Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted
Abstract
1. Background
2. Mechanisms of Cisplatin Toxicity
3. Cisplatin Derivatives
Platinum Loaded Nanoparticles
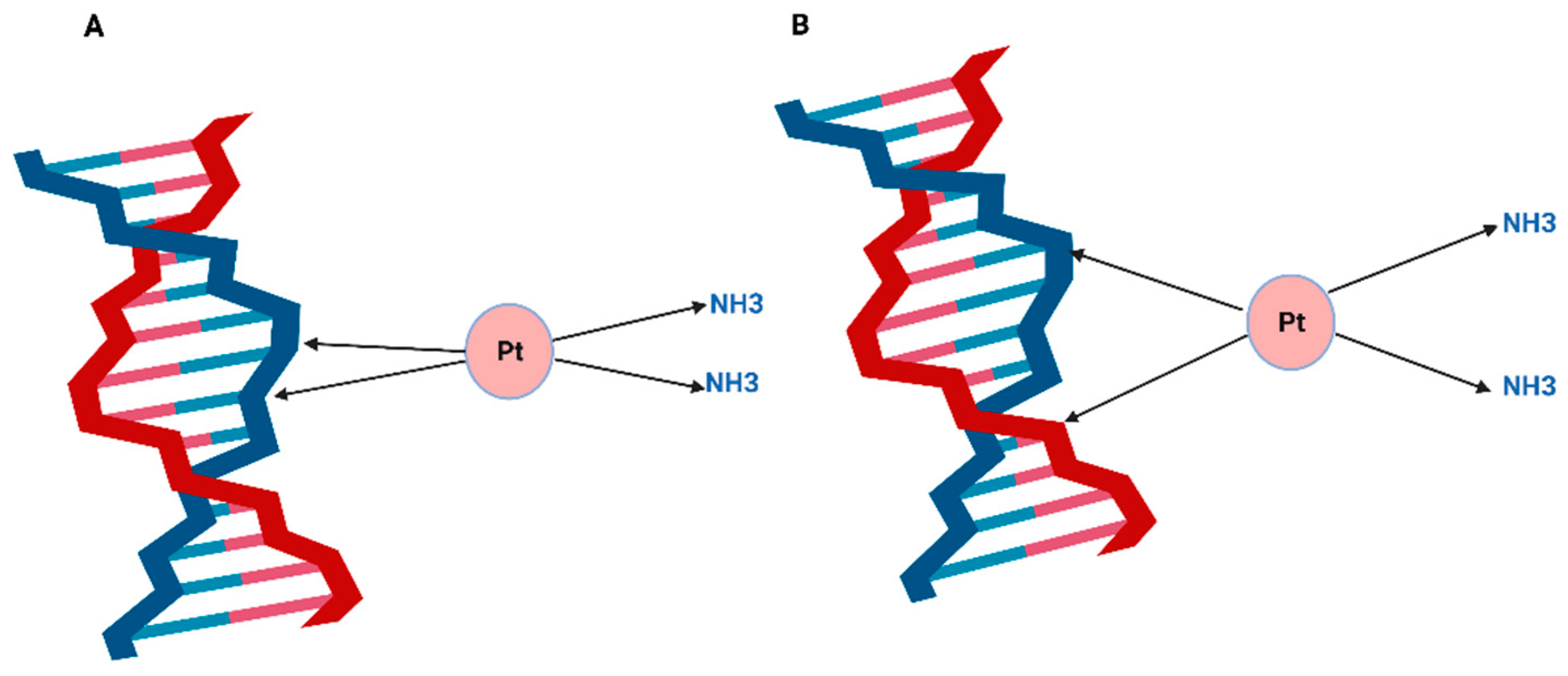
4. Cisplatin-Induced DNA Damage
4.1. Nucleotide Excision Repair Pathway
4.2. Mismatch Repair Pathway
5. Role of DSB Pathways in Repairing Cisplatin-Induced DNA Lesions
BRCA Mutations and Cisplatin Sensitivity
6. Cisplatin and Apoptosis
7. Mechanisms of Cisplatin Resistance
8. Cisplatin and Immune Response
9. Cisplatin Uptake Transporters
10. Increased Cisplatin Detoxification
11. Epigenetics Changes
12. Upregulation of DNA Repair Capacity
13. Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omez-Ruiz, S.G.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. On the Discovery, Biological Effects, and Use of Cisplatin and Metallocenes in Anticancer Chemotherapy. Bioinorg. Chem. Appl. 2012, 2012, 140284. [Google Scholar] [CrossRef]
- Alderden, R.A.; Hall, M.D.; Hambley, T.W.; Kauffman, G.B. Chemistry for Everyone the Discovery and Development of Cisplatin Products of Chemistry edited by. J. Chem. Educ. 2006, 83, 728. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Krishnamurthy, S. Cellular responses to cisplatin-induced DNA damage. J. Nucleic Acids 2010, 2010, 201367. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Townsend, D.M.; Hanigan, M.H. Inhibition of γ-glutamyl transpeptidase or cysteine sconjugate β-lyase activity blocks the nephrotoxicity of cisplatin in mice. J. Pharmacol. Exp. Ther. 2002, 300, 142–148. [Google Scholar] [CrossRef]
- Daubeuf, S.; Balin, D.; Leroy, P.; Visvikis, A. Different mechanisms for γ-glutamyltransferase-dependent resistance to carboplatin and cisplatin. Biochem. Pharmacol. 2003, 66, 595–604. [Google Scholar] [CrossRef]
- Ha, S.S.; Rubaina, K.; Lee, C.-S.; John, V.; Seetharamu, N. Amifostine is a Nephro-Protectant in Patients Receiving Treatment with Cisplatin- Myth, Mystery or Matter-of-Fact? J. Nephrol. Sci. 2021, 3, 4–8. [Google Scholar]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Vasconcellos, V.F.; Marta, G.N.; da Silva, E.M.K.; Gois, A.F.T.; de Castria, T.B.; Riera, R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2020, 1, CD009256. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Woodward, N.; Coward, J.I.G. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol. Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Holsinger, F.C.; Colevas, A.D.; Hara, W.; Le, Q.T.; Beadle, B.M. Cisplatin or Carboplatin-Based Chemoradiation for Locoregionally Advanced Squamous Cell Carcinoma of the Head and Neck: A Population-Based Comparison. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e371. [Google Scholar] [CrossRef]
- de Man, F.M.; van Eerden, R.A.G.; Oomen-De Hoop, E.; Veraart, J.N.; van Doorn, N.; van Doorn, L.; van der Gaast, A.; Mathijssen, R.H.J. Efficacy and Toxicity of Weekly Carboplatin and Paclitaxel as Induction or Palliative Treatment in Advanced Esophageal Cancer Patients. Cancers 2019, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Marr, B.P.; Dunkel, I.J.; Linker, A.; Abramson, D.H. Periocular carboplatin for retinoblastoma: Long-term report (12 years) on efficacy and toxicity. Br. J. Ophthalmol. 2012, 96, 881–883. [Google Scholar] [CrossRef]
- Murray, L.J.; Bridgewater, C.H.; Levy, D. Carboplatin Chemotherapy in Patients with Recurrent High-grade Glioma. Clin. Oncol. 2011, 23, 55–61. [Google Scholar] [CrossRef]
- Moens, S.; Zhao, P.; Baietti, M.F.; Marinelli, O.; Van Haver, D.; Impens, F.; Floris, G.; Marangoni, E.; Neven, P.; Annibali, D.; et al. The mitotic checkpoint is a targetable vulnerability of carboplatin-resistant triple negative breast cancers. Sci. Rep. 2021, 11, 3176. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers 2021, 13, 131. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Yoon, J.; Kim, T.-Y.; Bang, J.-H.; Nam, A.-R.; Oh, K.-S.; Kim, J.-M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Hussain, S.A.; Lester, J.F.; Jackson, R.; Gornall, M.; Qureshi, M.; Elliott, A.; Crabb, S.J.; Huddart, R.A.; Vasudev, N.; Birtle, A.J.; et al. Addition of nintedanib or placebo to neoadjuvant gemcitabine and cisplatin in locally advanced muscle-invasive bladder cancer (NEOBLADE): A double-blind, randomised, phase 2 trial. Lancet Oncol. 2022, 23, 650–658. [Google Scholar] [CrossRef]
- Bourhis, J.; Burtness, B.; Licitra, L.F.; Nutting, C.; Schoenfeld, J.D.; Omar, M.; Bouisset, F.; Nauwelaerts, H.; Urfer, Y.; Zanna, C.; et al. Xevinapant or placebo plus chemoradiotherapy in locally advanced squamous cell carcinoma of the head and neck: TrilynX phase III study design. Future Oncol. 2022, 18, 1669–1678. [Google Scholar] [CrossRef]
- Frankart, A.J.; Sadraei, N.H.; Huth, B.; Redmond, K.P.; Barrett, W.L.; Kurtzweil, N.; Riaz, M.K.; Wise-Draper, T.; Rodriguez, C.P.; Adelstein, D.J.; et al. A phase I/II trial of concurrent immunotherapy with chemoradiation in locally advanced larynx cancer. Laryngoscope Investig. Otolaryngol. 2022, 7, 437–443. [Google Scholar] [CrossRef]
- Kubicek, G.J.; Khrizman, P.; Squillante, C.; Callahan, K.; Xu, Q.; Abouzgheib, W.; Boujaoude, Z.; Patel, A.; Hageboutros, A. Stereotactic Body Radiotherapy and Systemic Dose Chemotherapy for Locally Advanced Lung Cancer: Single Arm Phase 2 Study. Am. J. Clin. Oncol. 2022, 45, 129–133. [Google Scholar] [CrossRef]
- Biswas, T.; Dowlati, A.; Kunos, C.A.; Pink, J.J.; Oleinick, N.L.; Malik, S.; Fu, P.; Cao, S.; Bruno, D.S.; Bajor, D.L.; et al. Adding Base-Excision Repair Inhibitor TRC102 to Standard Pemetrexed-Platinum-Radiation in Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer: Results of a Phase I Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 646–652. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Huang, H.Q.; Filiaci, V.L.; Randall, M.; DiSilvestro, P.A.; Moxley, K.M.; Fowler, J.M.; Powell, M.A.; Spirtos, N.M.; Tewari, K.S.; et al. Patient reported outcomes for cisplatin and radiation followed by carboplatin/paclitaxel versus carboplatin/paclitaxel for locally advanced endometrial carcinoma: An NRG oncology study. Gynecol. Oncol. 2022, 164, 428–436. [Google Scholar] [CrossRef]
- Xu, B.; Zeng, M.; Zeng, J.; Feng, J.; Yu, L. Meta-analysis of clinical trials comparing the efficacy and safety of liposomal cisplatin versus conventional nonliposomal cisplatin in nonsmall cell lung cancer (NSCLC) and squamous cell carcinoma of the head and neck (SCCHN). Medicine 2018, 97, e13169. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Q.; He, J.; Hu, H.; Du, J.; Zhu, Y.; Chen, J.; Liu, Z.; Wang, J.; Sun, L.; et al. Phase II study of anlotinib in combination with oxaliplatin and capecitabine for patients with RAS/BRAF wild-type metastatic colorectal adenocarcinoma as the first-line therapy. BMC Med. 2022, 20, 155. [Google Scholar] [CrossRef]
- Alcindor, T.; Beauger, N. Oxaliplatin: A review in the era of molecularly targeted therapy. Curr. Oncol. 2011, 18, 18–25. [Google Scholar] [CrossRef]
- Bruno, P.M.; Liu, Y.; Park, G.Y.; Murai, J.; Koch, C.E.; Eisen, T.J.; Pritchard, J.R.; Pommier, Y.; Lippard, S.J.; Hemann, M.T. A subset of platinum-containing chemotherapeutic agents kill cells by inducing ribosome biogenesis stress rather than by engaging a DNA damage response. Nat. Med. 2017, 23, 461. [Google Scholar] [CrossRef]
- Sutton, E.C.; DeRose, V.J. Early nucleolar responses differentiate mechanisms of cell death induced by oxaliplatin and cisplatin. J. Biol. Chem. 2021, 296, 100633. [Google Scholar] [CrossRef]
- Bordonaro, R.; Calvo, A.; Auriemma, A.; Hollebecque, A.; Rubovszky, G.; Saunders, M.P.; Pápai, Z.; Prager, G.; Stein, A.; André, T.; et al. Trifluridine/tipiracil in combination with oxaliplatin and either bevacizumab or nivolumab in metastatic colorectal cancer: A dose-expansion, phase I study. ESMO Open 2021, 6, 100270. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Itamochi, H.; Kigawa, J. Nedaplatin: A cisplatin derivative in cancer chemotherapy. Cancer Manag. Res. 2013, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, D.; Ivanova, S. Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases. Molecules 2022, 27, 2466. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.Z.; Xu, H.Y.; Zhao, Z.M.; Zhang, G.M.; Lin, F.W. Comparison of efficacy and toxicity between nedaplatin and cisplatin in treating malignant pleural effusion. OncoTargets Ther. 2018, 11, 5509–5512. [Google Scholar] [CrossRef]
- El-Shafie, S.; Fahmy, S.A.; Ziko, L.; Elzahed, N.; Shoeib, T.; Kakarougkas, A. Encapsulation of Nedaplatin in Novel PEGylated Liposomes Increases Its Cytotoxicity and Genotoxicity against A549 and U2OS Human Cancer Cells. Pharmaceutics 2020, 12, 863. [Google Scholar] [CrossRef]
- Hamilton, G.; Olszewski, U. Picoplatin pharmacokinetics and chemotherapy of non-small cell lung cancer. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1381–1390. [Google Scholar] [CrossRef]
- Eckardt, J.R.; Oncology, J.; Lipatov, O.N. Phase II Study of Picoplatin As Second-Line Therapy for Patients With Small-Cell Lung Cancer. Artic. J. Clin. Oncol. 2009, 27, 2046–2051. [Google Scholar] [CrossRef]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef]
- Ciuleanu, T.; Samarzjia, M.; Demidchik, Y.; Beliakouski, V.; Rancic, M.; Bentsion, D.L.; Orlov, S.V.; Schaeffler, B.A.; De Jager, R.L.; Breitz, H.B. Randomized phase III study (SPEAR) of picoplatin plus best supportive care (BSC) or BSC alone in patients (pts) with SCLC refractory or progressive within 6 months after first-line platinum-based chemotherapy. J. Clin. Oncol. 2010, 28, 7002. [Google Scholar] [CrossRef]
- Malik-Gajewska, M.; Trynda, J.; Zierkiewicz, W.; Helios, K.; Latajka, R.; Wietrzyk, J.; Michalska, D. Picoplatin-based complexes with the bioactive orotate and 5-fluoroorotate ligands: Synthesis, DFT calculations, structure, spectroscopic characterization and in vitro cytotoxicity. J. Mol. Struct. 2018, 1171, 155–167. [Google Scholar] [CrossRef]
- Boulikas, T. Clinical overview on LipoplatinTM: A successful liposomal formulation of cisplatin. Drugs 2009, 18, 1197–1218. [Google Scholar] [CrossRef]
- Farooq, M.A.; Aquib, M.; Farooq, A.; Haleem Khan, D.; Joelle Maviah, M.B.; Sied Filli, M.; Kesse, S.; Boakye-Yiadom, K.O.; Mavlyanova, R.; Parveen, A.; et al. Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: An overview. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1674–1692. [Google Scholar] [CrossRef]
- Song, H.; Quan, F.; Yu, Z.; Zheng, M.; Ma, Y.; Xiao, H.; Ding, F. Carboplatin prodrug conjugated Fe3O4 nanoparticles for magnetically targeted drug delivery in ovarian cancer cells. J. Mater. Chem. B 2019, 7, 433–442. [Google Scholar] [CrossRef]
- Esim, O.; Gedik, M.E.; Dogan, A.L.; Gunaydin, G.; Hascicek, C. Development of carboplatin loaded bovine serum albumin nanoparticles and evaluation of its effect on an ovarian cancer cell line. J. Drug Deliv. Sci. Technol. 2021, 64, 102655. [Google Scholar] [CrossRef]
- Roudsari, M.H.; Saeidi, N.; Kabiri, N.; Ahmadi, A.; Tabrizi, M.M.; Shahmabadi, H.E.; Khiyavi, A.A.; Reghbati, B. Investigation of Characteristics and Behavior of Loaded Carboplatin on the, Liposomes Nanoparticles, on the Lung and Ovarian Cancer: An In-Vitro Evaluation. Asian Pac. J. Cancer Biol. 2016, 1, 9. [Google Scholar] [CrossRef]
- Sadasivam, M.; Avci, P.; Gupta, G.K.; Lakshmanan, S.; Chandran, R.; Huang, Y.Y.; Kumar, R.; Hamblin, M.R. Self-assembled liposomal nanoparticles in photodynamic therapy. Eur. J. Nanomed. 2013, 5, 115–129. [Google Scholar] [CrossRef]
- Liu, D.; Poon, C.; Lu, K.; He, C.; Lin, W. Self-assembled nanoscale coordination polymers with trigger release properties for effective anticancer therapy. Nat. Commun. 2014, 5, 4182. [Google Scholar] [CrossRef]
- Mi, P.; Miyata, K.; Kataoka, K.; Cabral, H. Clinical translation of self-assembled cancer nanomedicines. Adv. Ther. 2021, 4, 2000159. [Google Scholar] [CrossRef]
- Yang, G.G.; Pan, Z.Y.; Zhang, D.Y.; Cao, Q.; Ji, L.N.; Mao, Z.W. Precisely assembled nanoparticles against cisplatin resistance via cancer-specific targeting of mitochondria and imaging-guided chemo-photothermal therapy. ACS Appl. Mater. Interfaces 2020, 12, 43444–43455. [Google Scholar] [CrossRef] [PubMed]
- Du, X.F.; Li, Y.; Long, J.; Zhang, W.; Wang, D.; Li, C.R.; Zhao, M.X.; Lai, Y. Fabrication of cisplatin-loaded polydopamine nanoparticles via supramolecular self-assembly for photoacoustic imaging guided chemo-photothermal cancer therapy. Appl. Mater. Today 2021, 23, 101019. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2011, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA repair pathways and cisplatin resistance: An intimate relationship. Clinics 2018, 73, e478s. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Guan, Y.-D.; Chen, X.-S.; Yang, J.-M.; Cheng, Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 2021, 11, 629266. [Google Scholar] [CrossRef] [PubMed]
- Saldivar, J.S.; Wu, X.; Follen, M.; Gershenson, D. Nucleotide excision repair pathway review I: Implications in ovarian cancer and platinum sensitivity. Gynecol. Oncol. 2007, 107, S56–S71. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Onishi, Y.; Tada, H.; Kurihara, F.; Kusao, K.; Furukawa, M.; Iwai, S.; Yokoi, M.; Sakai, W.; Sugasawa, K. Mechanism and regulation of DNA damage recognition in nucleotide excision repair. Genes Environ. 2019, 41, 2. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.; Kothandapani, A.; Zhitkovich, A.; Sobol, R.W.; Patrick, S.M. Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links. DNA Repair 2015, 35, 126. [Google Scholar] [CrossRef]
- Takahashi, M.; Koi, M.; Balaguer, F.; Boland, C.R.; Goel, A. MSH3 Mediates Sensitization of Colorectal Cancer Cells to Cisplatin, Oxaliplatin, and a Poly(ADP-ribose) Polymerase Inhibitor*. J. Biol. Chem. 2011, 286, 12157–12165. [Google Scholar] [CrossRef]
- Martin, L.P.; Hamilton, T.C.; Schilder, R.J. Platinum Resistance: The Role of DNA Repair Pathways. Clin. Cancer Res. 2008, 14, 1291–1295. [Google Scholar] [CrossRef]
- Tentori, L.; Muzi, A.; Dorio, A.S.; Dolci, S.; Campolo, F.; Vernole, P.; Lacal, P.M.; Praz, F.; Graziani, G. MSH3 expression does not influence the sensitivity of colon cancer HCT116 cell line to oxaliplatin and poly(ADP-ribose) polymerase (PARP) inhibitor as monotherapy or in combination. Cancer Chemother. Pharmacol. 2013, 72, 117–125. [Google Scholar] [CrossRef]
- Kunitomi, H.; Banno, K.; Yanokura, M.; Takeda, T.; Iijima, M.; Nakamura, K.; Iida, M.; Adachi, M.; Watanabe, K.; Matoba, Y.; et al. New use of microsatellite instability analysis in endometrial cancer. Oncol. Lett. 2017, 14, 3297. [Google Scholar] [CrossRef]
- Karamurzin, Y.; Rutgers, J.K.L. DNA mismatch repair deficiency in endometrial carcinoma. Int. J. Gynecol. Pathol. 2009, 28, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Alldredge, J.K.; Eskander, R.N. EZH2 inhibition in ARID1A mutated clear cell and endometrioid ovarian and endometrioid endometrial cancers. Gynecol. Oncol. Res. Pract. 2017, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.W.; Tang, J.J.; Sun, W.; Wang, H. PGK1 facilities cisplatin chemoresistance by triggering HSP90/ERK pathway mediated DNA repair and methylation in endometrial endometrioid adenocarcinoma. Mol. Med. 2019, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Fazal, Z.; Singh, R.; Fang, F.; Bikorimana, E.; Baldwin, H.; Corbet, A.; Tomlin, M.; Yerby, C.; Adra, N.; Albany, C.; et al. Hypermethylation and global remodelling of DNA methylation is associated with acquired cisplatin resistance in testicular germ cell tumours. Epigenetics 2021, 16, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Constâncio, V.; Leite-Silva, P.; Guimarães, R.; Cantante, M.; Braga, I.; Maurício, J.; Looijenga, L.H.J.; Henrique, R.; Jerónimo, C. Differential methylation EPIC analysis discloses cisplatin-resistance related hypermethylation and tumor-specific heterogeneity within matched primary and metastatic testicular germ cell tumor patient tissue samples. Clin. Epigenet. 2021, 13, 70. [Google Scholar] [CrossRef]
- Diggle, C.P.; Bentley, J.; Knowles, M.A.; Kiltie, A.E. Inhibition of double-strand break non-homologous end-joining by cisplatin adducts in human cell extracts. Nucleic Acids Res. 2005, 33, 2531–2539. [Google Scholar] [CrossRef][Green Version]
- Boeckman, H.J.; Trego, K.S.; Turchi, J.J. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of non-homologous end joining. Mol. Cancer Res. MCR 2005, 3, 277. [Google Scholar] [CrossRef]
- West, R.B.; Yaneva, M.; Lieber, M.R. Productive and Nonproductive Complexes of Ku and DNA-Dependent Protein Kinase at DNA Termini. Mol. Cell. Biol. 1998, 18, 5908. [Google Scholar] [CrossRef]
- Borrego-Soto, G.; Ortiz-López, R.; Rojas-Martínez, A. Ionizing radiation-induced DNA injury and damage detection in patientswith breast cancer. Genet. Mol. Biol. 2015, 38, 420. [Google Scholar] [CrossRef]
- Sears, C.R.; Turchi, J.J. Complex Cisplatin-Double Strand Break (DSB) Lesions Directly Impair Cellular Non-Homologous End-Joining (NHEJ) Independent of Downstream Damage Response (DDR) Pathways. J. Biol. Chem. 2012, 287, 24263. [Google Scholar] [CrossRef]
- Usanova, S.; Piée-Staffa, A.; Sied, U.; Thomale, J.; Schneider, A.; Kaina, B.; Köberle, B. Cisplatin sensitivity of testis tumour cells is due to deficiency in interstrand-crosslink repair and low ERCC1-XPF expression. Mol. Cancer 2010, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Kothandapani, A.; Tillison, K.; Kalman-Maltese, V.; Patrick, S.M. Downregulation of XPF–ERCC1 enhances cisplatin efficacy in cancer cells. DNA Repair 2010, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, S.; Muguruma, M.; Takano, N.; Miyahara, K.; Kawate, T.; Kaise, H.; Yamada, K.; Miyazawa, K.; Ishikawa, T. Association of BRCA Mutations and BRCAness Status With Anticancer Drug Sensitivities in Triple-Negative Breast Cancer Cell Lines. J. Surg. Res. 2020, 250, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Alli, E.; Sharma, V.B.; Hartman, A.R.; Lin, P.S.; McPherson, L.; Ford, J.M. Enhanced sensitivity to cisplatin and gemcitabine in Brca1-deficient murine mammary epithelial cells. BMC Pharmacol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Panzarino, N.J.; Krais, J.J.; Cong, K.; Peng, M.; Mosqueda, M.; Nayak, S.U.; Bond, S.M.; Calvo, J.A.; Doshi, M.B.; Bere, M.; et al. Replication gaps underlie BRCA deficiency and therapy response. Cancer Res. 2021, 81, 1388–1397. [Google Scholar] [CrossRef]
- Deo, K.M.; Ang, D.L.; McGhie, B.; Rajamanickam, A.; Dhiman, A.; Khoury, A.; Holland, J.; Bjelosevic, A.; Pages, B.; Gordon, C.; et al. Platinum coordination compounds with potent anticancer activity. Coord. Chem. Rev. 2018, 375, 148–163. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, X.; Yuan, H.; Guo, Y.; Song, D.; Qi, F.; Zhu, Z.; Wang, X.; Guo, Z. Interfering in apoptosis and DNA repair of cancer cells to conquer cisplatin resistance by platinumn (iv) prodrugs. Chem. Sci. 2020, 11, 3829–3835. [Google Scholar] [CrossRef]
- Sakai, W.; Swisher, E.M.; Karlan, B.Y.; Agarwal, M.K.; Higgins, J.; Friedman, C.; Villegas, E.; Jacquemont, C.; Farrugia, D.J.; Couch, F.J.; et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 2008, 451, 1116. [Google Scholar] [CrossRef]
- D’Andrea, A.D. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair 2018, 71, 172–176. [Google Scholar] [CrossRef]
- Jackson, L.M.; Dhoonmoon, A.; Hale, A.; Dennis, K.A.; Schleicher, E.M.; Nicolae, C.M.; Moldovan, G.L. Loss of MED12 activates the TGFβ pathway to promote chemoresistance and replication fork stability in BRCA-deficient cells. Nucleic Acids Res. 2021, 49, 12855–12869. [Google Scholar] [CrossRef]
- Miron, B.; Hoffman-Censits, J.H.; Anari, F.; O’Neill, J.; Geynisman, D.M.; Zibelman, M.R.; Kutikov, A.; Viterbo, R.; Greenberg, R.E.; Chen, D.; et al. Defects in DNA Repair Genes Confer Improved Long-term Survival after Cisplatin-based Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2020, 3, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Alblihy, A.; Miligy, I.M.; Alabdullah, M.L.; Alsaleem, M.; Toss, M.S.; Algethami, M.; Abdel-Fatah, T.; Moseley, P.; Chan, S.; et al. Molecular disruption of DNA polymerase β for platinum sensitisation and synthetic lethality in epithelial ovarian cancers. Oncogene 2021, 40, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, K.A.; Alabdullah, M.; Griffin, M.; Toss, M.S.; Fatah, T.M.A.A.; Alblihy, A.; Moseley, P.; Chan, S.Y.T.; Rakha, E.A.; Madhusudan, S. ERCC1-XPF deficiency is a predictor of olaparib induced synthetic lethality and platinum sensitivity in epithelial ovarian cancers. Gynecol. Oncol. 2019, 153, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Zazuli, Z.; Otten, L.S.; Drögemöller, B.I.; Medeiros, M.; Monzon, J.G.; Wright, G.E.B.; Kollmannsberger, C.K.; Bedard, P.L.; Chen, Z.; Gelmon, K.A.; et al. Outcome Definition Influences the Relationship between Genetic Polymorphisms of ERCC1, ERCC2, SLC22A2 and Cisplatin Nephrotoxicity in Adult Testicular Cancer Patients. Genes 2019, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, D.; Yi, Y.; Zeng, S.; Liu, S.; Li, P.; Xie, H.; Yu, P.; Jiang, G.; Liu, H. Histone Deacetylase Inhibitor Sensitizes ERCC1-High Non-small-Cell Lung Cancer Cells to Cisplatin via Regulating miR-149. Mol. Ther. Oncolytics 2020, 17, 448–459. [Google Scholar] [CrossRef]
- Pan, C.H.; Chen, S.Y.; Wang, J.Y.; Tsao, S.P.; Huang, H.Y.; Wei-Chen Chiu, P.; Wu, C.H. Sclareol ameliorated ERCC1-mediated cisplatin resistance in A549 human lung adenocarcinoma cells and a murine xenograft tumor model by suppressing AKT-GSK3β-AP1/Snail and JNK-AP1 pathways. Chem. Biol. Interact. 2020, 332, 109304. [Google Scholar] [CrossRef]
- Du, P.; Wang, Y.; Chen, L.; Gan, Y.; Wu, Q. High ERCC1 expression is associated with platinum-resistance, but not survival in patients with epithelial ovarian cancer. Oncol. Lett. 2016, 12, 857–862. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Chen, Y.C.; Qian, H.; Chen, Y.J.; Su, J.Y.; Wu, M.; Lan, T. The functional status of DNA repair pathways determines the sensitization effect to cisplatin in non-small cell lung cancer cells. Cell. Oncol. 2016, 39, 511–522. [Google Scholar] [CrossRef]
- Prieto-Garcia, C.; Hartmann, O.; Reissland, M.; Fischer, T.; Maier, C.R.; Rosenfeldt, M.; Schülein-Völk, C.; Klann, K.; Kalb, R.; Dikic, I.; et al. Inhibition of USP28 overcomes Cisplatin-resistance of squamous tumors by suppression of the Fanconi anemia pathway. Cell Death Differ. 2021, 29, 568–584. [Google Scholar] [CrossRef]
- Jacquemont, C.; Simon, J.A.; D’Andrea, A.D.; Taniguchi, T. Non-specific chemical inhibition of the Fanconi anemia pathway sensitizes cancer cells to cisplatin. Mol. Cancer 2012, 11, 26. [Google Scholar] [CrossRef]
- Kutuk, O.; Arisan, E.D.; Tezil, T.; Shoshan, M.C.; Basaga, H. Cisplatin overcomes Bcl-2-mediated resistance to apoptosis via preferential engagement of Bak: Critical role of Noxa-mediated lipid peroxidation. Carcinogenesis 2009, 30, 1517–1527. [Google Scholar] [CrossRef]
- Fraser, M.; Bai, T.; Tsang, B.K. Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int. J. Cancer 2008, 122, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Kilari, D.; Guancial, E.; Kim, E.S. Role of copper transporters in platinum resistance. World J. Clin. Oncol. 2016, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Kalayda, G.V.; Wagner, C.H.; Buß, I.; Reedijk, J.; Jaehde, U. Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer 2008, 8, 175. [Google Scholar] [CrossRef]
- Zhu, S.; Shanbhag, V.; Wang, Y.; Lee, J.; Petris, M. A Role for The ATP7A Copper Transporter in Tumorigenesis and Cisplatin Resistance. J. Cancer 2017, 8, 1952. [Google Scholar] [CrossRef]
- Li, Z.H.; Qiu, M.Z.; Zeng, Z.L.; Luo, H.Y.; Wu, W.J.; Wang, F.; Wang, Z.Q.; Zhang, D.S.; Li, Y.H.; Xu, R.H. Copper-transporting P-type adenosine triphosphatase (ATP7A) is associated with platinum-resistance in non-small cell lung cancer (NSCLC). J. Transl. Med. 2012, 10, 21. [Google Scholar] [CrossRef]
- Li, Z.H.; Zheng, R.; Chen, J.T.; Jia, J.; Qiu, M. The role of copper transporter ATP7A in platinum-resistance of esophageal squamous cell cancer (ESCC). J. Cancer 2016, 7, 2085. [Google Scholar] [CrossRef]
- Rébé, C.; Demontoux, L.; Pilot, T.; Ghiringhelli, F. Platinum Derivatives Effects on Anticancer Immune Response. Biomolecules 2019, 10, 13. [Google Scholar] [CrossRef]
- Okita, R.; Yukawa, T.; Nojima, Y.; Maeda, A.; Saisho, S.; Shimizu, K.; Nakata, M. MHC class I chain-related molecule A and B expression is upregulated by cisplatin and associated with good prognosis in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2016, 65, 499–509. [Google Scholar] [CrossRef]
- De Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-Induced Antitumor Immunomodulation: A Review of Preclinical and Clinical Evidence. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 5384. [Google Scholar] [CrossRef]
- Gameiro, S.R.; Caballero, J.A.; Hodge, J.W. Defining the molecular signature of chemotherapy-mediated lung tumor phenotype modulation and increased susceptibility to T-cell killing. Cancer Biother. Radiopharm. 2012, 27, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Pestka, S.; Jubin, R.G.; Lyu, Y.L.; Tsai, Y.C.; Liu, L.F. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE 2012, 7, e32542. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Meurisse, A.; Spehner, L.; Stouvenot, M.; François, E.; Buecher, B.; André, T.; Samalin, E.; Jary, M.; Nguyen, T.; et al. Pooled analysis of 115 patients from updated data of Epitopes-HPV01 and Epitopes-HPV02 studies in first-line advanced anal squamous cell carcinoma. Ther. Adv. Med Oncol. 2020, 12, 1758835920975356. [Google Scholar] [CrossRef] [PubMed]
- Spehner, L.; Boustani, J.; Cabel, L.; Doyen, J.; Vienot, A.; Borg, C.; Kim, S. Present and Future Research on Anal Squamous Cell Carcinoma. Cancers 2021, 13, 3895. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Meurisse, A.; Stouvenot, M.; Jary, M.; Hon, T.N.T.; Francois, E.; Buecher, B.; Andre, T.; Samalin, E.; Boulbair, F.; et al. Updated data of epitopes-HPV02 trial and external validation of efficacy of DCF in prospective epitopes-HPV01 study in advanced anal squamous cell carcinoma. Pooled analysis of 115 patients. Ann. Oncol. 2019, 30, v203. [Google Scholar] [CrossRef]
- Spehner, L.; Kim, S.; Vienot, A.; François, E.; Buecher, B.; Adotevi, O.; Vernerey, D.; Abdeljaoued, S.; Meurisse, A.; Borg, C. Anti-Telomerase CD4+ Th1 Immunity and Monocytic-Myeloid-Derived-Suppressor Cells Are Associated with Long-Term Efficacy Achieved by Docetaxel, Cisplatin, and 5-Fluorouracil (DCF) in Advanced Anal Squamous Cell Carcinoma: Translational Study of Epitopes-HPV01 and 02 Trials. Int. J. Mol. Sci. 2020, 21, 6838. [Google Scholar] [CrossRef]
- Kim, S.; François, E.; André, T.; Samalin, E.; Jary, M.; El Hajbi, F.; Baba-Hamed, N.; Pernot, S.; Kaminsky, M.C.; Bouché, O.; et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 1094–1106. [Google Scholar] [CrossRef]
- Chang, C.L.; Hsu, Y.T.; Wu, C.C.; Lai, Y.Z.; Wang, C.; Yang, Y.C.; Wu, T.C.; Hung, C.F. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res. 2013, 73, 119. [Google Scholar] [CrossRef]
- Grabosch, S.; Bulatovic, M.; Zeng, F.; Ma, T.; Zhang, L.; Ross, M.; Brozick, J.; Fang, Y.S.; Tseng, G.; Kim, E.; et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019, 38, 2380. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Wu, H.; Xie, H.; Zhang, T.; Deng, Z. Blocking the PD-1/PD-L1 axis enhanced cisplatin chemotherapy in osteosarcoma in vitro and in vivo. Environ. Health Prev. Med. 2019, 24, 79. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, L.; Li, W.; Fan, K.; Qian, W.; Hou, S.; Wang, H.; Dai, M.; Hellstrom, I.; Hellstrom, K.E.; et al. Combinatorial PD-1 Blockade and CD137 Activation Has Therapeutic Efficacy in Murine Cancer Models and Synergizes with Cisplatin. PLoS ONE 2013, 8, 84927. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Qian, J.; Wang, H.; Ren, L.; Yang, Y.; Chen, C.; Chen, X.; Huang, Y.; Liu, J.; Xu, N.; et al. Cisplatin plus anti-PD-1 antibody enhanced treatment efficacy in advanced esophageal squamous cell carcinoma. Am. J. Cancer Res. 2022, 12, 451. [Google Scholar]
- Sun, F.; Cui, L.; Li, T.; Chen, S.; Song, J.; Li, D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J. Recept. Signal Transduct. 2019, 39, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell. Mol. Med. 2019, 23, 4854. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Kepp, O.; Schlemmer, F.; Adjemian, S.; Tailler, M.; Shen, S.; Michaud, M.; Menger, L.; Gdoura, A.; Tajeddine, N.; et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 2010, 30, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2009, 29, 482–491. [Google Scholar] [CrossRef]
- Bains, S.J.; Abrahamsson, H.; Flatmark, K.; Dueland, S.; Hole, K.H.; Seierstad, T.; Redalen, K.R.; Meltzer, S.; Ree, A.H. Immunogenic cell death by neoadjuvant oxaliplatin and radiation protects against metastatic failure in high-risk rectal cancer. Cancer Immunol. Immunother. 2020, 69, 355. [Google Scholar] [CrossRef]
- Song, W.; Shen, L.; Wang, Y.; Liu, Q.; Goodwin, T.J.; Li, J.; Dorosheva, O.; Liu, T.; Liu, R.; Huang, L. Indeed, self-assembled nanomedicine for cisplatin and lipoplatin as well as other chemotherapeutic drugs is an accelerating field of research that is believed to overcome the major toxicity problem limiting their use. Molecular self- assembly is a process. Nat. Commun. 2018, 9, 2237. [Google Scholar] [CrossRef]
- Zhu, H.; Shan, Y.; Ge, K.; Lu, J.; Kong, W.; Jia, C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell. Oncol. 2020, 43, 1203–1214. [Google Scholar] [CrossRef]
- Sprowl, J.A.; Lancaster, C.S.; Pabla, N.; Hermann, E.; Kosloske, A.M.; Gibson, A.A.; Li, L.; Zeeh, D.; Schlatter, E.; Janke, L.J.; et al. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin. Cancer Res. 2014, 20, 4026–4035. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G.; Deuster, D.; Knief, A.; Sperling, M.; Holtkamp, M.; Edemir, B.; Pavenstädt, H.; Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen, A.A.; Schinkel, A.H.; et al. Organic Cation Transporter 2 Mediates Cisplatin-Induced Oto- and Nephrotoxicity and Is a Target for Protective Interventions. Am. J. Pathol. 2010, 176, 1169. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, H.; Yamashita, M.; Katsura, H.; Yu, J.; Waki, Y.; Nagata, N.; Sai, Y.; Miyamoto, K.I. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol. Pharm. Bull. 2010, 33, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef]
- Blair, B.G.; Larson, C.; Safaei, R.; Howell, S.B. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clin. Cancer Res. 2009, 15, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; McCormick, F.; Smith-McCune, K.; Hanahan, D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 2010, 17, 574. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Tang, X.M.; Peterson, D.R.; Kilari, D.; Chow, C.W.; Fujimoto, J.; Kalhor, N.; Swisher, S.G.; Stewart, D.J.; Wistuba, I.I.; et al. Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer 2014, 85, 88–93. [Google Scholar] [CrossRef]
- Rose, M.C.; Kostyanovskaya, E.; Huang, R.S. Pharmacogenomics of Cisplatin Sensitivity in Non-small Cell Lung Cancer. Genom. Proteom. Bioinform. 2014, 12, 198–209. [Google Scholar] [CrossRef]
- Sawers, L.; Ferguson, M.J.; Ihrig, B.R.; Young, H.C.; Chakravarty, P.; Wolf, C.R.; Smith, G. Glutathione S-transferase P1 (GSTP1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines. Br. J. Cancer 2014, 111, 1150–1158. [Google Scholar] [CrossRef]
- Wülfing, C.; van Ahlen, H.; Eltze, E.; Piechota, H.; Hertle, L.; Schmid, K.W. Metallothionein in bladder cancer: Correlation of overexpression with poor outcome after chemotherapy. World J. Urol. 2007, 25, 199–205. [Google Scholar] [CrossRef]
- Werynska, B.; Pula, B.; Muszczynska-Bernhard, B.; Gomulkiewicz, A.; Piotrowska, A.; Prus, R.; Podhorska-Okolow, M.; Jankowska, R.; Dziegiel, P. Metallothionein 1F and 2A overexpression predicts poor outcome of non-small cell lung cancer patients. Exp. Mol. Pathol. 2013, 94, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.A.M.; Michalkova, H.; Strmiska, V.; Casar, B.; Crespo, P.; de los Rios, V.; Ignacio Casal, J.; Haddad, Y.; Guran, R.; Eckschlager, T.; et al. Metallothionein-3 promotes cisplatin chemoresistance remodelling in neuroblastoma. Sci. Rep. 2021, 11, 5496. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, Y.; Yang, D.; Zheng, L. MicroRNA-591 Functions as a Tumor Suppressor in Hepatocellular Carcinoma by Lowering Drug Resistance through Inhibition of Far-Upstream Element-Binding Protein 2-Mediated Phosphoinositide 3-Kinase/Akt/Mammalian Target of Rapamycin Axis. Pharmacology 2019, 104, 173–186. [Google Scholar] [CrossRef]
- Williams, L.A.; Mills, L.; Hooten, A.J.; Langer, E.; Roesler, M.; Frazier, A.L.; Krailo, M.; Nelson, H.H.; Bestrashniy, J.; Amatruda, J.F.; et al. Differences in DNA methylation profiles by histologic subtype of paediatric germ cell tumours: A report from the Children’s Oncology Group. Br. J. Cancer 2018, 119, 864–872. [Google Scholar] [CrossRef]
- Vander Broek, R.; Snow, G.E.; Chen, Z.; Van Waes, C. Chemoprevention of Head and Neck Squamous Cell Carcinoma through Inhibition of NF-κB Signaling. Oral Oncol. 2014, 50, 930. [Google Scholar] [CrossRef]
- Steele, N.; Finn, P.; Brown, R.; Plumb, J.A. Combined inhibition of DNA methylation and histone acetylation enhances gene re-expression and drug sensitivity in vivo. Br. J. Cancer 2009, 100, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, S.; Redon, C.E.; Peer, C.J.; Bryla, C.; Lee, M.J.; Trepel, J.B.; Tomita, Y.; Rajan, A.; Giaccone, G.; Bonner, W.M.; et al. Phase I trial of belinostat with cisplatin and etoposide in advanced solid tumors, with a focus on neuroendocrine and small cell cancers of the lung. Anti-Cancer Drugs 2018, 29, 457. [Google Scholar] [CrossRef]
- Lobo, J.; Guimarães-Teixeira, C.; Barros-Silva, D.; Miranda-Gonçalves, V.; Camilo, V.; Guimarães, R.; Cantante, M.; Braga, I.; Maurício, J.; Oing, C.; et al. Efficacy of HDAC Inhibitors Belinostat and Panobinostat against Cisplatin-Sensitive and Cisplatin-Resistant Testicular Germ Cell Tumors. Cancers 2020, 12, 2903. [Google Scholar] [CrossRef]
- Kim, T.M.; Laird, P.W.; Park, P.J. XThe landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell 2013, 155, 858. [Google Scholar] [CrossRef]
- Watanabe, Y.; Koi, M.; Hemmi, H.; Hoshai, H.; Noda, K. A change in microsatellite instability caused by cisplatin-based chemotherapy of ovarian cancer. Br. J. Cancer 2001, 85, 1064–1069. [Google Scholar] [CrossRef]
- Nicolay, N.H.; Helleday, T.; Sharma, R.A. Biological Relevance of DNA Polymerase Beta and Translesion Synthesis Polymerases to Cancer and its Treatment. Curr. Mol. Pharmacol. 2011, 5, 54–67. [Google Scholar] [CrossRef]
- Shilkin, E.S.; Boldinova, E.O.; Stolyarenko, A.D.; Goncharova, R.I.; Chuprov-Netochin, R.N.; Smal, M.P.; Makarova, A.V. Translesion DNA Synthesis and Reinitiation of DNA Synthesis in Chemotherapy Resistance. Biochemistry 2020, 85, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Ling, H. Structural Basis for Human DNA Polymerase Kappa to Bypass Cisplatin Intrastrand Cross-Link (Pt-GG) Lesion as an Efficient and Accurate Extender. J. Mol. Biol. 2018, 430, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Doles, J.; Oliver, T.G.; Cameron, E.R.; Hsu, G.; Jacks, T.; Walker, G.C.; Hemann, M.T. Suppression of Rev3, the catalytic subunit of Pol?, sensitizes drug-resistant lung tumors to chemotherapy. Proc. Natl. Acad. Sci. USA 2010, 107, 20786–20791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, Y.W.; Liu, X.; Chu, P.; Loria, S.; Wang, Y.; Yen, Y.; Chou, K.M. Expression of DNA Translesion Synthesis Polymerase η in Head and Neck Squamous Cell Cancer Predicts Resistance to Gemcitabine and Cisplatin-Based Chemotherapy. PLoS ONE 2013, 8, e83978. [Google Scholar] [CrossRef]
- Einhorn, L.H. Treatment of testicular cancer: A new and improved model. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1990, 8, 1777–1781. [Google Scholar] [CrossRef]
- de Vries, G.; Rosas-Plaza, X.; van Vugt, M.A.T.M.; Gietema, J.A.; de Jong, S. Testicular cancer: Determinants of cisplatin sensitivity and novel therapeutic opportunities. Cancer Treat. Rev. 2020, 88, 102054. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Hsia, T.C.; Tsai, C.M.; Tsang, K.; Chang, G.C.; Chang, J.W.C.; Thitiya, S.; Sriuranpong, V.; Thongprasert, S.; Chua, D.T.; et al. Efficacy of bevacizumab with cisplatin and gemcitabine in Asian patients with advanced or recurrent non-squamous non-small cell lung cancer who have not received prior chemotherapy: A substudy of the Avastin in Lung trial. Asia Pac. J. Clin. Oncol. 2011, 7, 4–12. [Google Scholar] [CrossRef]
- Chu, G.; Liu, X.; Yu, W.; Chen, M.; Dong, L. Cisplatin plus paclitaxel chemotherapy with or without bevacizumab in postmenopausal women with previously untreated advanced cervical cancer: A retrospective study. BMC Cancer 2021, 21, 133. [Google Scholar] [CrossRef]
- Yarchoan, M.; Myzak, M.C.; Johnson, B.A.; De Jesus-Acosta, A.D.; Le, D.T.; Jaffee, E.M.; Azad, N.S.; Donehower, R.C.; Zheng, L.; Oberstein, P.E.; et al. Olaparib in combination with irinotecan, cisplatin, and mitomycin C in patients with advanced pancreatic cancer. Oncotarget 2017, 8, 44073–44081. [Google Scholar] [CrossRef]
- Arita, M.; Watanabe, S.; Aoki, N.; Kuwahara, S.; Suzuki, R.; Goto, S.; Abe, Y.; Takahashi, M.; Sato, M.; Hokari, S.; et al. Combination therapy of cisplatin with cilastatin enables an increased dose of cisplatin, enhancing its antitumor effect by suppression of nephrotoxicity. Sci. Rep. 2021, 11, 750. [Google Scholar] [CrossRef] [PubMed]

| Drug | Cancer | Outcome | Year | Reference |
|---|---|---|---|---|
| Gemcitabine and cisplatin plus durvalumab with or without tremelimumab | Advanced biliary tract cancer | Gemcitabine and cisplatin plus durvalumab are still being evaluated. However, Gemcitabine and cisplatin plus immunotherapy showed an acceptable level of safety and are considered a potential effective first-line therapy for advanced biliary tract cancer patients | 2022 | [19] |
| Addition of nintedanib or placebo to neoadjuvant gemcitabine and cisplatin | Advanced muscle-invasive bladder cancer | Nintedanib addition was safe but did not show improvement in the pathological response for the targeted bladder cancer patients. | 2022 | [20] |
| Xevinapant or placebo plus cisplatin | Advanced squamous cell carcinoma of the head and neck | No results have been released yet | 2022 | [21] |
| Pembrolizumab plus a high dose of cisplatin and radiation | Larynx cancer | The therapy was safe and showed effectiveness, but more data and longer-term monitoring are needed | 2022 | [22] |
| Stereotactic body radiotherapy and a full dose of cisplatin or carboplatin | Lung cancer | The therapy was effective and safe | 2022 | [23] |
| TRC102 in combination with pemetrexed, cisplatin, and radiotherapy. | Lung cancer | The therapy was safe with specific doses of TRC12 (200 mg along with cisplatin) | 2022 | [24] |
| Cisplatin coupled with radiation followed by carboplatin/paclitaxel vs carboplatin/paclitaxel | Endometrial carcinoma | Chemoradiotherapy showed some toxicity in comparison to chemotherapy alone | 2022 | [25] |
| Liposomal cisplatin versus conventional non-liposomal cisplatin | Lung cancer and squamous cell carcinoma of the head and neck | Liposomal cisplatin showed a significant reduction in toxicity compared to conventional therapy | 2018 | [26] |
| Anlotinib, in combination with oxaliplatin and capecitabine | Colorectal adenocarcinoma | Anlotinib combined with capecitabine and oxaliplatin exhibited significant effectiveness as first-line therapy with manageable toxicity | 2022 | [27] |
| Molecule | Cancer Type | Status | NCT Number | Year |
|---|---|---|---|---|
| Atezolizumab, Bevacizumab, Placebo, Cisplatin, Gemcitabine | Biliary Tract Cancer | Phase II | NCT04677504 | 2021 |
| Pembrolizumab Carboplatin Paclitaxel Placebo for pembrolizumab docetaxel Cisplatin Radiation: External Beam Radiotherapy (EBRT) Cisplatin (as radiosensitiser) Radiation: Brachytherapy | Endometrial Neoplasms | Phase III | NCT04634877 | 2021 |
| Pembrolizumab Lenvatinib Cisplatin 5-FU Oxaliplatin Leucovorin Levoleucovorin Paclitaxel | Metastatic Esophageal Squamous Cell Carcinoma | Phase III | NCT04949256 | 2021 |
| Zanidatamab Tislelizumab Trastuzumab Capecitabine Oxaliplatin Cisplatin 5-FluorouracilDiagnostic Test: In situ hybridisation (ISH)-based companion diagnostic assayDiagnostic Test: Immunohistochemistry (IHC)-based companion diagnostic assay | Gastric Neoplasms Gastroesophageal Adenocarcinoma Esophageal Adenocarcinoma | Phase III | NCT05152147 | 2021 |
| SavolitinibDrug: Osimertinib Pemetrexed Cisplatin Carboplatin | Carcinoma Non-Small-Cell Lung | Phase III | NCT05261399 | 2022 |
| Olaparib Radiation: Pelvic external beam radiotherapy Cisplatin Durvalumab Medroxyprogesterone Acetate Megestrol Acetate Other: Observation | Endometrial Cancer | Phase II & III | NCT05255653 | 2022 |
| Nab-paclitaxel Gemcitabine Cisplatin Irinotecan Capecitabine Pembrolizumab Olaparib | Metastatic Pancreatic Cancer | Phase II | NCT04753879 | 2021 |
| Bintrafusp Alfa Pemetrexed Carboplatin Cisplatin | Locally Advanced Lung Non-Squamous Non-Small Cell Carcinoma. Metastatic Lung Non-Squamous Non-Small Cell Carcinoma. Unresectable Lung Non-Squamous Non-Small Cell Carcinoma | Phase II | NCT04971187 | 2021 |
| AMG 510 Cisplatin Carboplatin Pemetrexed | Lung Cancer | Phase II | NCT05118854 | 2022 |
| Cisplatin | SCCHN Squamous Cell Carcinoma | Phase II | NCT04595981 | 2022 |
| Modified GCN+TTF treatment | Metastatic Pancreatic Cancer. Pancreatic Adenocarcinoma. Metastatic Adenocarcinoma | Phase I & Phase II | NCT04605913 | 2022 |
| BET Bromodomain Inhibitor ZEN-3694 Cisplatin Etoposide | Advanced NUT Carcinoma Metastatic NUT Carcinoma Unresectable NUT Carcinoma | Phase I & Phase II | NCT05019716 | 2022 |
| Cisplatin and immunotherapy | Cholangiocarcinoma | Phase I & Phase II | NCT04989218 | 2022 |
| Sasanlimab Radiation: Stereotactic Body Radiation Therapy Procedure: Radical Cystectomy + pelvic lymph node dissection + urinary diversion | Urothelial Carcinoma Bladder | Phase II | NCT05241340 | 2022 |
| Zimberelimab Etrumadenant Cisplatin Radiation | Head and Neck Cancer. Squamous Cell Carcinoma of Head and Neck. Oral Cavity Squamous Cell Carcinoma. Oropharynx Squamous Cell Carcinoma. Larynx Cancer. Pharynx Cancer. Hypopharynx Cancer. Hypopharynx Squamous Cell Carcinoma | Phase I | NCT04892875 | 2022 |
| Molecule | Cancer Type | Status | NCT Number | Year |
|---|---|---|---|---|
| Carboplatin and with combinations and other drugs versus Sacituzumab Govitecan-hziy | Triple-Negative Breast Cancer PD-L1 Negative | Phase III | NCT05382299 | 2022 |
| Carboplatin and other drugs versus Sacituzumab govitecan-hziy (SG) and pembrolizumab | Triple-Negative Breast Cancer PD-L1 Positive | Phase III | NCT05382286 | 2022 |
| Olvi-Vec followed by platinum-doublet chemotherapy (carboplatin or cisplatin) and bevacizumab compared to the Active Comparator Arm with platinum-doublet chemotherapy (carboplatin or cisplatin) and bevacizumab | Platinum-resistant Ovarian Cancer Platinum-refractory Ovarian Cancer Fallopian Tube Cancer Primary Peritoneal Cancer High-grade Serous Ovarian Cancer Endometrioid Ovarian Cancer Ovarian Clear Cell Carcinoma | Phase III | NCT05281471 | 2022 |
| Pembrolizumab/vibostolimab (MK-7684A) in combination with other drugs including Cisplatin or Carboplatin versus pembrolizumab in combination with other drugs including Cisplatin or Carboplatin | Metastatic Non-Small Cell Lung Cancer | Phase III | NCT05226598 | 2022 |
| Pembrolizumab (MK-3475) compared to a combination of carboplatin and paclitaxel | Endometrial Neoplasms | Phase III | NCT05173987 | 2022 |
| Efficacy and safety of Dato-DXd compared with Investigator’s choice chemotherapy such as carboplatin | Breast Cancer | Phase III | NCT05374512 | 2022 |
| Fixed-dose of pembrolizumab/vibostolimab co-formulation (MK-7684A) with etoposide/platinum chemotherapy (cisplatin, carboplatin, or others) followed by MK-7684A compared to the combination of atezolizumab with etoposide/platinum chemotherapy (cisplatin, carboplatin, or others) followed by atezolizumab | Small Cell Lung Carcinoma | Phase III | NCT05224141 | 2022 |
| Patritumab Deruxtecan versus Platinum-based chemotherapy | Non-squamous Non-small Cell Lung Cancer EGFR L858R | Phase III | NCT05338970 | 2022 |
| Pembrolizumab/vibostolimab (MK-7684A) in combination with concurrent chemoradiotherapy including cisplatin or carboplatin followed by pembrolizumab/vibostolimab versus chemoradiotherapy including cisplatin or carboplatin followed by durvalumab | Carcinoma, Non-Small-Cell Lung cancer | Phase III | NCT05298423 | 2022 |
| Savolitinib in combination with osimertinib versus platinum-based doublet chemotherapy including cisplatin or carboplatin | Carcinoma Non-Small-Cell Lung Phase 3 | Phase III | NCT05261399 | 2022 |
| Different strategies using the following: Olaparib Radiation: Pelvic external beam radiotherapy Chemotherapy, including cisplatin and carboplatin Durvalumab Medroxyprogesterone Acetate Megestrol Acetate Other: Observation | Endometrial Cancer | Phase II & III | NCT05255653 | 2022 |
| Trials with different combinations of the following drugs: Tucatinib Trastuzumab Bevacizumab Cetuximab Oxaliplatin Leucovorin Levoleucovorin Fluorouracil | Colorectal Neoplasms | Phase III | NCT05253651 | 2022 |
| Specifying the best therapy among the following: mFOLFOX6 3–6-month CAPOX 3-month mFOLFIRINOX mFOLFOX6 6 month CAPOX 6 month | Colon Cancer | Phase III | NCT05174169 | 2022 |
| Bemarituzumab combined with oxaliplatin and 5-fluorouracil (5-FU) (mFOLFOX6) versus placebo plus mFOLFOX6 | Gastric Cancer | Phase III | NCT05052801 | 2022 |
| Testing the efficacy of zilovertamab vedotin in combination with other drugs such as oxaliplatin | Diffuse Large B-Cell Lymphoma | Phase II & III | NCT05139017 | 2022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, R.; Aouida, M.; Alhaj Sulaiman, A.; Madhusudan, S.; Ramotar, D. Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted. Int. J. Mol. Sci. 2022, 23, 7241. https://doi.org/10.3390/ijms23137241
Ali R, Aouida M, Alhaj Sulaiman A, Madhusudan S, Ramotar D. Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted. International Journal of Molecular Sciences. 2022; 23(13):7241. https://doi.org/10.3390/ijms23137241
Chicago/Turabian StyleAli, Reem, Mustapha Aouida, Abdallah Alhaj Sulaiman, Srinivasan Madhusudan, and Dindial Ramotar. 2022. "Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted" International Journal of Molecular Sciences 23, no. 13: 7241. https://doi.org/10.3390/ijms23137241
APA StyleAli, R., Aouida, M., Alhaj Sulaiman, A., Madhusudan, S., & Ramotar, D. (2022). Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted. International Journal of Molecular Sciences, 23(13), 7241. https://doi.org/10.3390/ijms23137241







