BK Polyomavirus bkv-miR-B1-5p: A Stable Micro-RNA to Monitor Active Viral Replication after Kidney Transplantation
Abstract
:1. Introduction
2. Results
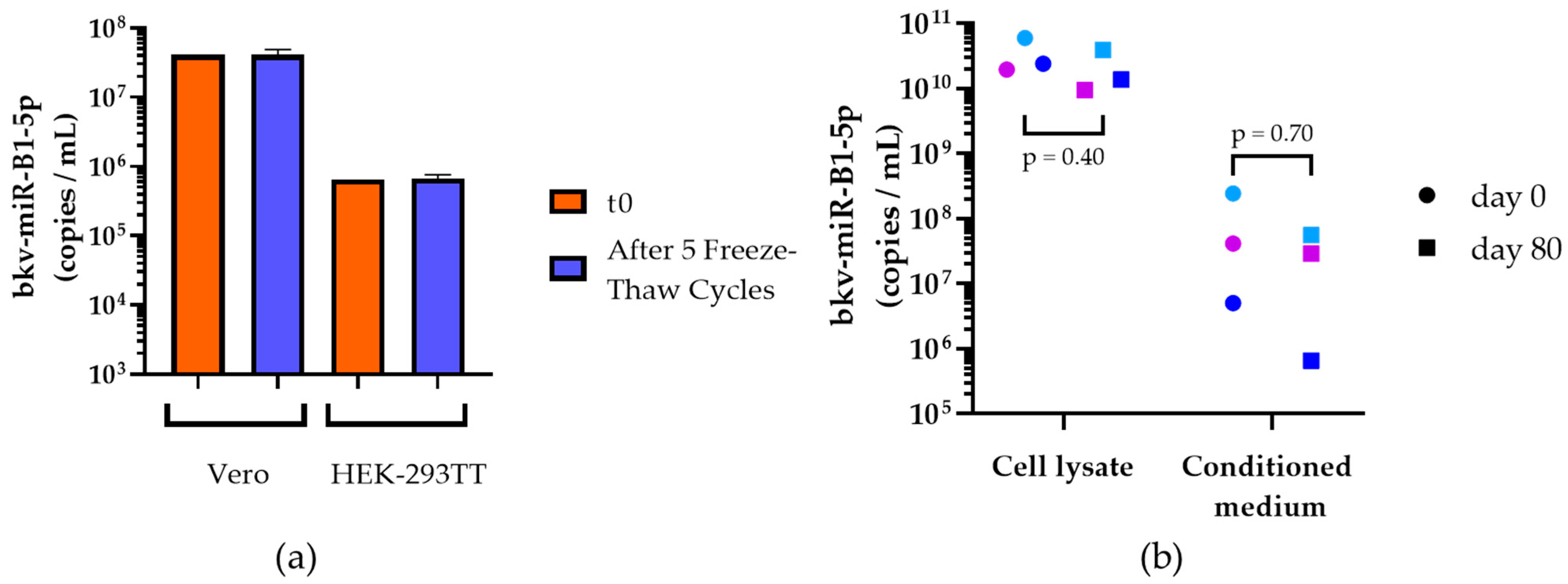
2.1. Stability of In Vitro Generated bkv-miR-B1-5p
2.2. Stability of bkv-miR-B1-5p in Human Urine
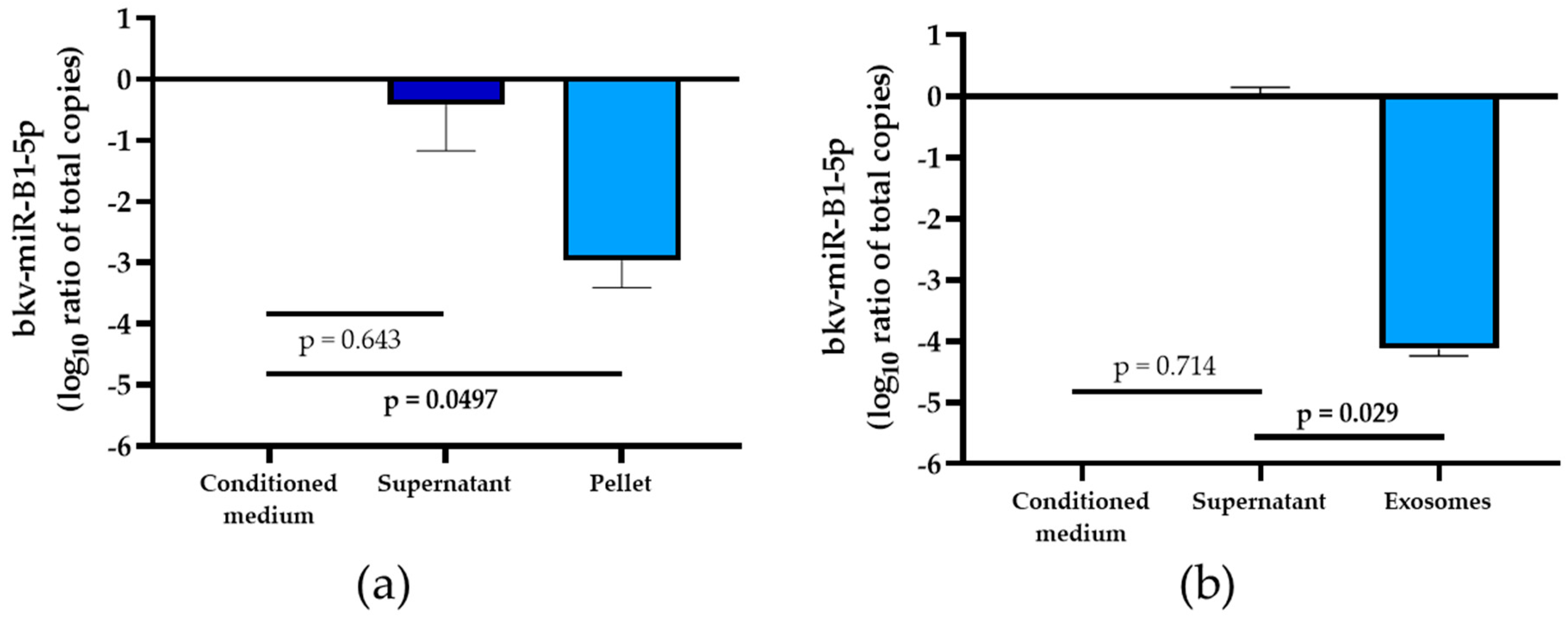
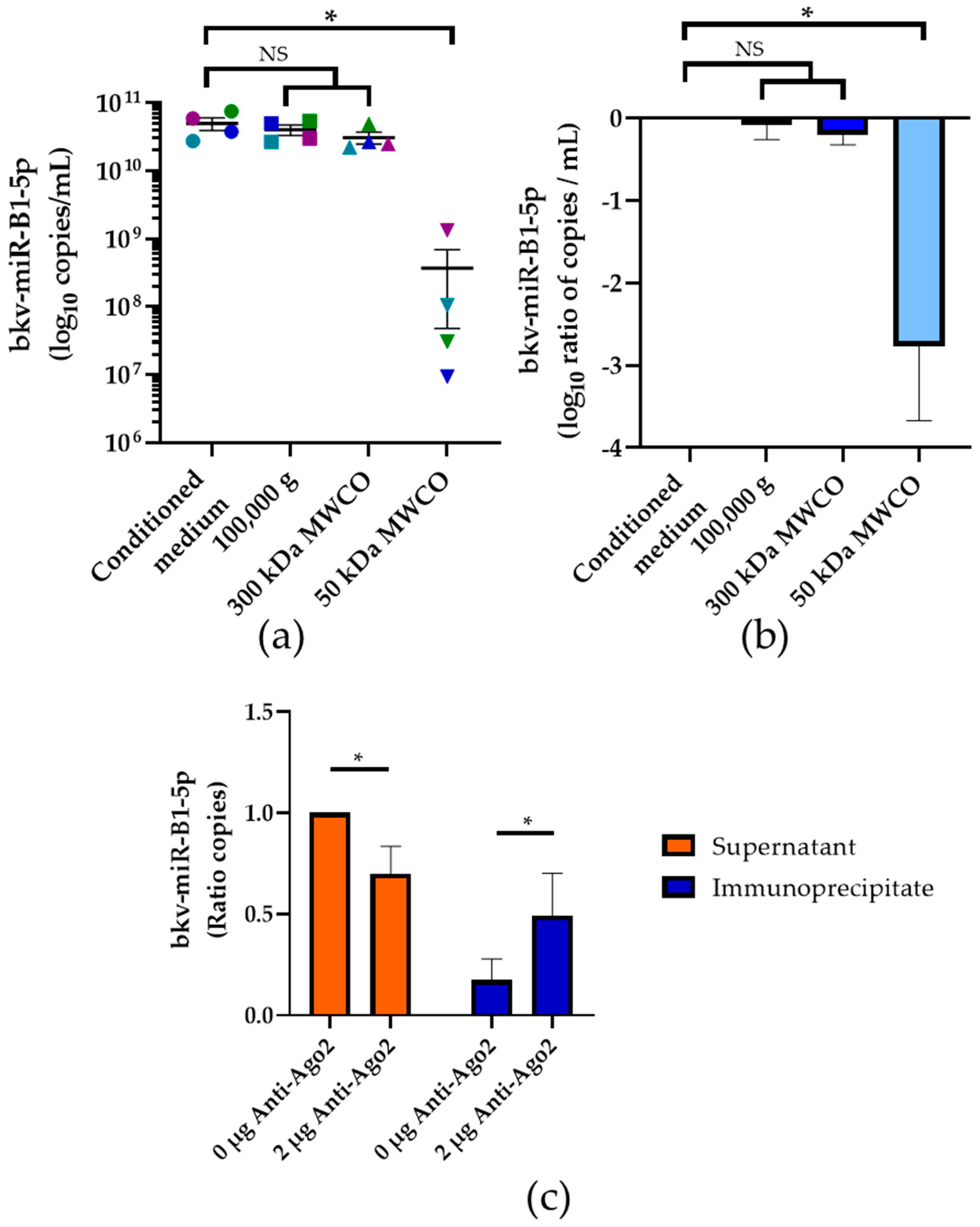
2.3. Part of Exosomal bkv-miR-B1-5p in Extracellular Compartment
2.4. Association of bkv-miR-B1-5p with Ago2 Protein
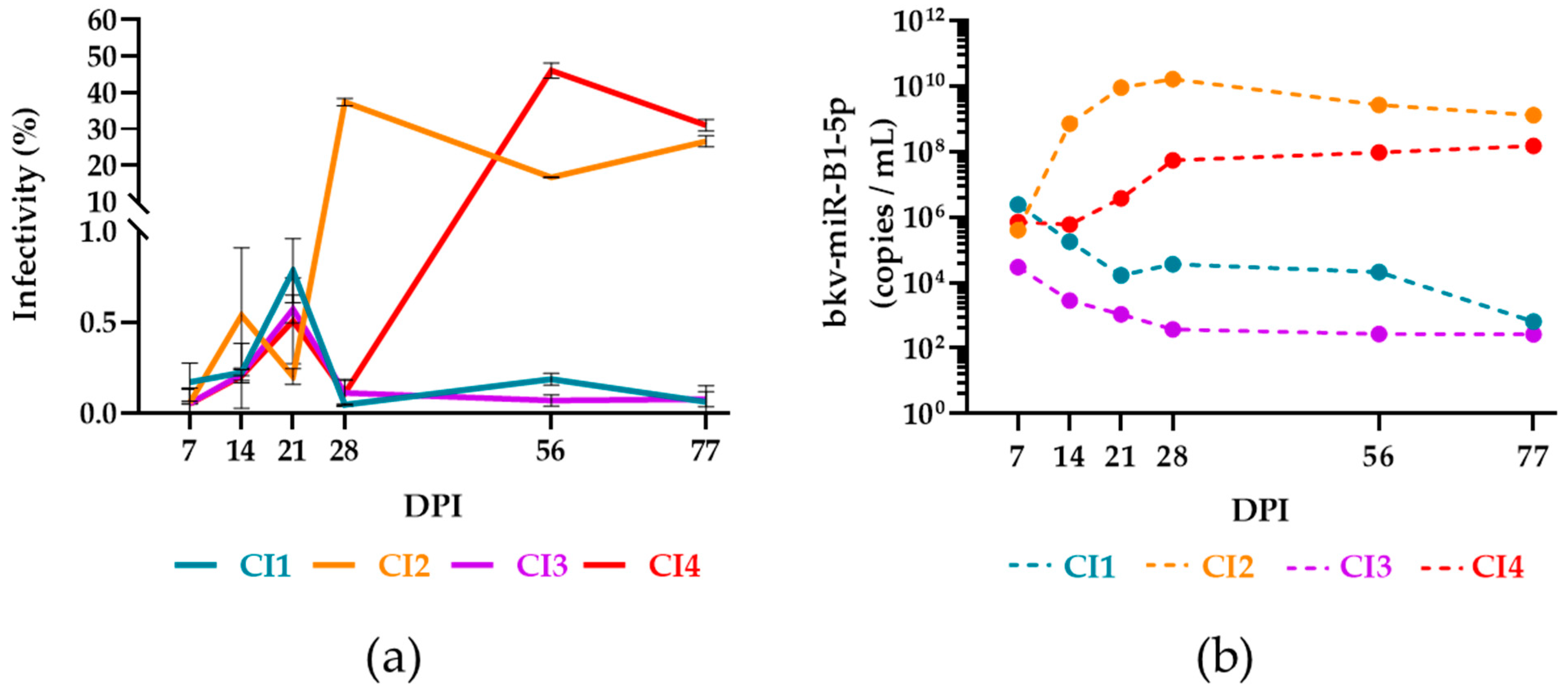
2.5. bkv-miR-B1-5p to Distinguish Active and Persistent Infections
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Human Samples
4.3. Physico-Chemical Stability
4.4. miRNA Purification
4.5. miRNA RT-PCR Assay and BKPyV DNA qPCR
4.6. Antibodies and Reagents
4.7. Immunostaining
4.8. Ultracentrifugation and Ultrafiltration
4.9. Immunoprecipitation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirsch, H.H.; Steiger, J. Polyomavirus BK. Lancet Infect. Dis. 2003, 3, 611–623. [Google Scholar] [CrossRef]
- Helle, F.; Brochot, E.; Handala, L.; Martin, E.; Castelain, S.; Francois, C.; Duverlie, G. Biology of the BKPyV: An Update. Viruses 2017, 9, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressollette-Bodin, C.; Coste-Burel, M.; Hourmant, M.M.; Renaudin, K.; Imbert-Marcille, B.M. Le Virus BK: État Des Connaissances En 2003 et Particularités de l‘infection En Transplantation Rénale. Virologie 2003, 7, 433–444. [Google Scholar]
- Mori, Y.; Miyamoto, T.; Kato, K.; Kamezaki, K.; Kuriyama, T.; Oku, S.; Takenaka, K.; Iwasaki, H.; Harada, N.; Shiratsuchi, M.; et al. Different Risk Factors Related to Adenovirus- or BK Virus-Associated Hemorrhagic Cystitis Following Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Nickeleit, V.; Hirsch, H.H.; Binet, I.F.; Gudat, F.; Prince, O.; Dalquen, P.; Thiel, G.; Mihatsch, M.J. Polyomavirus Infection of Renal Allograft Recipients: From Latent Infection to Manifest Disease. J. Am. Soc. Nephrol. 1999, 10, 1080–1089. [Google Scholar] [CrossRef]
- Sharif, A.; Alachkar, N.; Bagnasco, S.; Geetha, D.; Gupta, G.; Womer, K.; Arend, L.; Racusen, L.; Montgomery, R.; Kraus, E. Incidence and Outcomes of BK Virus Allograft Nephropathy among ABO- and HLA-Incompatible Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 1320–1327. [Google Scholar] [CrossRef]
- Rinaldo, C.H.; Hirsch, H.H. Antivirals for the Treatment of Polyomavirus BK Replication. Expert Rev. Anti Infect. Ther. 2007, 5, 105–115. [Google Scholar] [CrossRef]
- Song, T.-R.; Rao, Z.-S.; Qiu, Y.; Liu, J.-P.; Huang, Z.-L.; Wang, X.-D.; Lin, T. Fluoroquinolone Prophylaxis in Preventing BK Polyomavirus Infection after Renal Transplant: A Systematic Review and Meta-Analysis. Kaohsiung J. Med. Sci. 2016, 32, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.A.M.; Khalil, M.A.U.; Tan, J.; Khan, T.F.T. Fluoroquinolones and BK Virus Nephropathy: A Myth or a Reality. Indian J. Nephrol. 2018, 28, 257–264. [Google Scholar] [CrossRef]
- Randhawa, P.S.; Schonder, K.; Shapiro, R.; Farasati, N.; Huang, Y. Polyomavirus BK Neutralizing Activity in Human Immunoglobulin Preparations. Transplantation 2010, 89, 1462–1465. [Google Scholar] [CrossRef] [Green Version]
- Bernhoff, E.; Gutteberg, T.J.; Sandvik, K.; Hirsch, H.H.; Rinaldo, C.H. Cidofovir Inhibits Polyomavirus BK Replication in Human Renal Tubular Cells Downstream of Viral Early Gene Expression. Am. J. Transplant. 2008, 8, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.H.; Gosert, R.; Bernhoff, E.; Finstad, S.; Hirsch, H.H. 1-O-Hexadecyloxypropyl Cidofovir (CMX001) Effectively Inhibits Polyomavirus BK Replication in Primary Human Renal Tubular Epithelial Cells. Antimicrob. Agents Chemother. 2010, 54, 4714–4722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.W.; Mital, D.; Chong, A.; Kottayil, A.; Millis, M.; Longstreth, J.; Huang, W.; Brady, L.; Jensik, S. Experiences with Leflunomide in Solid Organ Transplantation. Transplantation 2002, 73, 358–366. [Google Scholar] [CrossRef]
- Hardinger, K.L.; Koch, M.J.; Bohl, D.J.; Storch, G.A.; Brennan, D.C. BK-Virus and the Impact of Pre-Emptive Immunosuppression Reduction: 5-Year Results. Am. J. Transplant. 2010, 10, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaub, S.; Hirsch, H.H.; Dickenmann, M.; Steiger, J.; Mihatsch, M.J.; Hopfer, H.; Mayr, M. Reducing Immunosuppression Preserves Allograft Function in Presumptive and Definitive Polyomavirus-Associated Nephropathy. Am. J. Transplant. 2010, 10, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Wu, B.-S.; Lien, T.-J.; Yang, A.-H.; Yang, C.-Y. BK Polyomavirus Nephropathy in Kidney Transplantation: Balancing Rejection and Infection. Viruses 2021, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- EDQM International Figures on Donation and Transplantation 2020; Cover; Council of Europe and Organización Nacional de Trasplantes: Madrid, Spain, 2021.
- Hirsch, H.H.; Randhawa, P.S.; AST Infectious Diseases Community of Practice. BK Polyomavirus in Solid Organ Transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13528. [Google Scholar] [CrossRef]
- Demey, B.; Tinez, C.; François, C.; Helle, F.; Choukroun, G.; Duverlie, G.; Castelain, S.; Brochot, E. Risk Factors for BK Virus Viremia and Nephropathy after Kidney Transplantation: A Systematic Review. J. Clin. Virol. 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Kiberd, B.A. Screening to Prevent Polyoma Virus Nephropathy: A Medical Decision Analysis. Am. J. Transplant. 2005, 5, 2410–2416. [Google Scholar] [CrossRef]
- Brochot, E.; Descamps, V.; Handala, L.; Faucher, J.; Choukroun, G.; Helle, F.; Castelain, S.; François, C.; Duverlie, G.; Touzé, A. BK Polyomavirus in the Urine for Follow-up of Kidney Transplant Recipients. Clin. Microbiol. Infect. 2018, 25, 112.e1–112.e5. [Google Scholar] [CrossRef] [Green Version]
- Broekema, N.M.; Imperiale, M.J. MiRNA Regulation of BK Polyomavirus Replication during Early Infection. Proc. Natl. Acad. Sci. USA 2013, 110, 8200–8205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, W.; Vue, G.S.; Assetta, B.; Manza, H.; Atwood, W.J.; Imperiale, M.J. Control of Archetype BK Polyomavirus MicroRNA Expression. J. Virol. 2020, 95, e01589-20. [Google Scholar] [CrossRef] [PubMed]
- Nomburg, J.; Zou, W.; Frost, T.C.; Datta, C.; Vasudevan, S.; Starrett, G.J.; Imperiale, M.J.; Meyerson, M.; DeCaprio, J.A. Long-Read Sequencing Reveals Complex Patterns of Wraparound Transcription in Polyomaviruses. PLoS Pathog. 2022, 18, e1010401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.Z.; McNicholas, K.; Yong, T.Y.; Rao, N.; Coates, P.T.H.; Higgins, G.D.; Carroll, R.P.; Woodman, R.J.; Michael, M.Z.; Gleadle, J.M. BK Virus Encoded MicroRNAs Are Present in Blood of Renal Transplant Recipients With BK Viral Nephropathy: BK Virus MicroRNAs in Renal Transplant Recipients. Am. J. Transplant. 2014, 14, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Lagatie, O.; Tritsmans, L.; Stuyver, L.J. The MiRNA World of Polyomaviruses. Virol. J. 2013, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Wang, Z.; Huang, Y.; Abedin, Z.; Liu, Y.; Randhawa, P. Cellular and Viral MiRNA Expression in Polyomavirus BK Infection. Transpl. Infect. Dis. 2019, 21, e13159. [Google Scholar] [CrossRef]
- Demey, B.; Descamps, V.; Presne, C.; Helle, F.; Francois, C.; Duverlie, G.; Castelain, S.; Brochot, E. BK Polyomavirus Micro-RNAs: Time Course and Clinical Relevance in Kidney Transplant Recipients. Viruses 2021, 13, 351. [Google Scholar] [CrossRef]
- Martelli, F.; Mencarini, J.; Rocca, A.; Malva, N.D.; Bartolozzi, D.; Giannecchini, S. Polyomavirus MicroRNA in Saliva Reveals Persistent Infectious Status in the Oral Cavity. Virus Res. 2018, 249, 1–7. [Google Scholar] [CrossRef]
- Pietilä, T.; Nummi, M.; Auvinen, P.; Mannonen, L.; Auvinen, E. Expression of BKV and JCV Encoded MicroRNA in Human Cerebrospinal Fluid, Plasma and Urine. J. Clin. Virol. 2015, 65, 1–5. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.H.; Seo, J.-W.; Moon, H.; Kim, J.S.; Kim, Y.G.; Jeong, K.-H.; Moon, J.-Y.; Lee, T.W.; Ihm, C.-G.; et al. Urinary Exosomal Viral MicroRNA as a Marker of BK Virus Nephropathy in Kidney Transplant Recipients. PLoS ONE 2017, 12, e0190068. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, M.; Chen, Y.-F.A.; Hüls, C.; Gärtner, K.; Tagawa, T.; Mejias-Perez, E.; Keppler, O.T.; Göbel, C.; Zeidler, R.; Shein, M.; et al. MicroRNAs Are Minor Constituents of Extracellular Vesicles That Are Rarely Delivered to Target Cells. PLoS Genet. 2021, 17, e1009951. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of Extracellular Circulating MicroRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 Complexes Carry a Population of Circulating MicroRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mall, C.; Rocke, D.M.; Durbin-Johnson, B.; Weiss, R.H. Stability of MiRNA in Human Urine Supports Its Biomarker Potential. Biomark. Med. 2013, 7, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Zealy, R.W.; Wrenn, S.P.; Davila, S.; Min, K.-W.; Yoon, J.-H. MicroRNA-Binding Proteins: Specificity and Function. WIREs RNA 2017, 8, e1414. [Google Scholar] [CrossRef]
- Henriksen, S.; Mittelholzer, C.; Gosert, R.; Hirsch, H.H.; Rinaldo, C.H. Human BK Polyomavirus Plasmid PBKV (34-2) (Dunlop) Contains Mutations Not Found in the Originally Published Sequences. Genome Announc. 2015, 3, e00046-15. [Google Scholar] [CrossRef] [Green Version]
- Addetia, A.; Phung, Q.; Bradley, B.T.; Lin, M.J.; Zhu, H.; Xie, H.; Huang, M.-L.; Greninger, A.L. In Vivo Generation of BK and JC Polyomavirus Defective Viral Genomes in Human Urine Samples Associated with Higher Viral Loads. J. Virol. 2021, 95, e00250-21. [Google Scholar] [CrossRef]
- Moriyama, T.; Sorokin, A. BK Virus (BKV): Infection, Propagation, Quantitation, Purification, Labeling, and Analysis of Cell Entry. Curr. Protoc. Cell Biol. 2009, 42, 26.2.1–26.2.13. [Google Scholar] [CrossRef] [Green Version]
- Tribolet, L.; Kerr, E.; Cowled, C.; Bean, A.G.D.; Stewart, C.R.; Dearnley, M.; Farr, R.J. MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Front. Microbiol. 2020, 11, 1197. [Google Scholar] [CrossRef]
- Bazié, W.W.; Boucher, J.; Traoré, I.T.; Kania, D.; Somé, D.Y.; Alary, M.; Gilbert, C. Vesicular MicroRNA as Potential Biomarkers of Viral Rebound. Cells 2022, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreno, R.; Torre-Cisneros, J.; Cantisán, S. Human Cytomegalovirus (HCMV)-Encoded MicroRNAs: Potential Biomarkers and Clinical Applications. RNA Biol. 2021, 18, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Talaya, A.; Giménez, E.; Pascual, M.J.; Gago, B.; Piñana, J.L.; Hernández-Boluda, J.C.; Vázquez, L.; García, M.; Serrano, D.; Hernández, M.; et al. An Investigation of the Utility of Plasma Cytomegalovirus (CMV) MicroRNA Detection to Predict CMV DNAemia in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Med. Microbiol. Immunol. 2020, 209, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lagatie, O.; Van Loy, T.; Tritsmans, L.; Stuyver, L.J. Viral MiRNAs in Plasma and Urine Divulge JC Polyomavirus Infection. Virol. J. 2014, 11, 158. [Google Scholar] [CrossRef] [Green Version]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Janas, M.M.; Wang, B.; Harris, A.S.; Aguiar, M.; Shaffer, J.M.; Subrahmanyam, Y.V.B.K.; Behlke, M.A.; Wucherpfennig, K.W.; Gygi, S.P.; Gagnon, E.; et al. Alternative RISC Assembly: Binding and Repression of MicroRNA–MRNA Duplexes by Human Ago Proteins. RNA 2012, 18, 2041–2055. [Google Scholar] [CrossRef] [Green Version]
- Beltrami, C.; Clayton, A.; Newbury, L.J.; Corish, P.; Jenkins, R.H.; Phillips, A.O.; Fraser, D.J.; Bowen, T. Stabilization of Urinary MicroRNAs by Association with Exosomes and Argonaute 2 Protein. Non-Coding RNA 2015, 1, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Gardner, S.; Field, A.; Coleman, D.; Hulme, B. New Human Papovavirus (B.K.) Isolated from Urine after Renal Transplantation. Lancet 1971, 297, 1253–1257. [Google Scholar] [CrossRef]
- Moens, U.; Johansen, T.; Johnsen, J.I.; Seternes, O.M.; Traavik, T. Noncoding Control Region of Naturally Occurring BK Virus Variants: Sequence Comparison and Functional Analysis. Virus Genes 1995, 10, 261–275. [Google Scholar] [CrossRef]
- Rinaldo, C.H.; Hansen, H.; Traavik, T. Human Endothelial Cells Allow Passage of an Archetypal BK Virus (BKV) Strain—A Tool for Cultivation and Functional Studies of Natural BKV Strains. Arch. Virol. 2005, 150, 1449–1458. [Google Scholar] [CrossRef]
- Nukuzuma, S.; Takasaka, T.; Zheng, H.-Y.; Zhong, S.; Chen, Q.; Kitamura, T.; Yogo, Y. Subtype I BK Polyomavirus Strains Grow More Efficiently in Human Renal Epithelial Cells than Subtype IV Strains. J. Gen. Virol. 2006, 87, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Takasaka, T.; Hasegawa, M.; Zheng, H.-Y.; Chen, Q.; Sugimoto, C.; Kitamura, T.; Yogo, Y. Evolution of BK Virus Based on Complete Genome Data. J. Mol. Evol. 2006, 63, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, F.; Rookmaaker, M.B.; Margaritis, T.; Rios, A.; Ammerlaan, C.; Jansen, J.; Gijzen, L.; Vormann, M.; Vonk, A.; Viveen, M.; et al. Tubuloids Derived from Human Adult Kidney and Urine for Personalized Disease Modeling. Nat. Biotechnol. 2019, 37, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Fiore, T.; Martin, E.; Descamps, V.; Brochot, E.; Morel, V.; Handala, L.; Dakroub, F.; Castelain, S.; Duverlie, G.; Helle, F.; et al. Indoleamine 2,3-Dioxygenase Is Involved in Interferon Gamma’s Anti-BKPyV Activity in Renal Cells. Viruses 2020, 12, 865. [Google Scholar] [CrossRef]
- Handala, L.; Fiore, T.; Rouillé, Y.; Helle, F. QuantIF: An ImageJ Macro to Automatically Determine the Percentage of Infected Cells after Immunofluorescence. Viruses 2019, 11, 165. [Google Scholar] [CrossRef] [Green Version]
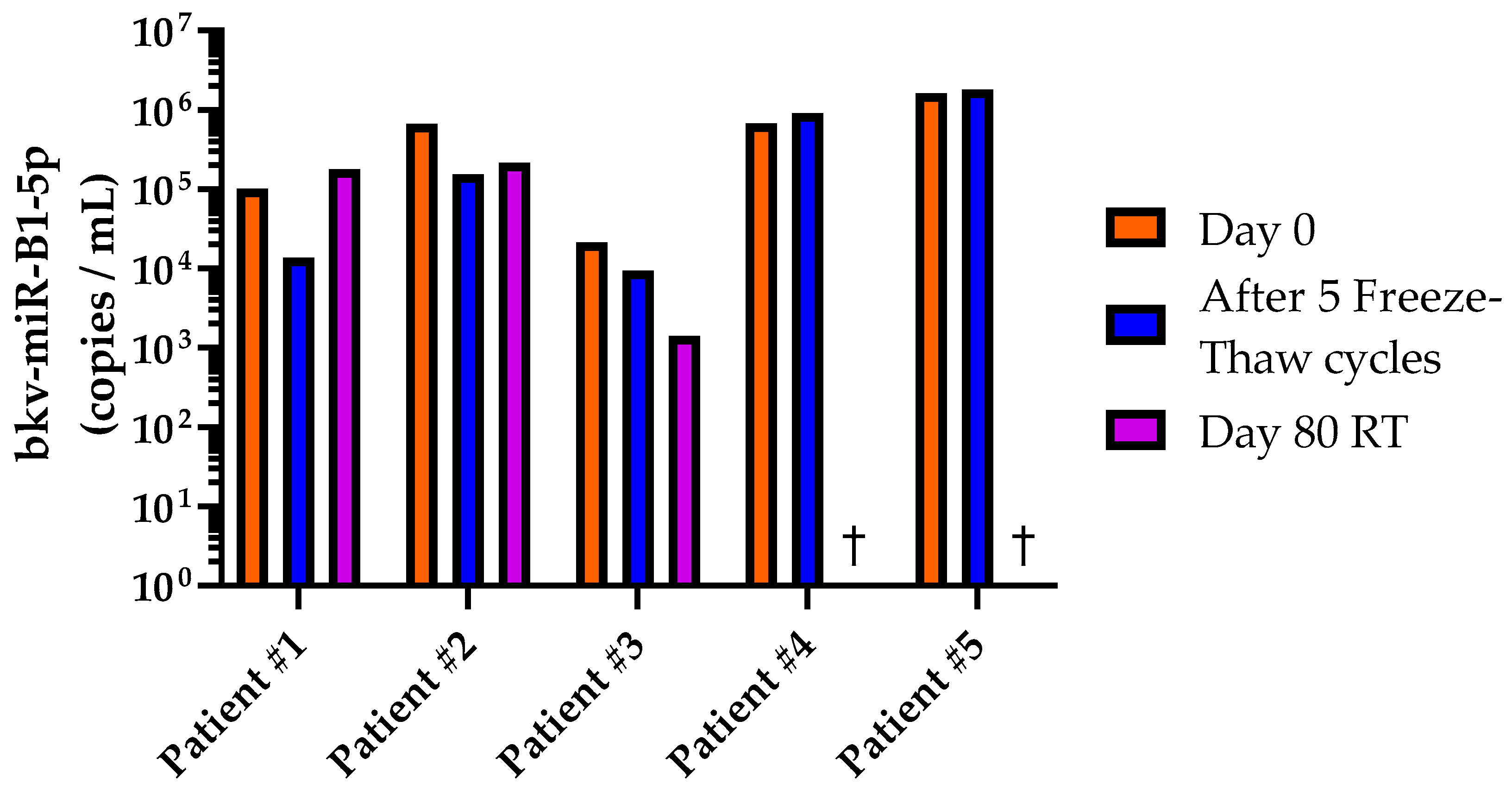
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demey, B.; Bentz, M.; Descamps, V.; Morel, V.; Francois, C.; Castelain, S.; Helle, F.; Brochot, E. BK Polyomavirus bkv-miR-B1-5p: A Stable Micro-RNA to Monitor Active Viral Replication after Kidney Transplantation. Int. J. Mol. Sci. 2022, 23, 7240. https://doi.org/10.3390/ijms23137240
Demey B, Bentz M, Descamps V, Morel V, Francois C, Castelain S, Helle F, Brochot E. BK Polyomavirus bkv-miR-B1-5p: A Stable Micro-RNA to Monitor Active Viral Replication after Kidney Transplantation. International Journal of Molecular Sciences. 2022; 23(13):7240. https://doi.org/10.3390/ijms23137240
Chicago/Turabian StyleDemey, Baptiste, Marine Bentz, Véronique Descamps, Virginie Morel, Catherine Francois, Sandrine Castelain, Francois Helle, and Etienne Brochot. 2022. "BK Polyomavirus bkv-miR-B1-5p: A Stable Micro-RNA to Monitor Active Viral Replication after Kidney Transplantation" International Journal of Molecular Sciences 23, no. 13: 7240. https://doi.org/10.3390/ijms23137240
APA StyleDemey, B., Bentz, M., Descamps, V., Morel, V., Francois, C., Castelain, S., Helle, F., & Brochot, E. (2022). BK Polyomavirus bkv-miR-B1-5p: A Stable Micro-RNA to Monitor Active Viral Replication after Kidney Transplantation. International Journal of Molecular Sciences, 23(13), 7240. https://doi.org/10.3390/ijms23137240






