. Activated microglia contribute to convergent nociceptive inputs to spinal dorsal horn neurons and the development of neuropathic pain. Neurochem. Res. 2015, 40, 1000–1012. [Google Scholar] [CrossRef]
- Clark, A.K.; Yip, P.K.; Grist, J.; Gentry, C.; Staniland, A.A.; Marchand, F.; Dehvari, M.; Wotherspoon, G.; Winter, J.; Ullah, J.; et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 10655–10660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beggs, S.; Salter, M.W. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav. Immun. 2007, 21, 624–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, A.F.; Davies, C.L.; Holloway, R.K.; Labrak, Y.; Ireland, G.; Carradori, D.; Dillenburg, A.; Borger, E.; Soong, D.; Richardson, J.C.; et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 2019, 22, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef]
- Newton, K.; Manning, G. Necroptosis and Inflammation. Annu. Rev. Biochem. 2016, 85, 743–763. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.F.; Mao, L.; Xia, Y.P.; Jin, H.J.; Li, Y.N.; You, M.F.; et al. Microglia-derived TNF-alpha mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019, 10, 487. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Jitkaew, S.; Zhao, J.; Chiang, H.C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.G.; Liu, Z.G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2014, 16, 55–65. [Google Scholar] [CrossRef]
- Feoktistova, M.; Makarov, R.; Yazdi, A.S.; Panayotova-Dimitrova, D. RIPK1 and TRADD Regulate TNF-Induced Signaling and Ripoptosome Formation. Int. J. Mol. Sci. 2021, 22, 12459. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Chan, F.K.; Kroemer, G. Necroptosis: Mechanisms and Relevance to Disease. Annu. Rev. Pathol. 2017, 12, 103–130. [Google Scholar] [CrossRef]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kos, R.; Garssen, J.; Redegeld, F. Molecular Insights into the Mechanism of Necroptosis: The Necrosome As a Potential Therapeutic Target. Cells 2019, 8, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef] [Green Version]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Oberst, A.; Dillon, C.P.; Weinlich, R.; McCormick, L.L.; Fitzgerald, P.; Pop, C.; Hakem, R.; Salvesen, G.S.; Green, D.R. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011, 471, 363–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.F.; Wang, F.S.; Wang, X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Shamash, S.; Reichert, F.; Rotshenker, S. The cytokine network of Wallerian degeneration: Tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J. Neurosci. 2002, 22, 3052–3060. [Google Scholar] [CrossRef] [Green Version]
- Sacerdote, P.; Franchi, S.; Trovato, A.E.; Valsecchi, A.E.; Panerai, A.E.; Colleoni, M. Transient early expression of TNF-alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathy. Neurosci. Lett. 2008, 436, 210–213. [Google Scholar] [CrossRef]
- Schafers, M.; Sorkin, L.S.; Geis, C.; Shubayev, V.I. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci. Lett. 2003, 347, 179–182. [Google Scholar] [CrossRef]
- Wei, X.H.; Zang, Y.; Wu, C.Y.; Xu, J.T.; Xin, W.J.; Liu, X.G. Peri-sciatic administration of recombinant rat TNF-alpha induces mechanical allodynia via upregulation of TNF-alpha in dorsal root ganglia and in spinal dorsal horn: The role of NF-kappa B pathway. Exp. Neurol. 2007, 205, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Covey, W.C.; Ignatowski, T.A.; Renauld, A.E.; Knight, P.R.; Nader, N.D.; Spengler, R.N. Expression of neuron-associated tumor necrosis factor alpha in the brain is increased during persistent pain. Reg. Anesth. Pain Med. 2002, 27, 357–366. [Google Scholar] [PubMed]
- Ignatowski, T.A.; Covey, W.C.; Knight, P.R.; Severin, C.M.; Nickola, T.J.; Spengler, R.N. Brain-derived TNFalpha mediates neuropathic pain. Brain Res. 1999, 841, 70–77. [Google Scholar] [CrossRef]
- Covey, W.C.; Ignatowski, T.A.; Knight, P.R.; Spengler, R.N. Brain-derived TNFalpha: Involvement in neuroplastic changes implicated in the conscious perception of persistent pain. Brain Res. 2000, 859, 113–122. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Yang, H.W.; Yang, T.S.; Xie, W.X.; Hu, Z.H. TNF-alpha-mediated peripheral and central inflammation are associated with increased incidence of PND in acute postoperative pain. Bmc Anesthesiol. 2021, 21, 79. [Google Scholar] [CrossRef]
- Clark, I.A. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007, 18, 335–343. [Google Scholar] [CrossRef]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Nei, H.; Dougherty, P.M. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J. Neurosci. 2010, 30, 12844–12855. [Google Scholar] [CrossRef]
- Opree, A.; Kress, M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: Effects on heat-evoked calcitonin gene-related peptide release from rat skin. J. Neurosci. 2000, 20, 6289–6293. [Google Scholar] [CrossRef]
- Junger, H.; Sorkin, L.S. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain 2000, 85, 145–151. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Brull, S.J.; Zhang, J.M. Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J. Neurophysiol. 2002, 88, 1393–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafers, M.; Lee, D.H.; Brors, D.; Yaksh, T.L.; Sorkin, L.S. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J. Neurosci. 2003, 23, 3028–3038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Li, H.; Liu, B.; Brull, S.J. Acute topical application of tumor necrosis factor alpha evokes protein kinase A-dependent responses in rat sensory neurons. J. Neurophysiol. 2002, 88, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Ding, H.H.; Xu, T.; Liu, M.; Ma, C.; Wu, S.L.; Wei, J.Y.; Liu, C.C.; Zhang, S.B.; Xin, W.J. Palmitoylation of delta-catenin promotes kinesin-mediated membrane trafficking of Nav1.6 in sensory neurons to promote neuropathic pain. Sci. Signal. 2018, 11, eaar4394. [Google Scholar] [CrossRef] [Green Version]
- Leo, M.; Argalski, S.; Schafers, M.; Hagenacker, T. Modulation of Voltage-Gated Sodium Channels by Activation of Tumor Necrosis Factor Receptor-1 and Receptor-2 in Small DRG Neurons of Rats. Mediat. Inflamm. 2015, 2015, 124942. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Wang, S.; Gruber, S.; Mata, M.; Fink, D.J. Full-length membrane-bound tumor necrosis factor-alpha acts through tumor necrosis factor receptor 2 to modify phenotype of sensory neurons. Pain 2013, 154, 1778–1782. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Gereau, R.W.t. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci. 2006, 26, 246–255. [Google Scholar] [CrossRef]
- Sole, L.; Wagnon, J.L.; Akin, E.J.; Meisler, M.H.; Tamkun, M.M. The MAP1B Binding Domain of Nav1.6 Is Required for Stable Expression at the Axon Initial Segment. J. Neurosci. 2019, 39, 4238–4251. [Google Scholar] [CrossRef] [Green Version]
- Sittl, R.; Lampert, A.; Huth, T.; Schuy, E.T.; Link, A.S.; Fleckenstein, J.; Alzheimer, C.; Grafe, P.; Carr, R.W. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc. Natl. Acad. Sci. USA 2012, 109, 6704–6709. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Zhang, J.; Strong, J.A.; Zhang, J.M. Role of NaV1.6 and NaVbeta4 Sodium Channel Subunits in a Rat Model of Low Back Pain Induced by Compression of the Dorsal Root Ganglia. Neuroscience 2019, 402, 51–65. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Benson, C.; Lankford, K.L.; Zhao, P.; Carrara, J.; Tan, A.M.; Kocsis, J.D.; Waxman, S.G.; Dib-Hajj, S.D. Sodium channel Nav1.6 in sensory neurons contributes to vincristine-induced allodynia. Brain 2020, 143, 2421–2436. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Strong, J.A.; Ye, L.; Mao, J.X.; Zhang, J.M. Knockdown of sodium channel NaV1.6 blocks mechanical pain and abnormal bursting activity of afferent neurons in inflamed sensory ganglia. Pain 2013, 154, 1170–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Huang, J.; Zhao, P.; Persson, A.K.; Dib-Hajj, F.B.; Cheng, X.; Tan, A.; Waxman, S.G.; Dib-Hajj, S.D. Conditional knockout of NaV1.6 in adult mice ameliorates neuropathic pain. Sci Rep 2018, 8, 3845. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.H.; Zhang, S.B.; Lv, Y.Y.; Ma, C.; Liu, M.; Zhang, K.B.; Ruan, X.C.; Wei, J.Y.; Xin, W.J.; Wu, S.L. TNF-alpha/STAT3 pathway epigenetically upregulates Nav1.6 expression in DRG and contributes to neuropathic pain induced by L5-VRT. J. Neuroinflamm. 2019, 16, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894–898. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 2010, 33, 325–347. [Google Scholar] [CrossRef] [Green Version]
- McDermott, L.A.; Weir, G.A.; Themistocleous, A.C.; Segerdahl, A.R.; Blesneac, I.; Baskozos, G.; Clark, A.J.; Millar, V.; Peck, L.J.; Ebner, D.; et al. Defining the Functional Role of NaV1.7 in Human Nociception. Neuron 2019, 101, 905–919.e908. [Google Scholar] [CrossRef] [Green Version]
- Fertleman, C.R.; Baker, M.D.; Parker, K.A.; Moffatt, S.; Elmslie, F.V.; Abrahamsen, B.; Ostman, J.; Klugbauer, N.; Wood, J.N.; Gardiner, R.M.; et al. SCN9A mutations in paroxysmal extreme pain disorder: Allelic variants underlie distinct channel defects and phenotypes. Neuron 2006, 52, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Minett, M.S.; Nassar, M.A.; Clark, A.K.; Passmore, G.; Dickenson, A.H.; Wang, F.; Malcangio, M.; Wood, J.N. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat. Commun. 2012, 3, 791. [Google Scholar] [CrossRef] [Green Version]
- Nassar, M.A.; Stirling, L.C.; Forlani, G.; Baker, M.D.; Matthews, E.A.; Dickenson, A.H.; Wood, J.N. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA 2004, 101, 12706–12711. [Google Scholar] [CrossRef] [Green Version]
- Shields, S.D.; Cheng, X.; Uceyler, N.; Sommer, C.; Dib-Hajj, S.D.; Waxman, S.G. Sodium channel Na(v)1.7 is essential for lowering heat pain threshold after burn injury. J. Neurosci. 2012, 32, 10819–10832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; North, R.Y.; Rhines, L.D.; Tatsui, C.E.; Rao, G.; Edwards, D.D.; Cassidy, R.M.; Harrison, D.S.; Johansson, C.A.; Zhang, H.; et al. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J. Neurosci. 2018, 38, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Minett, M.S.; Falk, S.; Santana-Varela, S.; Bogdanov, Y.D.; Nassar, M.A.; Heegaard, A.M.; Wood, J.N. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. 2014, 6, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, R.; Nemoto, T.; Maruta, T.; Onizuka, S.; Yanagita, T.; Wada, A.; Murakami, M.; Tsuneyoshi, I. Up-regulation of NaV1.7 sodium channels expression by tumor necrosis factor-alpha in cultured bovine adrenal chromaffin cells and rat dorsal root ganglion neurons. Anesth. Analg. 2014, 118, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.X.; Zhang, X.L.; Xu, J.; Zeng, W.A.; Li, D.; Xu, T.; Pang, R.P.; Ma, K.; Liu, X.G. Nuclear Factor-kappaB Gates Nav1.7 Channels in DRG Neurons via Protein-Protein Interaction. iScience 2019, 19, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Deng, T.; Shang, Z.; Wang, D.; Xiao, Y. Blocking TRPA1 and TNF-alpha Signal Improves Bortezomib-Induced Neuropathic Pain. Cell. Physiol. Biochem. 2018, 51, 2098–2110. [Google Scholar] [CrossRef]
- Constantin, C.E.; Mair, N.; Sailer, C.A.; Andratsch, M.; Xu, Z.Z.; Blumer, M.J.; Scherbakov, N.; Davis, J.B.; Bluethmann, H.; Ji, R.R.; et al. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J. Neurosci. 2008, 28, 5072–5081. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Feng, C.; He, H.; He, J.; Wang, J.; Li, X.; Wang, S.; Li, W.; Hou, J.; Liu, T.; et al. Sensitization of TRPV1 receptors by TNF-alpha orchestrates the development of vincristine-induced pain. Oncol. Lett. 2018, 15, 5013–5019. [Google Scholar]
- Czeschik, J.C.; Hagenacker, T.; Schafers, M.; Busselberg, D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci. Lett. 2008, 434, 293–298. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1 beta, interleukin-6, and tumor necrosis factor-beta in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Zhou, L.J.; Hu, N.W.; Xu, J.T.; Wu, C.Y.; Zhang, T.; Li, Y.Y.; Liu, X.G. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: The role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology 2007, 52, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Schoffnegger, D.; Drdla-Schutting, R.; Honigsperger, C.; Wunderbaldinger, G.; Gassner, M.; Sandkuhler, J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J. Neurosci. 2013, 33, 6540–6551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garraway, S.M.; Woller, S.A.; Huie, J.R.; Hartman, J.J.; Hook, M.A.; Miranda, R.C.; Huang, Y.J.; Ferguson, A.R.; Grau, J.W. Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: Role of tumor necrosis factor alpha and apoptosis. Pain 2014, 155, 2344–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, H.; Kobayashi, K.; Yamanaka, H.; Okubo, M.; Noguchi, K. Microglial TNFalpha Induces COX2 and PGI2 Synthase Expression in Spinal Endothelial Cells during Neuropathic Pain. eNeuro 2017, 4, ENEURO.0064-17.2017. [Google Scholar] [CrossRef]
- Kroenke, K.; Bair, M.J.; Damush, T.M.; Wu, J.; Hoke, S.; Sutherland, J.; Tu, W. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: A randomized controlled trial. JAMA 2009, 301, 2099–2110. [Google Scholar] [CrossRef] [Green Version]
- Page, G.G.; Opp, M.R.; Kozachik, S.L. Reduced sleep, stress responsivity, and female sex contribute to persistent inflammation-induced mechanical hypersensitivity in rats. Brain Behav. Immun. 2014, 40, 244–251. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhou, L.J.; Wu, Y.; Li, F.; Shen, K.F.; Pang, R.P.; Wei, X.H.; Li, Y.Y.; Liu, X.G. Magnesium L-threonate Prevents and Restores Memory Deficits Associated with Neuropathic Pain by Inhibition of TNF-alpha. Pain Physician 2013, 16, E563–E575. [Google Scholar]
- Berryman, C.; Stanton, T.R.; Jane Bowering, K.; Tabor, A.; McFarlane, A.; Lorimer Moseley, G. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain 2013, 154, 1181–1196. [Google Scholar] [CrossRef]
- Oka, T.; Wakugawa, Y.; Hosoi, M.; Oka, K.; Hori, T. Intracerebroventricular injection of tumor necrosis factor-alpha induces thermal hyperalgesia in rats. Neuroimmunomodulation 1996, 3, 135–140. [Google Scholar] [CrossRef]
- Coelho, A.; Fioramonti, J.; Bueno, L. Brain interleukin-1beta and tumor necrosis factor-alpha are involved in lipopolysaccharide-induced delayed rectal allodynia in awake rats. Brain Res. Bull. 2000, 52, 223–228. [Google Scholar] [CrossRef]
- Wei, F.; Guo, W.; Zou, S.; Ren, K.; Dubner, R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J. Neurosci. 2008, 28, 10482–10495. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.M.; Mehrabani, S.; Liu, S.; Taylor, A.J.; Cahill, C.M. Topography of microglial activation in sensory- and affect-related brain regions in chronic pain. J. Neurosci. Res 2017, 95, 1330–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, M.D.; Guginski, G.; Sato, K.L.; Sanada, L.S.; Sluka, K.A.; Santos, A.R.S. Persistent pain induces mood problems and memory loss by the involvement of cytokines, growth factors, and supraspinal glial cells. Brain Behav. Immun. Health 2020, 7, 100118. [Google Scholar] [CrossRef]
- Barcelon, E.E.; Cho, W.H.; Jun, S.B.; Lee, S.J. Brain Microglial Activation in Chronic Pain-Associated Affective Disorder. Front. Neurosci. 2019, 13, 213. [Google Scholar] [CrossRef] [Green Version]
- Ren, K.; Dubner, R. Neuron-glia crosstalk gets serious: Role in pain hypersensitivity. Curr. Opin. Anesthesiol. 2008, 21, 570–579. [Google Scholar] [CrossRef] [Green Version]
- Stellwagen, D.; Beattie, E.C.; Seo, J.Y.; Malenka, R.C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 2005, 25, 3219–3228. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Zhao, H.; Gao, H.; Liu, D.; Li, J. Participation of pro-inflammatory cytokines in neuropathic pain evoked by chemotherapeutic oxaliplatin via central GABAergic pathway. Mol. Pain 2018, 14, 1744806918783535. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yu, J.; Wang, J.; Ding, C.P.; Han, S.P.; Zeng, X.Y.; Wang, J.Y. The Red Nucleus TNF-alpha Participates in the Initiation and Maintenance of Neuropathic Pain Through Different Signaling Pathways. Neurochem. Res. 2015, 40, 1360–1371. [Google Scholar] [CrossRef]
- Butler, M.P.; O’Connor, J.J.; Moynagh, P.N. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience 2004, 124, 319–326. [Google Scholar] [CrossRef]
- Cunningham, A.J.; Murray, C.A.; O’Neill, L.A.; Lynch, M.A.; O’Connor, J.J. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci. Lett. 1996, 203, 17–20. [Google Scholar] [CrossRef]
- Griffin, R.; Nally, R.; Nolan, Y.; McCartney, Y.; Linden, J.; Lynch, M.A. The age-related attenuation in long-term potentiation is associated with microglial activation. J. Neurochem. 2006, 99, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Pickering, M.; Cumiskey, D.; O’Connor, J.J. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 2005, 90, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Pickering, M.; O’Connor, J.J. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog. Brain Res. 2007, 163, 339–354. [Google Scholar] [PubMed]
- Tancredi, V.; D’Arcangelo, G.; Grassi, F.; Tarroni, P.; Palmieri, G.; Santoni, A.; Eusebi, F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci. Lett. 1992, 146, 176–178. [Google Scholar] [CrossRef]
- Thorburn, A. Death receptor-induced cell killing. Cell. Signal. 2004, 16, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, V.; Mohand-Said, S.; Hanoteau, N.; Fuchs, C.; Pfizenmaier, K.; Eisel, U. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: Opposite roles of TNF receptor 1 and TNF receptor 2. J. Neurosci. 2002, 22, RC216. [Google Scholar] [CrossRef]
- Robertson, J.; Beaulieu, J.M.; Doroudchi, M.M.; Durham, H.D.; Julien, J.P.; Mushynski, W.E. Apoptotic death of neurons exhibiting peripherin aggregates is mediated by the proinflammatory cytokine tumor necrosis factor-alpha. J. Cell Biol. 2001, 155, 217–226. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Sekiguchi, Y.; Konno, S.; Kobayashi, H.; Homma, Y.; Kikuchi, S. Comparison of neuropathic pain and neuronal apoptosis following nerve root or spinal nerve compression. Eur. Spine J. 2009, 18, 1978–1985. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.F.; Yeh, S.R.; Lu, K.T.; Hsu, J.L.; Chao, P.K.; Hsu, H.C.; Peng, C.H.; Lee, Y.L.; Hung, Y.H.; Ro, L.S. Interactions between Autophagy, Proinflammatory Cytokines, and Apoptosis in Neuropathic Pain: Granulocyte Colony Stimulating Factor as a Multipotent Therapy in Rats with Chronic Constriction Injury. Biomedicines 2021, 9, 542. [Google Scholar] [CrossRef]
- Laster, S.M.; Wood, J.G.; Gooding, L.R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 1988, 141, 2629–2634. [Google Scholar] [PubMed]
- Kawahara, A.; Ohsawa, Y.; Matsumura, H.; Uchiyama, Y.; Nagata, S. Caspase-independent cell killing by Fas-associated protein with death domain. J. Cell Biol. 1998, 143, 1353–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, F.K.; Shisler, J.; Bixby, J.G.; Felices, M.; Zheng, L.; Appel, M.; Orenstein, J.; Moss, B.; Lenardo, M.J. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 2003, 278, 51613–51621. [Google Scholar] [CrossRef] [Green Version]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J.H. RIP3, an Energy Metabolism Regulator That Switches TNF-Induced Cell Death from Apoptosis to Necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef] [Green Version]
- Ofengeim, D.; Ito, Y.; Najafov, A.; Zhang, Y.; Shan, B.; DeWitt, J.P.; Ye, J.; Zhang, X.; Chang, A.; Vakifahmetoglu-Norberg, H.; et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015, 10, 1836–1849. [Google Scholar] [CrossRef] [Green Version]
- Re, D.B.; Le Verche, V.; Yu, C.H.; Amoroso, M.W.; Politi, K.A.; Phani, S.; Ikiz, B.; Hoffmann, L.; Koolen, M.; Nagata, T.; et al. Necroptosis Drives Motor Neuron Death in Models of Both Sporadic and Familial ALS. Neuron 2014, 81, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- Caccamo, A.; Branca, C.; Piras, I.S.; Ferreira, E.; Huentelman, M.J.; Liang, W.S.; Readhead, B.; Dudley, J.T.; Spangenberg, E.E.; Green, K.N.; et al. Necroptosis activation in Alzheimer’s disease. Nat. Neurosci. 2017, 20, 1236–1246. [Google Scholar] [CrossRef]
- Wang, P.; Shao, B.Z.; Deng, Z.; Chen, S.; Yue, Z.; Miao, C.Y. Autophagy in ischemic stroke. Prog. Neurobiol. 2018, 163-164, 98–117. [Google Scholar] [CrossRef]
- Zhou, H.; Li, D.D.; Zhu, P.J.; Ma, Q.; Toan, S.; Wang, J.; Hu, S.Y.; Chen, Y.D.; Zhang, Y.M. Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J. Pineal Res. 2018, 65, e12503. [Google Scholar] [CrossRef] [PubMed]
- Abdalkader, M.; Lampinen, R.; Kanninen, K.M.; Malm, T.M.; Liddell, J.R. Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration. Front. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingledine, R.; Varvel, N.H.; Dudek, F.E. When and how do seizures kill neurons, and is cell death relevant to epileptogenesis? Adv. Exp. Med. Biol. 2014, 813, 109–122. [Google Scholar] [PubMed] [Green Version]
- Summers, D.W.; DiAntonio, A.; Milbrandt, J. Mitochondrial Dysfunction Induces Sarm1-Dependent Cell Death in Sensory Neurons. J. Neurosci. 2014, 34, 9338–9350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Liu, Z.G. Execution of RIPK3-regulated necrosis. Mol. Cell Oncol 2014, 1, e960759. [Google Scholar] [CrossRef] [Green Version]
- Newton, K. RIPK1 and RIPK3: Critical regulators of inflammation and cell death. Trends in Cell Biology 2015, 25, 347–353. [Google Scholar] [CrossRef]
- Picon, C.; Jayaraman, A.; James, R.; Beck, C.; Gallego, P.; Witte, M.E.; van Horssen, J.; Mazarakis, N.D.; Reynolds, R. Neuron-specific activation of necroptosis signaling in multiple sclerosis cortical grey matter. Acta Neuropathol. 2021, 141, 585–604. [Google Scholar] [CrossRef]
- Xu, C.; Wu, J.; Wu, Y.; Ren, Z.; Yao, Y.; Chen, G.; Fang, E.F.; Noh, J.H.; Liu, Y.U.; Wei, L.; et al. TNF-alpha-dependent neuronal necroptosis regulated in Alzheimer’s disease by coordination of RIPK1-p62 complex with autophagic UVRAG. Theranostics 2021, 11, 9452–9469. [Google Scholar] [CrossRef]
- Dionisio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Molecular mechanisms of necroptosis and relevance for neurodegenerative diseases. Cell Death Regul. Health Dis.—Pt C 2020, 353, 31–82. [Google Scholar]
- Jayaraman, A.; Htike, T.T.; James, R.; Picon, C.; Reynolds, R. TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathol. Commun. 2021, 9, 159. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Wang, Y.S.; Yang, J.R.; Yin, Y.; Liu, M.L.; Shi, Z.L.; Mu, N.; Yu, L.; Ma, H. Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis. J. Mol. Cell. Cardiol. 2018, 125, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yabal, M.; Muller, N.; Adler, H.; Knies, N.; Gross, C.J.; Damgaard, R.B.; Kanegane, H.; Ringelhan, M.; Kaufmann, T.; Heikenwalder, M.; et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014, 7, 1796–1808. [Google Scholar] [CrossRef] [Green Version]
- Wallach, D.; Kang, T.B.; Dillon, C.P.; Green, D.R. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 2016, 352, aaf2154. [Google Scholar] [CrossRef]
- Koehler, H.; Cotsmire, S.; Langland, J.; Kibler, K.V.; Kalman, D.; Upton, J.W.; Mocarski, E.S.; Jacobs, B.L. Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proc. Natl. Acad. Sci. USA 2017, 114, 11506–11511. [Google Scholar] [CrossRef] [Green Version]
- Shan, B.; Pan, H.; Najafov, A.; Yuan, J. Necroptosis in development and diseases. Genes Dev. 2018, 32, 327–340. [Google Scholar] [CrossRef]
- Liang, Y.X.; Wang, N.N.; Zhang, Z.Y.; Juan, Z.D.; Zhang, C. Necrostatin-1 Ameliorates Peripheral Nerve Injury-Induced Neuropathic Pain by Inhibiting the RIP1/RIP3 Pathway. Front. Cell. Neurosci. 2019, 13, 211. [Google Scholar] [CrossRef]
- Ma, D.; Zhao, S.; Liu, X.; Li, Z.; Li, H.; Liu, J.; Cao, J.; Wang, X. RIP3/MLKL pathway-regulated necroptosis: A new mechanism of paclitaxel-induced peripheral neuropathy. J. Biochem. Mol. Toxicol. 2021, 35, e22834. [Google Scholar] [CrossRef]
- Fang, P.; Sun, G.Q.; Wang, J.Y. RIP3-mediated necroptosis increases neuropathic pain via microglia activation: Necrostatin-1 has therapeutic potential. FEBS Open Bio 2021, 11, 2858–2865. [Google Scholar] [CrossRef]
- Lagana, A.S.; La Rosa, V.L.; Rapisarda, A.M.C.; Valenti, G.; Sapia, F.; Chiofalo, B.; Rossetti, D.; Ban Frangez, H.; Vrtacnik Bokal, E.; Vitale, S.G. Anxiety and depression in patients with endometriosis: Impact and management challenges. Int. J. Womens Health 2017, 9, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Hampton, S.N.; Nakonezny, P.A.; Richard, H.M.; Wells, J.E. Pain catastrophizing, anxiety, and depression in hip pathology. Bone Joint J 2019, 101-B, 800–807. [Google Scholar] [CrossRef]
- Blackburn-Munro, G.; Blackburn-Munro, R.E. Chronic pain, chronic stress and depression: Coincidence or consequence? J. Neuroendocrinol. 2001, 13, 1009–1023. [Google Scholar] [CrossRef]
- Boersma, K.; Sodermark, M.; Hesser, H.; Flink, I.K.; Gerdle, B.; Linton, S.J. Efficacy of a transdiagnostic emotion-focused exposure treatment for chronic pain patients with comorbid anxiety and depression: A randomized controlled trial. Pain 2019, 160, 1708–1718. [Google Scholar] [CrossRef]
- Rogers, A.H.; Orr, M.F.; Shepherd, J.M.; Bakhshaie, J.; Ditre, J.W.; Buckner, J.D.; Zvolensky, M.J. Anxiety, depression, and opioid misuse among adults with chronic pain: The role of emotion dysregulation. J. Behav. Med. 2021, 44, 66–73. [Google Scholar] [CrossRef]
- Dahl, J.; Ormstad, H.; Aass, H.C.; Malt, U.F.; Bendz, L.T.; Sandvik, L.; Brundin, L.; Andreassen, O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 2014, 45, 77–86. [Google Scholar] [CrossRef]
- Hayley, S.; Hakim, A.M.; Albert, P.R. Depression, dementia and immune dysregulation. Brain 2021, 144, 746–760. [Google Scholar] [CrossRef]
- Biggs, J.E.; Lu, V.B.; Stebbing, M.J.; Balasubramanyan, S.; Smith, P.A. Is BDNF sufficient for information transfer between microglia and dorsal horn neurons during the onset of central sensitization? Mol. Pain 2010, 6, 1744–8069. [Google Scholar] [CrossRef] [Green Version]
- Rana, T.; Behl, T.; Sehgal, A.; Srivastava, P.; Bungau, S. Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. J. Mol. Neurosci. 2021, 71, 2008–2021. [Google Scholar] [CrossRef]
- Rainville, P.; Duncan, G.H.; Price, D.D.; Carrier, B.; Bushnell, M.C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997, 277, 968–971. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Ko, H.G.; Chen, T.; Descalzi, G.; Koga, K.; Wang, H.; Kim, S.S.; Shang, Y.; Kwak, C.; Park, S.W.; et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010, 330, 1400–1404. [Google Scholar] [CrossRef]
- Xiao, X.; Ding, M.; Zhang, Y.Q. Role of the Anterior Cingulate Cortex in Translational Pain Research. Neurosci. Bull. 2021, 37, 405–422. [Google Scholar] [CrossRef]
- Lee, J.A.; Miao, Z.; Chen, Q.Y.; Li, X.H.; Zhuo, M. Multiple synaptic connections into a single cortical pyramidal cell or interneuron in the anterior cingulate cortex of adult mice. Mol. Brain 2021, 14, 88. [Google Scholar] [CrossRef]
- Li, Z.Z.; Han, W.J.; Sun, Z.C.; Chen, Y.; Sun, J.Y.; Cai, G.H.; Liu, W.N.; Wang, T.Z.; Xie, Y.D.; Mao, H.H.; et al. Extracellular matrix protein laminin beta1 regulates pain sensitivity and anxiodepression-like behaviors in mice. J. Clin. Investig. 2021, 131, e146323. [Google Scholar] [CrossRef]
- Song, Y.; Yao, M.; Kemprecos, H.; Byrne, A.; Xiao, Z.; Zhang, Q.; Singh, A.; Wang, J.; Chen, Z.S. Predictive coding models for pain perception. J. Comput. Neurosci. 2021, 49, 107–127. [Google Scholar] [CrossRef]
- Barthas, F.; Humo, M.; Gilsbach, R.; Waltisperger, E.; Karatas, M.; Leman, S.; Hein, L.; Belzung, C.; Boutillier, A.L.; Barrot, M.; et al. Cingulate Overexpression of Mitogen-Activated Protein Kinase Phosphatase-1 as a Key Factor for Depression. Biol. Psychiatry 2017, 82, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Barthas, F.; Sellmeijer, J.; Hugel, S.; Waltisperger, E.; Barrot, M.; Yalcin, I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol. Psychiatry 2015, 77, 236–245. [Google Scholar] [CrossRef]
- Su, D.J.; Li, L.F.; Wang, S.Y.; Yang, Q.; Wu, Y.J.; Zhao, M.G.; Yang, L. Pra-C exerts analgesic effect through inhibiting microglial activation in anterior cingulate cortex in complete Freund’s adjuvant-induced mouse model. Mol. Pain 2021, 17, 1744806921990934. [Google Scholar] [CrossRef]
- Sun, T.; Wang, J.; Li, X.; Li, Y.J.; Feng, D.; Shi, W.L.; Zhao, M.G.; Wang, J.B.; Wu, Y.M. Gastrodin relieved complete Freund’s adjuvant-induced spontaneous pain by inhibiting inflammatory response. Int. Immunopharmacol. 2016, 41, 66–73. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Z.; Zhang, J.; Chen, J.L.; Yao, P.W.; Mai, C.L.; Mai, J.Z.; Zhang, H.; Liu, X.G. Chronic Oral Administration of Magnesium-L-Threonate Prevents Oxaliplatin-Induced Memory and Emotional Deficits by Normalization of TNF-alpha/NF-kappaB Signaling in Rats. Neurosci. Bull. 2021, 37, 55–69. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Landau, S.; Kerwin, R.; Everall, I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry 2001, 58, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Cotter, D.; Mackay, D.; Chana, G.; Beasley, C.; Landau, S.; Everall, I.P. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex 2002, 12, 386–394. [Google Scholar] [CrossRef]
- Deng, X.X.; Li, S.S.; Sun, F.Y. Necrostatin-1 Prevents Necroptosis in Brains after Ischemic Stroke via Inhibition of RIPK1-Mediated RIPK3/MLKL Signaling. Aging Dis. 2019, 10, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Gu, W.W.; Liu, Z.H.; Zhu, Y.M.; Rong, J.G.; Kent, T.A.; Li, M.; Qiao, S.G.; An, J.Z.; Zhang, H.L. RIP1K Contributes to Neuronal and Astrocytic Cell Death in Ischemic Stroke via Activating Autophagic-lysosomal Pathway. Neuroscience 2018, 371, 60–74. [Google Scholar] [CrossRef]
- Cao, H.; Zuo, C.; Huang, Y.; Zhu, L.; Zhao, J.; Yang, Y.; Jiang, Y.; Wang, F. Hippocampal proteomic analysis reveals activation of necroptosis and ferroptosis in a mouse model of chronic unpredictable mild stress-induced depression. Behav. Brain Res. 2021, 407, 113261. [Google Scholar] [CrossRef]
- Yang, R.; Hu, K.; Chen, J.; Zhu, S.; Li, L.; Lu, H.; Li, P.; Dong, R. Necrostatin-1 protects hippocampal neurons against ischemia/reperfusion injury via the RIP3/DAXX signaling pathway in rats. Neurosci. Lett. 2017, 651, 207–215. [Google Scholar] [CrossRef]
- Sakry, D.; Neitz, A.; Singh, J.; Frischknecht, R.; Marongiu, D.; Biname, F.; Perera, S.S.; Endres, K.; Lutz, B.; Radyushkin, K.; et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol. 2014, 12, e1001993. [Google Scholar] [CrossRef]
- Birey, F.; Kloc, M.; Chavali, M.; Hussein, I.; Wilson, M.; Christoffel, D.J.; Chen, T.; Frohman, M.A.; Robinson, J.K.; Russo, S.J.; et al. Genetic and Stress-Induced Loss of NG2 Glia Triggers Emergence of Depressive-like Behaviors through Reduced Secretion of FGF2. Neuron 2015, 88, 941–956. [Google Scholar] [CrossRef] [Green Version]
- Tkachev, D.; Mimmack, M.L.; Ryan, M.M.; Wayland, M.; Freeman, T.; Jones, P.B.; Starkey, M.; Webster, M.J.; Yolken, R.H.; Bahn, S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 2003, 362, 798–805. [Google Scholar] [CrossRef]
- Atmaca, M.; Onalan, E.; Yildirim, H.; Yuce, H.; Koc, M.; Korkmaz, S. The association of myelin oligodendrocyte glycoprotein gene and white matter volume in obsessive-compulsive disorder. J. Affect. Disord. 2010, 124, 309–313. [Google Scholar] [CrossRef]
- Stewart, S.E.; Platko, J.; Fagerness, J.; Birns, J.; Jenike, E.; Smoller, J.W.; Perlis, R.; Leboyer, M.; Delorme, R.; Chabane, N.; et al. A genetic family-based association study of OLIG2 in obsessive-compulsive disorder. Arch. Gen. Psychiatry 2007, 64, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Dwork, A.J.; Mancevski, B.; Rosoklija, G. White matter and cognitive function in schizophrenia. Int. J. Neuropsychopharmacol. 2007, 10, 513–536. [Google Scholar] [CrossRef]
- Mechawar, N.; Savitz, J. Neuropathology of mood disorders: Do we see the stigmata of inflammation? Transl. Psychiatry 2016, 6, e946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuigan, C.; Hutchinson, M. Unrecognised symptoms of depression in a community-based population with multiple sclerosis. J. Neurol. 2006, 253, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, X.; Ou, Z.; Wang, Y.; Chen, W.; Zhao, T.; Liu, C.; Chen, Y. Dysmyelination by Oligodendrocyte-Specific Ablation of Ninj2 Contributes to Depressive-Like Behaviors. Adv. Sci. 2022, 9, e2103065. [Google Scholar] [CrossRef]
- Dong, H.S.; Han, C.; Jeon, S.W.; Yoon, S.; Jeong, H.G.; Huh, Y.J.; Pae, C.U.; Patkar, A.A.; Steffens, D.C. Characteristics of neurocognitive functions in mild cognitive impairment with depression. Int. Psychogeriatr. 2016, 28, 1181–1190. [Google Scholar] [CrossRef] [Green Version]
- Czeh, B.; Lucassen, P.J. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur. Arch Psychiatry Clin. Neurosci. 2007, 257, 250–260. [Google Scholar] [CrossRef]
- Khera, T.; Rangasamy, V. Cognition and Pain: A Review. Front. Psychol. 2021, 12, 1819. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef] [Green Version]
- Mutso, A.A.; Radzicki, D.; Baliki, M.N.; Huang, L.; Banisadr, G.; Centeno, M.V.; Radulovic, J.; Martina, M.; Miller, R.J.; Apkarian, A.V. Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. 2012, 32, 5747–5756. [Google Scholar] [CrossRef] [Green Version]
- Mao, C.P.; Bai, Z.L.; Zhang, X.N.; Zhang, Q.J.; Zhang, L. Abnormal Subcortical Brain Morphology in Patients with Knee Osteoarthritis: A Cross-sectional Study. Front. Aging Neurosci. 2016, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Oosterman, J.M.; Derksen, L.C.; van Wijck, A.J.; Veldhuijzen, D.S.; Kessels, R.P. Memory functions in chronic pain: Examining contributions of attention and age to test performance. Clin. J. Pain 2011, 27, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.P.; Martelli, M.F.; Zasler, N.D. Chronic pain and neuropsychological functioning. Neuropsychol. Rev. 2000, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.E.; Navratilova, E.; Porreca, F. Chronic Pain Produces Reversible Memory Deficits That Depend on Task Difficulty in Rats. J. Pain 2021, 22, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Gerges, N.Z. Neurogranin Regulates Metaplasticity. Front. Mol. Neurosci. 2019, 12, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin Ibrahim, M.Z.; Benoy, A.; Sajikumar, S. Long-term plasticity in the hippocampus: Maintaining within and ‘tagging’ between synapses. FEBS J. 2022, 289, 2176–2201. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.A.; Manzhulo, I.V.; Konovalova, S.P.; Zagliadkina, A.A. Neuropathic Pain Causes a Decrease in the Dendritic Tree Complexity of Hippocampal CA3 Pyramidal Neurons. Cells Tissues Organs 2019, 208, 89–100. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.J.; Wang, J.; Li, D.; Ren, W.J.; Peng, J.; Wei, X.; Xu, T.; Xin, W.J.; Pang, R.P.; et al. TNF-alpha Differentially Regulates Synaptic Plasticity in the Hippocampus and Spinal Cord by Microglia-Dependent Mechanisms after Peripheral Nerve Injury. J. Neurosci. 2017, 37, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.J.; Liu, Y.; Zhou, L.J.; Li, W.; Zhong, Y.; Pang, R.P.; Xin, W.J.; Wei, X.H.; Wang, J.; Zhu, H.Q.; et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacology 2011, 36, 979–992. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Li, D.; Zhou, X.; Ouyang, H.D.; Zhou, L.J.; Zhou, H.; Zhang, H.M.; Wei, X.H.; Liu, G.; Liu, X.G. Oral Application of Magnesium-L-Threonate Attenuates Vincristine-induced Allodynia and Hyperalgesia by Normalization of Tumor Necrosis Factor-alpha/Nuclear Factor-kappaB Signaling. Anesthesiology 2017, 126, 1151–1168. [Google Scholar] [CrossRef]
- Saffarpour, S.; Janzadeh, A.; Rahimi, B.; Ramezani, F.; Nasirinezhad, F. Chronic nanocurcumin treatment ameliorates pain-related behavior, improves spatial memory, and reduces hippocampal levels of IL-1beta and TNFalpha in the chronic constriction injury model of neuropathic pain. Psychopharmacology 2021, 238, 877–886. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Li, Y.; Xu, L.; Yu, X.; Ge, L.; Li, J.; Zhu, Y.; He, S. Necroptosis mediates TNF-induced toxicity of hippocampal neurons. Biomed. Res. Int. 2014, 2014, 290182. [Google Scholar] [CrossRef] [Green Version]
- Telegina, D.V.; Suvorov, G.K.; Kozhevnikova, O.S.; Kolosova, N.G. Mechanisms of Neuronal Death in the Cerebral Cortex during Aging and Development of Alzheimer’s Disease-Like Pathology in Rats. Int. J. Mol. Sci. 2019, 20, 5632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.H.; Koh, R.Y.; Lim, C.L.; Leong, C.O. Receptor-Interacting Protein Kinase 1 (RIPK1) as a Potential Therapeutic Target: An Overview of Its Possible Role in the Pathogenesis of Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Jia, N.; Yang, F.; Liu, Z.; Li, R.; Jiang, Y.; Zhao, J.; Wang, L.; Zhang, S.; Zhang, Z.; et al. Hydrogen Alleviates Necroptosis and Cognitive Deficits in Lithium-Pilocarpine Model of Status Epilepticus. Cell Mol. Neurobiol. 2019, 39, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.M.K.; Abdel-Nasser, Z.M.; Shahin, N.N. Ameliorative Effect of Necrosulfonamide in a Rat Model of Alzheimer’s Disease: Targeting Mixed Lineage Kinase Domain-like Protein-Mediated Necroptosis. ACS Chem. Neurosci. 2020, 11, 3386–3397. [Google Scholar] [CrossRef]
- Nasseri, B.; Zareian, P.; Alizade, H. Apelin attenuates streptozotocin-induced learning and memory impairment by modulating necroptosis signaling pathway. Int. Immunopharmacol. 2020, 84, 106546. [Google Scholar] [CrossRef]
- Colucci, M.; Stefanucci, A.; Mollica, A.; Aloisi, A.M.; Maione, F.; Pieretti, S. New Insights on Formyl Peptide Receptor Type 2 Involvement in Nociceptive Processes in the Spinal Cord. Life 2022, 12, 500. [Google Scholar] [CrossRef]



| Rank | Popular Journals | Co-Citations (n) | IF(2021) | Research Directions |
|---|---|---|---|---|
| 1 | Cell | 341 | 41.584/Q1 | Cell biology/stem cells |
| 2 | J biol chem | 310 | 5.157/Q2 | Signal transduction |
| 3 | J neurosci | 275 | 6.167/Q1 | Neuroscience/electrophysiology |
| 4 | P natlacad sci usa | 274 | 11.205/Q1 | Biology/physics |
| 5 | Cell death differ | 256 | 15.828/Q1 | Molecular biology/cell differentiation |
| 6 | Nature | 244 | 49.962/Q1 | Life sciences/natural science |
| 7 | J neurochem | 206 | 5.372/Q1 | Neuroinflammation/microglia |
| 8 | Brain res | 187 | 3.252/Q3 | Neuroscience/neuroprotection |
| 9 | Stroke | 181 | 7.914/Q1 | Stroke/cardio cerebrovascular diseases |
| 10 | Science | 169 | 47.728/Q1 | Catalysis/inheritance |
| Rank | Source | Citations (n) | Main Results | Research Directions | Ref. |
|---|---|---|---|---|---|
| 1 | Nat Chem Biol | 74 | Identification of necroptosis and its inhibitor Nec-1 | New pathway of cell death | [65] |
| 2 | Nat Chem Biol | 48 | Necrostatinsarea family offirst-in-class inhibitors of RIP1 kinase, the key upstream kinase involved in the activation of necroptosis | Inhibition of necroptosis and its mechanism | [144] |
| 3 | Nat Rev Mol Cell Biol | 43 | Necroptosis can occur in a regulated manner | Molecular mechanisms of necroptosis | [151] |
| 4 | Cell | 42 | MLKL is a key mediator of necroptosis signalling downstream of RIP3 kinase | Molecular mechanisms of necroptosis | [152] |
| 5 | Cell | 32 | RIP3 controls programmed necroptosis by initiating the pronecrotic kinase cascade | Molecular mechanisms of necroptosis | [153] |
| 6 | Science | 29 | RIP3 is a molecular switch between TNF-induced apoptosis and necrosis and is required for RIP1-mediated necrosis | Molecular mechanisms of necroptosis | [154] |
| 7 | Cell | 29 | RIP3 as a determinant for cellular necrosis is recruited to RIPK1 to form a necrosis-inducing complex | Molecular mechanisms of necroptosis | [155] |
| 8 | Cell rep | 28 | Necroptosis is involved in multiple sclerosis (MS), and RIPK1 may represent a therapeutic strategy | Role of necroptosis in MS | [156] |
| 9 | Nature | 28 | A review of the regulatory mechanisms of necroptosis and its potential role in inflammation and diseases | Molecular mechanisms of necroptosis and its role in inflammation | [61] |
| 10 | Nat Immunol. | 24 | RIP is required for caspase-independent necrotic death induced by Pas, TNF and TRAIL | Molecular mechanisms of cell death | [54] |
| Rank | Source | Citations (n) | Main Results | Research Directions | Ref. |
|---|---|---|---|---|---|
| 1 | Neuron | 244 | Necroptosis drives motor neuron death in models of both sporadic and familial amyotrophic lateral sclerosis (ALS) | Role of necroptosis in ALS | [157] |
| 2 | Nat Rev Neurosci | 197 | Review of necroptosis in neurological diseases | Role of necroptosis in neurological diseases | [158] |
| 3 | Nat Neurosci | 152 | Genes regulated by RIPK1 overlap with multiple transcriptomic signatures of Alzheimer’s disease (AD) | Role of necroptosis in AD | [159] |
| 4 | Prog Neurobiol. | 148 | Review of the regulation of autophagy in neurons, glia, and brain microvascular cells in response to ischemia stress | Crosstalk between autophagy, necroptosis and apoptosis | [160] |
| 5 | J Pineal Res | 136 | Melatonin inhibits the Ripk3–PGAM5–CypD–mPTP cascade and hence reduces necroptosis | Role of necroptosis in ischaemia–reperfusion injury | [161] |
| 6 | J Neuroinflammation | 131 | Review of the molecular mechanisms of necroptosis and its relevance to diseases | The molecular mechanisms of necroptosis and its role in disease | [147] |
| 7 | Front Neurosci | 131 | Review of targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration | Neuroprotective signalling pathways | [162] |
| 8 | Adv Exp Med Biol | 92 | Review stating that the biochemical pathways causing programmed neurodegeneration, instead of neuronal death per se, are responsible for epileptogenesis | Reprogramming of neuronal death pathways in epileptogenesis | [163] |
| 9 | J Neurosci | 88 | The axodestructive factor Sarm1 is required for mitochondrial depolarisation-induced axon degeneration and cell death | A novel form of programmed cell destruction called sarmoptosis | [164] |
| 10 | Nat Neurosci | 87 | Efficient remyelination requires the death of microglia followed by their repopulation to a pro-regenerative state | Role of microglia in white matter regeneration | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.-W.; Chen, S.-X.; Li, Q.-Y.; Zang, Y. Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-α-Necroptosis Pathway. Int. J. Mol. Sci. 2022, 23, 7191. https://doi.org/10.3390/ijms23137191
Duan Y-W, Chen S-X, Li Q-Y, Zang Y. Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-α-Necroptosis Pathway. International Journal of Molecular Sciences. 2022; 23(13):7191. https://doi.org/10.3390/ijms23137191
Chicago/Turabian StyleDuan, Yi-Wen, Shao-Xia Chen, Qiao-Yun Li, and Ying Zang. 2022. "Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-α-Necroptosis Pathway" International Journal of Molecular Sciences 23, no. 13: 7191. https://doi.org/10.3390/ijms23137191
APA StyleDuan, Y.-W., Chen, S.-X., Li, Q.-Y., & Zang, Y. (2022). Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-α-Necroptosis Pathway. International Journal of Molecular Sciences, 23(13), 7191. https://doi.org/10.3390/ijms23137191





