Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model
Abstract
:1. Introduction
2. Results
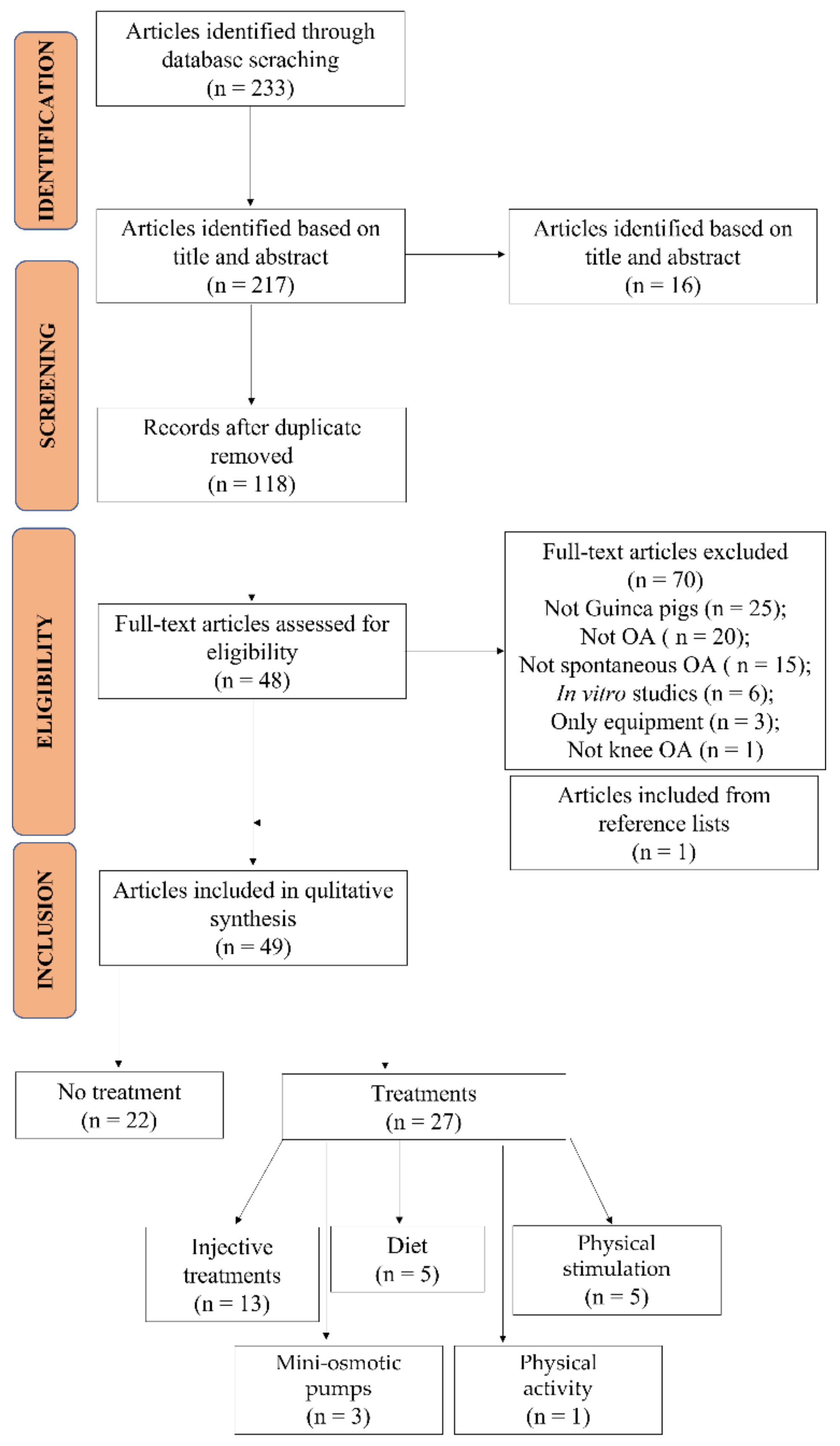
2.1. Studies Selection and Characteristics
2.2. Naturally Occurring OA in Guinea Pigs
2.3. Treatments in Naturally Occurring OA in DH Guinea Pigs
2.3.1. Injective Treatments
2.3.2. Diet
2.3.3. Physical Stimulation
2.3.4. Mini-Osmotic Pumps
2.3.5. Physical Activity
2.3.6. Risk of Bias
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Eligibility Criteria
5.2. Information Sources and Search Strategies
5.3. Studies Selection and Data Extraction
| ANIMALS (Age) | AIM | EVALUATIONS | RESULTS | REF. |
|---|---|---|---|---|
| 21 female DH guinea pigs (3, 5 and 9 mo) | To describe the age dependent cartilage degeneration in OA progression | MRI Histology/histomorphometry | At 9 mo ↓ stain intensity, CT; ↑ cartilage degeneration | [12] |
| 12 female DH guinea pigs (6.5, 8, 9.5 and 11 mo) | To describe structural features of OA and the progressive changes during aging | Micro-CT Histology/histomorphometry | Continuous OA changes gradually occur from 9.5 mo. At 6.5 mo ↓ distribution of chondrocytes in superficial zone; At 8 mo rough and irregular surface. At 9.5 mo chondrocytes disappeared in cartilage. At 11 mo depletion of cartilage in the adjacent region with the SB. During ageing ↓ Alcian blue; ↑ Mankin score | [13] |
| 60 female DH guinea pigs (8, 10 and 12 mo) | To observe chondrocyte and matrix degradation in the superficial surface cartilage during aging | Histology/histomorphometry IHC ELISA of serum WB | At 10 mo ↑ surface hyperthrophic chondrocytes, COLLX, MMP13, Caspase3. At 12 mo cartilage not stained in some areas, damaged with longitudinal cracks, hypertrophic chondrocytes, empty cartilage lacunae; ↓ Aggrecan; ↑ COLL X, MMP13, Caspase3, OARSI score, serum CTXII | [15] |
| 30 male DH guinea pigs (2, 3, 5, 9 and 15 mo) | To evaluate joint changes in spontaneous OA | Micro-CT Histology/histomorphometry | At 2 and 3 mo mild articular surface irregularities, slight ↓ PG. At 5 mo severe PG loss, occasional fissures, mild hypocellularity. At 9 mo more accentuated hypocellularity, chondrocyte clustering, tidemark duplication. At 15 mo complete loss of cartilage. During ageing ↑ OARSI score, TbTh; ↓ BV/TV; ↑ Cortical thickness | [17] |
| 40 male DH guinea pigs (1, 3, 6 and 9 mo) | To investigate the association between changes in SB and sGAG content of articular cartilage in spontaneous OA | Micro-CT Histology/histomorphometry Raman Spectroscopy | At 3 mo ↓ chondrocytes. At 6 mo ↓ chondrocytes; ↑ fibrillation. At 9 mo chondrocyte and tidemark disappeared; ↑ fissures. During ageing ↓ sGAG, TBPf, SMI; ↑ BMD, SBT, phosphate, TbTh | [18] |
| 24 male DH guinea pigs (2, 3, 5 and 7 mo) | To evaluate changes in the quadriceps skeletal muscle in spontaneous OA | Histology/histomorphometry ELISA of serum RT-PCR ICDH and LDH enzyme activities | At 2 mo free from OA. At 3 mo ↑ MHC IIX 3, 5 mo: PG loss in mild zone, cartilage surface irregularities; ↑ serum RANTES. 7 mo: ↑ PG loss and irregularities. During ageing: ↓ serum CTXII | [19] |
| 18 male DH guinea pigs (2.5, 5 and 7.5 mo) | To determine structural changes and OPG/RANKL during development of OA | Micro-CT Histology/histomorphometry IHC | During ageing ↑ SBT, OPG, cartilage fibrillation, PG loss, OARSI score; ↓ RANKL, cartilage cellularity | [20] |
| 60 male DH guinea pigs (1, 3, 5, 7 and 9 mo) | To study the progression of spontaneous OA | Histology/histomorphometry Biomechanics | During ageing ↑ OARSI score, instantaneous modulus; ↓ CT | [22] |
| 10 male DH guinea pigs (21.2 ± 2.9 mo) | To characterize association between SB circulation and bone structure and cartilage degeneration in spontaneous OA | Macroscopy Histology/histomorphometry Subchondral vessels IHC | Mild OA than severe OA, no intraosseous thrombi | [24] |
| 66 male and 66 female DH guinea pigs (1–11 mo) | To investigate the structural alterations in spontaneous OA | Micro-CT | From 5 mo ↑ degeneration of the articular cartilage. 1–6 mo ↑ BMD, BV/TV, TbTh. Male ↑ cartilage degeneration than female | [25] |
| 18 DH and 18 BS2 guinea pigs (1, 2 and 3 mo) | To investigate the spatial and temporal SB change of spontaneous OA at early stage | Micro-CT Histology/histomorphometry IHC | DH ↑ SB BMD, SBT, BMD, BV/TV, Osterix positive cells, TbTh; ↓ porosity, SMI, TbPf, TbSp, DA than BS2 | [26] |
| 21 female DH and 21 female BS2 guinea pigs (1, 2 and 3 mo) | To evaluate dynamic changes in the rod-and-plate microstructure | Micro-CT Histology/histomorphometry | DH ↑ BV/TV than BS2. DH show dynamic changes in the rod-and-plate microstructure | [27] |
| 24 male DH and 24 male BS2 guinea pigs (10 wks and 4, 6 and 7.5 mo) | To determine the role of chondrocyte apoptosis in spontaneous OA | Histology/histomorphometry IHC | DH ↑ OARSI score, fibrillation, PG loss, apoptotic cells than BS2. During ageing ↑ caspase-3 positive chondrocytes | [28] |
| 24 male DH and 24 male BS2 guinea pigs (10 wks and 4, 6 and 7.5 mo) | To investigate the association between bone remodeling and cartilage degradation with chondrocyte apoptosis in spontaneous OA | Micro-CT Histology/histomorphometry IHC | DH ↑ TbTh, TbN, BMD, BV/TV, cartilage degradation; ↓ TbSp than BS2. During ageing ↑ TbTh, BMD, cartilage degradation, chondrocyte apoptosis | [11] |
| 24 male DH and 24 BS2 guinea pigs (10 wks and 4, 6 and 7.5 mo) | To investigate the temporal and the spatial relationship between bone remodeling in Sbp and Tb in spontaneous OA | Micro-CT | DH ↓ GpTh during aging. DH ↑ SbpTh, TbTh, BMD than BS2. During ageing growth plate completely closed at 7.5 mo; ↑ SbpTh, TbTh | [29] |
| 10 male and female DH and 10 male and female Strain 13 guinea pigs (12 mo) | To determine the association of cartilage degeneration with subchondral BMD in spontaneous OA | Macroscopy Histology/histomorphometry DXA Atomic absorption spectrophometry | DH male and Strain 13 female ↑ severe cartilage degeneration, surface fibrillation, cartilage clefts, PG loss, Mankin score, BMD than DH female and Strain 13 male | [30] |
| 18 male DH and 18 male Strain 13 guinea pigs (5, 9 and 15 mo) | To evaluate skeletal muscle dysfunction, articular cartilage degeneration, and bone loss during aging in spontaneous OA | MRI Fiber angle Histology/histomorphometry IHC Skeletal muscle protein synthesis rates | DH ↑ OARSI, gastrocnemius and soleus mass; ↓ fiber angle than Strain 13. DH ↓ gastrocnemius and soleus density, type II myofibers, gastrocnemius and soleus myofibrillar, cytosolic, and mitochondrial fractional synthesis rates at 15 mo. During ageing ↑ OARSI; ↓ gastrocnemius collagen synthesis | [31] |
| 40 female DH guinea pigs (1, 3, 6, 9 and 12 mo) | To evaluate age-related changes in articular cartilage, BMD, and estradiol levels | ELISA of serum Histology/histmorphometry SEM and TEM IHC DXA | At 3 mo mild ulceration, focal PG loss and fibrillation, formation of microcilia. At 6 mo ulcerations, matrix loss, chondrocyte hyperthrophy and death, rough surface. At 9 mo ostephyte initial development, fibrillation and PG loss, collagenous fibers degenerated into thick bundles and cracks. At 12 mo ↑ ulcerations, matrix loss, ostephytes, erosion, severe cracks. During ageing ↑ Mankin score, MMP3, SB BMD, serum estradiol; ↓ GAG | [2] |
| 32 female DH guinea pigs (3, 6, 9 and 12 mo) | To evaluate TGF-β activity in OA progression | Micro-CT Histology/histomorphometry IHC | At 3 mo superficial zone undulation with matrix loss, pSmad2/3 in all zones. At 6 mo fissures; ↓ PG in superficial zone; ↑ SbPTh. At 9 mo fissures; ↓ PG in middle zone. At 12 mo fractures in deep zones, severe loss of PG, cracks. During ageing: ↑ Mankin score, TbTh, TbSp, TRAP staining, pSmad1/5/8; ↓ TbN, DA, pSmad2/3 | [14] |
| 20 male DH guinea pigs (2, 4, 8 and 12 mo) | To evaluate the expression of PPARγ, and H- and L-PGDS during spontaneous OA | Histology/histomorphometry IHC RT-PCR | At 4 mo minor surface irregularity, initial Safranin O decrease. At 8 mo surface erosion, markedly reduced cellularity and Safranin O, initial development of osteophytes. At 12 mo severe surface erosion, PG loss, osteophytes formation, SB sclerosis. During ageing ↓ PPAR γ; ↑ L-PGDS | [16] |
| 16 male DH guinea pigs (6 and 12 mo) | To determine the association between cartilage IGF-1 with loss of chondrocyte and ECM breakdown in spontaneous OA | Histology/histomorphometry RT-PCR | During ageing ↓ cellularity, matrix integrity, IGF-1; ↑ cartilage disruption, PG loss, Mankin score | [21] |
| 25 male DH guinea pigs (1, 3, 6, 9 and 12 mo) | To investigate the expression of MGP in spontaneous OA | Proteomics analysis Histology/histomorphometry IHC | During ageing ↑ Mankin score, MGP than 1, 3, 6, 9 mo | [23] |
| ANIMALS (Age) | TREATMENTS | EVALUATIONS | RESULTS | REF. |
|---|---|---|---|---|
| INJECTIVE TREATMENTS | ||||
| 24 female DH guinea pigs (3 and 6 mo) | i.a. injections of PBS or EGCG 1/wk for 3 mo | Endurance test Macroscopy Histology/histomorphometry IHC | EGCG ↑ running endurance, COLL II; ↓ roughness, ulceration, osteophyte, OARSI score, surface erosion, PG and GAG loss, MMP13, p16Ink4a. During ageing ↓ running endurance, GAG, COLL II; ↑ OA severity with erosion, ulceration, osteophyte, fissures, fibrillation, PG loss, OARSI, MMP13, p16Ink4a | [32] |
| 24 male DH guinea pigs (3 and 6 mo) | i.a. injections of saline solution or PRP 1/wk for 3 times | ELISA Histology/histomorphometry | PRP ↓ COMP, OARSI score, synovitis score, synovial vascularity | [33] |
| 32 DH guinea pigs (8 and 11 mo) | 1 or 3 (1/wk) i.a. injections of PRP | Histology/histomorphometry | At 8 mo: PRP ↓ synovitis. 3 PRP injections ↓ articular cartilage damage. At 11 mo: 3 PRP injections ↓ synovitis | [34] |
| 42 DH guinea pigs (3 and 11 mo) | i.a. injections of HA ± CCL25 (63, 693 or 6993 pg) 1/wk for 5 mo | Histology/histomorphometry | HA + CCL25 (693 and 6993 pg) ↓ OARSI score than HA alone | [35] |
| 60 male DH guinea pigs (7 mo) | i.a. injections of PBS, HA, PBS + hMSCs or HA + hMSCs | Macroscopy Histology IHC WB | HA weak matrix staining and cell depletion. PBS, HA and PBS + hMSCs ↑ rough surface. PBS, PBS + hMSCs ↓ chondrocytes, matrix fibrillation. HA + hMSCs ↑ numbers of chondrocytes with cluster formations, smooth surface, COLL II, Mankin score; ↓ Macroscopic score, MMP13 | [36] |
| 9 DH strain 051 guinea pigs | i.a. injection of Pa-MSCs or Re-MSCs | ELISA Histology/histomorphometry RT-PCR | Re-MSCs ↓ TNFα, RANTES, OARSI score. Pa-MSCs ↓ TNFα | [37] |
| 6 male DH guinea pigs (2 mo) | i.a. injections of TV and PBS or TV and NTV for 2 mo | RT-PCR | TV ↓ IL1β expression than PBS and NTC | [38] |
| 32 male DH guinea pigs (2 mo) | i.a. injections of TV and NTV, Ad-Luc and Ad-hIRAP or TV and PBS for 2 or 4 mo | Histology/histomorphometry IHC RT-PCR | TV and Ad-hIRAP ↓ TNFα, IL8, INFy, IL1β expression; ↑ TGFβ1 than NTV and Ad-Luc. TV ↓ TNFα, IL8, MMP13 expression than PBS | [39] |
| 50 female SPF-grade DH albino guinea pigs (9, 10 and 11 mo) | i.a. injections of rapa, saline or 3-MA | Histology/histomorphometry Macroscopy FMT IHC RT-PCR | 3-MA ↑ OARSI score, MMP13, Glycogenin 1, Caspase3, Tunel; ↓ Aggrecan, Beclin 1. rapa ↓ OARSI score, MMP13, Glycogenin 1, Caspase3, Tunel; ↑ Aggrecan, Beclin 1 | [40] |
| 27 male DH guinea pigs (6 and 7 mo) | i.a. injections of PTH (1–34) for 3 mo | Micro-CT Histology/histomorphometry IHC | PTH (1–34) ↑ GAG; ↓ OARSI score, apoptosis rate | [41] |
| 48 female DH guinea pigs (1 and 3 mo) | s.c. injections of saline solution or PTH for 3 or 6 mo | Histology/histomorphometry IHC Micro-CT | During aging ↑ MMP13, SOST; ↓ COLL II. PTH ↓ roughness, ulceration, osteophytes, OARSI score, MMP13, SOST, RANKL; ↑ COLL II, PTH1R, OPG, OPG/RANKL ratio, BMD, BV/TV, SMI | [42] |
| 16 male DH guinea pigs (3.5 mo) | s.c injection of sodium lactate solution or DFO for 7.5 mo | CBC Histology/histomorphometry RT-PCR IHC Overhead enclosure monitoring | DFO ↑ OARSI score, mTOR, NF-κB p65, PTGS-2, BAD, BAX, BAK, Caspase-9, Caspase-3, COLL II, ACAN, MMP2, MMP9, MMP13; ↓ 4-HNE, p-AMPKα, TIMP2, change in distance traveled, change in average speed | [43] |
| 48 male DH guinea pigs (1.5 and 3 mo) | s.c. injections of risedronate for 1.5, 3 or 6 mo | Macroscopy Histology/histomorphometry ELISA Indentation test | Risedronate ↓ OS/BS, BFR/BS, Sb.Th, serum CTX-II, MS/BS | [44] |
| DIET | ||||
| 27 male DH guinea pigs (5 mo) | Rapa ± metformin for 3 mo | Micro-CT Histology/histomorphometry IHC WB | Rapa ± metformin ↑ OARSI score, PG loss, cortical th; ↓ P-RPS6. Rapa + metformin ↑ cartilage damage; ↓ P/T RPS6. Rapa ↓ P/T AMPK than rapa + metformin | [45] |
| 24 male DH guinea pigs (2 mo) | Calorie-restricted, HFD or calorie-restricted HFD for 3 mo | Micro-CT Histology/histomorphometry IHC ELISA | Regular chow ↑ surface fibrillation, fissures. Calorie-restricted mild superficial PG loss, no MCP1. HFD superficial PG loss, occasional chondrocyte clustering, and focal cell loss. Calorie-restricted ↓ Micro-CT scoring system, small enthesophytes and/or osteophytes, SB sclerosis, OARSI score, serum C3, cartilage MCP1 than HDF | [46] |
| 65 DH guinea pigs (1 mo) | Oleuropein, Rutin or Rutin + curcumin for 8 mo | ELISA Histology/histomorphometry | Oleuropein, Rutin or Rutin + curcumin ↓ OARSI score, synovial score, serum PGE2, Fib3-1 and Fib3-2, Coll2-1NO2 kinetic curve, cellularity; ↑ surface integrity, PG during aging. Oleuropein ↓ osteophyte, lining and infiltrated cells. Rutin or Rutin + curcumin ↓ Coll2-1 kinetic curve, ARGS. Rutin + curcumin ↓ Fib3-1 kinetic curve. During aging ↓ serum ARGS | [47] |
| 21 DH giunea pigs (3, 6 and 12 mo) | CM-01 until 6, 12 or 18 mo | Radiograph Histology/histomorphometry IHC | CM-01 ↓ meniscal calcification, osteophytes, surface lesions, PG and chondrocyte loss, cartilage degeneration, Mankin score, MMP13; ↑ cartilage bars in SB, CT | [48] |
| 50 female DH guinea pigs (3 wks, 1 and 8 mo) | D-GlcN or CS Na until 8, 12 or 18 mo | Histology/histomorphometry RT-PCR | D-GlcN and CS Na ↓ Mankin score, MMP3; ↑ total RNA, chondrocytes. During ageing ↑ TUNEL-positive cells, MMP8; ↓ RNA, ACAN, COLL II, MMP13 | [49] |
| PHYSICAL STIMULATION | ||||
| 18 male DH guinea pigs (3 and 17 mo) | EA for 1 mo | Nociceptive Behavioral Test Histology/histomorphometry WB ELISA | EA ↑ mechanical withdraw threshold; ↓ fibrillation, NLRP3, Caspase-1 and IL1β, serum TNFα and IL1β, cartilage MMP13 | [50] |
| 10 male DH guinea pigs | EA for 3 wks | Open-field enclosure monitoring parameters Treadmill-based gait analysis Histology/histomorphometry ELISA RT-PCR | EA ↑ average speed, maximum speed, total distance traveled, stride length, COLL II, FGF18, TGFβ1, TIMP1, SOD2 | [51] |
| 25 female DH guinea pigs (5, 9 and 18 mo) | Hyperthermia | Histology/histomorphometry IHC | Hyperthermia ↑ HSP70; ↓ Mankin score, ULK1, Beclin1 positive cells | [52] |
| 15 DH guinea pigs (21 mo) | PEMFs at 37 or 75 Hz for 3 mo | Histology/histomorphometry | PEMFs at 37 and 75 Hz ↓ cartilage degeneration, Mankin score, FI, SBT, TbN; ↑ TbTh, TbSp. PEMFs at 75 Hz ↓ Mankin score, FI, TbTh; ↑ CT, TbN than PEMFs at 37 Hz | [53] |
| 56 DH guinea pigs (6 mo) | Pure rapa ± LIPUS, L-rapa ± LIPUS, or LIPUS for 2 mo | Histology/histomorphometry IHC ELISA Cell count AST, ALT, BUN, creatinine, electrolytes sodium, potassium, calcium, inorganic phosphorous, chloride | L-rapa ± LIPUS ↑ GAG, cartilage COLL II; ↓ OARSI score, cartilage MMP13. L-rapa + LIPUS ↓ serum C2C. Pure rapa ± LIPUS, L-rapa ± LIPUS, or LIPUS normal cell count, serum AST, ALT, BUN, creatinine and sodium, potassium, calcium, inorganic phosphorous, chloride | [54] |
| MINI-OSMOTIC PUMPS | ||||
| 86 male DH guinea pigs (6 mo) | A subcutaneous mini-osmotic pump filled with TN14003, T140 or AMD3100 for 3 mo | ELISA RT-PCR WB | TN14003 ↓ serum SDF1, MMP3, MMP9, MMP13; ↑ COLL II, ACAN. T140 ↓ serum SDF1, MMP3, MMP9, MMP13; ↑ COLL II, ACAN than AMD3100. AMD3100 ↓ serum SDF1, MMP3, MMP9, MMP13; ↑ COLL II, ACAN than no treatment | [55] |
| 36 male DH guinea pigs (9 mo) | A subcutaneous mini-osmotic pump filled with T140 or PBS for 3 mo | ELISA Histology/histomorphometry RT-PCR WB | T140 ↓ serum SDF1, OA changes and severity, Mankin score, cartilage damage, MMP3, MMP9, MMP13 expression, serum SDF1; ↑ COLL II, ACAN expression, COLL II protein | [56] |
| 35 male DH guinea pigs (9 mo) | A subcutaneous mini-osmotic pump filled with AMD3100 or PBS for 3 mo | ELISA DMMB assay Macroscopy Histology/histomorphometry | PBS deep and wide fissures. AMD3100 ↓ cartilage damage, Mankin score, GAG, SDF1, pro-MMP1, active MMP13, IL1β of SF and serum | [57] |
| PHYSICAL ACTIVITY | ||||
| 36 male DH guinea pigs (2 mo) | Physical activity on a flatbed treadmill at a rate of 20–25 m/min, 5 days/wk for 22 wks | Histology/histomorphometry Biomechanics Biochemistry | Physical activity ↑ Aggrecan; ↓ depth of cartilage degeneration | [58] |
| Ref. | Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Blinding | Random Outcome Assessment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| [12] | NO | YES | NO | NO | NO | YES | YES | YES | YES | UNCLEAR |
| [13] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [15] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [17] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [18] | NO | YES | NO | NO | NO | NO | YES | YES | YES | UNCLEAR |
| [19] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [20] | NO | YES | NO | NO | NO | YES | NO | YES | YES | UNCLEAR |
| [22] | NO | YES | NO | NO | NO | NO | YES | YES | YES | UNCLEAR |
| [24] | NO | YES | NO | NO | YES | NO | YES | YES | YES | UNCLEAR |
| [25] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [26] | NO | YES | NO | NO | NO | YES | YES | YES | YES | UNCLEAR |
| [27] | NO | YES | NO | NO | NO | NO | YES | YES | YES | UNCLEAR |
| [28] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| [11] | YES | YES | NO | NO | NO | NO | YES | YES | YES | UNCLEAR |
| [29] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [30] | YES | YES | NO | YES | NO | YES | NO | YES | YES | YES |
| [31] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [2] | NO | UNCLEAR | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [14] | NO | YES | NO | NO | YES | YES | YES | YES | YES | UNCLEAR |
| [16] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [21] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [23] | NO | YES | NO | NO | NO | YES | YES | YES | YES | YES |
| [32] | NO | YES | NO | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [33] | NO | YES | NO | NO | YES | NO | YES | YES | YES | YES |
| [34] | YES | YES | YES | YES | NO | YES | NO | YES | YES | UNCLEAR |
| [35] | NO | YES | NO | NO | NO | NO | YES | YES | YES | UNCLEAR |
| [36] | NO | YES | NO | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [37] | YES | YES | YES | YES | NO | YES | YES | YES | YES | UNCLEAR |
| [38] | NO | YES | NO | NO | NO | NO | YES | YES | YES | UNCLEAR |
| [39] | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| [40] | NO | YES | NO | NO | NO | YES | NO | YES | YES | UNCLEAR |
| [41] | YES | YES | YES | YES | NO | YES | NO | YES | YES | UNCLEAR |
| [42] | NO | YES | NO | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [43] | NO | YES | YES | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [44] | NO | YES | YES | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [45] | YES | YES | YES | YES | NO | YES | YES | YES | YES | UNCLEAR |
| [46] | NO | YES | NO | YES | YES | YES | YES | YES | YES | UNCLEAR |
| [47] | NO | YES | NO | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [48] | NO | YES | NO | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [49] | NO | YES | NO | NO | NO | YES | NO | YES | YES | UNCLEAR |
| [50] | NO | YES | NO | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [51] | NO | YES | YES | YES | NO | YES | YES | YES | YES | UNCLEAR |
| 52 | NO | YES | NO | NO | NO | NO | NO | YES | YES | UNCLEAR |
| 53 | NO | YES | NO | YES | NO | NO | NO | YES | YES | UNCLEAR |
| [54] | NO | YES | NO | YES | NO | NO | NO | YES | YES | UNCLEAR |
| [55] | NO | YES | NO | YES | NO | YES | NO | YES | YES | UNCLEAR |
| [56] | NO | YES | NO | YES | NO | YES | NO | YES | YES | UNCLEAR |
| [57] | NO | YES | NO | YES | NO | NO | YES | YES | YES | UNCLEAR |
| [58] | NO | YES | NO | YES | NO | NO | YES | YES | YES | UNCLEAR |
| Ref. | TITLE | ABSTRACT | INTRODUCTION | METHODS | RESULTS | DISCUSSION | TOTAL |
|---|---|---|---|---|---|---|---|
| [12] | 1 | 2 | 3 | 13 | 6 | 5 | 30 |
| [13] | 0 | 2 | 2 | 12 | 2 | 1 | 19 |
| [15] | 1 | 1 | 1 | 11 | 2 | 4 | 20 |
| [17] | 1 | 2 | 3 | 7 | 2 | 2 | 17 |
| [18] | 1 | 2 | 2 | 15 | 4 | 5 | 29 |
| [19] | 1 | 2 | 3 | 10 | 2 | 5 | 23 |
| [20] | 1 | 2 | 3 | 12 | 2 | 3 | 23 |
| [22] | 1 | 2 | 2 | 8 | 2 | 3 | 13 |
| [24] | 1 | 2 | 3 | 12 | 5 | 3 | 28 |
| [25] | 1 | 2 | 3 | 8 | 2 | 5 | 21 |
| [26] | 1 | 2 | 2 | 11 | 2 | 2 | 20 |
| [27] | 1 | 2 | 1 | 11 | 2 | 5 | 22 |
| [28] | 1 | 2 | 3 | 16 | 7 | 5 | 34 |
| [11] | 1 | 2 | 3 | 11 | 6 | 4 | 27 |
| [29] | 1 | 2 | 1 | 8 | 3 | 1 | 16 |
| [30] | 1 | 2 | 3 | 13 | 2 | 2 | 23 |
| [31] | 1 | 2 | 2 | 9 | 1 | 3 | 18 |
| [2] | 1 | 2 | 2 | 9 | 2 | 3 | 19 |
| [14] | 1 | 2 | 3 | 12 | 2 | 1 | 22 |
| [16] | 1 | 2 | 2 | 11 | 2 | 1 | 19 |
| [21] | 1 | 2 | 2 | 11 | 2 | 1 | 19 |
| [23] | 1 | 2 | 3 | 13 | 2 | 5 | 26 |
| [32] | 1 | 2 | 3 | 14 | 2 | 3 | 25 |
| [33] | 1 | 2 | 2 | 10 | 2 | 5 | 22 |
| [34] | 1 | 2 | 3 | 10 | 3 | 1 | 20 |
| [35] | 1 | 2 | 2 | 12 | 1 | 3 | 21 |
| [36] | 1 | 2 | 3 | 12 | 1 | 3 | 22 |
| [37] | 1 | 2 | 3 | 11 | 5 | 4 | 26 |
| [38] | 1 | 2 | 2 | 10 | 1 | 2 | 18 |
| [39] | 1 | 2 | 2 | 9 | 2 | 2 | 18 |
| [40] | 1 | 2 | 3 | 13 | 3 | 4 | 26 |
| [41] | 1 | 2 | 3 | 12 | 2 | 4 | 24 |
| [42] | 1 | 2 | 3 | 13 | 5 | 3 | 27 |
| [43] | 1 | 2 | 3 | 16 | 6 | 4 | 32 |
| [44] | 1 | 2 | 3 | 12 | 7 | 4 | 29 |
| [45] | 0 | 2 | 3 | 16 | 3 | 4 | 28 |
| [46] | 1 | 2 | 3 | 13 | 4 | 3 | 26 |
| [47] | 1 | 2 | 2 | 14 | 5 | 5 | 29 |
| [48] | 1 | 2 | 2 | 12 | 2 | 3 | 22 |
| [49] | 0 | 1 | 2 | 8 | 2 | 1 | 14 |
| [50] | 1 | 2 | 2 | 12 | 2 | 3 | 22 |
| [51] | 1 | 2 | 3 | 16 | 6 | 3 | 31 |
| [52] | 1 | 2 | 2 | 11 | 3 | 3 | 22 |
| [53] | 1 | 2 | 2 | 13 | 3 | 3 | 24 |
| [54] | 0 | 2 | 3 | 13 | 4 | 2 | 24 |
| [55] | 0 | 2 | 2 | 13 | 2 | 2 | 21 |
| [56] | 0 | 2 | 2 | 13 | 2 | 2 | 21 |
| [57] | 0 | 2 | 2 | 12 | 3 | 2 | 21 |
| [58] | 1 | 2 | 3 | 12 | 2 | 1 | 21 |
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Y.; Tian, F.M.; Wang, W.Y.; Cheng, Y.; Xu, H.F.; Song, H.P.; Zhang, Y.Z.; Zhang, L. Age Dependent Changes in Cartilage Matrix, Subchondral Bone Mass, and Estradiol Levels in Blood Serum, in Naturally Occurring Osteoarthritis in Guinea Pigs. Int. J. Mol. Sci. 2014, 15, 13578–13595. [Google Scholar] [CrossRef]
- Serra, C.I.; Soler, C. Animal Models of Osteoarthritis in Small Mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 211–221. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular senescence in osteoarthritis pathology. Aging Cell 2017, 16, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bapat, S.; Hubbard, D.; Munjal, A.; Hunter, M.; Fulzele, S. Pros and cons of mouse models for studying osteoarthritis. Clin. Transl. Med. 2018, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cope, P.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Van der Kraan, P.; Matta, C.; Mobasheri, A. Age related alterations in signaling pathways in articular chondrocytes: Implications for the pathogenesis and progression of osteoarthritis a mini review. Gerontology 2017, 63, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamli, Z.; Brown, K.R.; Tarlton, J.F.; Adams, M.A.; Torlot, G.E.; Cartwright, C.; Cook, W.A.; Vassilevskaja, K.; Sharif, M. Subchondral Bone Plate Thickening Precedes Chondrocyte Apoptosis and Cartilage Degradation in Spontaneous Animal Models of Osteoarthritis. BioMed Res. Int. 2014, 2014, 606870. [Google Scholar] [CrossRef] [PubMed]
- Fenty, M.C.; Dodge, G.R.; Kassey, V.B.; Witschey, W.R.T.; Borthakur, A.; Reddy, R. Quantitative Cartilage Degeneration Associated with Spontaneous Osteoarthritis in a Guinea Pig Model. J. Magn. Reson. Imaging 2012, 35, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Song, D.H.; Kim, S.H.; Jung, Y.; Kim, S.J. Development and characterization of various osteoarthritis models for tissue engineering. PLoS ONE 2018, 13, e0194288. [Google Scholar] [CrossRef]
- Zhao, W.W.; Wang, T.; Luo, Q.; Chen, Y.; Leung, V.Y.L.; Wen, C.; Shah, M.F.; Pan, H.; Chiu, K.Y.; Cao, X.; et al. Cartilage Degeneration and Excessive Subchondral Bone Formation in Spontaneous Osteoarthritis Involves Altered TGF-b Signaling. J. Orthop. Res. 2016, 34, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Wei, L.; Xue, Y.; Li, R.S. Experimental observation of the sequence of tibial plateau chondrocyte and matrix degeneration in spontaneous osteoarthritis in Guinea pigs. BMC Musculoskelet. Disord. 2021, 22, 395. [Google Scholar] [CrossRef] [PubMed]
- Nebbaki, S.S.; El Mansouri, F.E.; Afif, H.; Kapoor, M.; Benderdour, M.; Pelletier, J.P.; Martel-Pelletier, J.; Fahmi, H. Expression of Peroxisome Proliferator-activated Receptors α, ß, γ, and H- and L-Prostaglandin D Synthase During Osteoarthritis in the Spontaneous Hartley Guinea Pig and Experimental Dog Models. J. Rheumatol. 2013, 40, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Radakovich, L.B.; Marolf, A.J.; Shannon, J.P.; Pannone, S.C.; Sherk, V.D.; Santangelo, K.S. Development of a microcomputed tomography scoring system to characterize disease progression in the Hartley guinea pig model of spontaneous osteoarthritis. Connect. Tissue Res. 2018, 59, 523–533. [Google Scholar] [CrossRef]
- Ren, P.; Niu, H.; Cen, H.; Jia, S.; Gong, H.; Fan, Y. Biochemical and Morphological Abnormalities of Subchondral Bone and Their Association with Cartilage Degeneration in Spontaneous Osteoarthritis. Calcif. Tissue Int. 2021, 109, 179–189. [Google Scholar] [CrossRef]
- Tonge, D.P.; Bardsley, R.G.; Parr, T.; Maciewicz, R.A.; Jones, S.W. Evidence of changes to skeletal muscle contractile properties during the initiation of disease in the ageing guinea pig model of osteoarthritis. Longev. Healthspan 2013, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Akman, A.A.; Talib, A.A.; Yusof, A.M.; Khalid, K.A.; Yusof, N.A.; Ghani, R.A.; Hamdan, A.H.; Zamli, Z. Structural changes and the differential expression of osteoprotegerin (opg) and receptor activator of nuclear factor kb ligand (rankl) in subchondral bone during the development of osteoarthritis. Malays. Appl. Biol. 2022, 47, 87–96. [Google Scholar]
- Wei, F.Y.; Lee, J.K.; Wei, L.; Qu, F.; Zhang, J.Z. Correlation of insulin-like growth factor 1 and osteoarthritic cartilage degradation: A spontaneous osteoarthritis in guinea-pig. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4493–4500. [Google Scholar] [PubMed]
- Legrand, C.; Ahmed, U.; Anwar, A.; Rajpoot, K.; Pasha, S.; Lambert, C.; Davidson, R.K.; Clark, I.M.; Thornalley, P.J.; Henrotin, Y.; et al. Glycation marker glucosepane increases with the progression of osteoarthritis and correlates with morphological and functional changes of cartilage in vivo. Arthritis Res. Ther. 2018, 20, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Zhang, Z.; Kang, X.; Deng, C.; Sun, Y.; Li, Y.; Huang, D.; Liu, X. Defining matrix Gla protein expression in the Dunkin-Hartley guinea pig model of spontaneous osteoarthritis. BMC Musculoskelet. Disord. 2021, 22, 870. [Google Scholar] [CrossRef] [PubMed]
- Dyke, J.P.; Synan, M.; Ezell, P.; Ballon, D.; Racine, J.; Aaron, R.K. Characterization of Bone Perfusion by Dynamic Contrast-Enhanced Magnetic Resonance Imaging and Positron Emission Tomography in the Dunkin-Hartley Guinea Pig Model of Advanced Osteoarthritis. J. Orthop. Res. 2015, 33, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wu, C.; Tao, J.; Zhao, D.; Jiang, X.; Tian, W. Differential proteomic analysis of tibial subchondral bone from male and female guinea pigs with spontaneous osteoarthritis. Exp. Ther. Med. 2021, 21, 633. [Google Scholar] [CrossRef]
- Wang, T.; Wen, C.Y.; Yan, C.H.; Lu, W.W.; Chiu, K.Y. Spatial and temporal changes of subchondral bone proceed to microscopic articular cartilage degeneration in guinea pigs with spontaneous osteoarthritis. Osteoarthr. Cartil. 2013, 21, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hu, Y.; Yu, Y.E.; Zhang, X.; Watts, T.; Zhou, B.; Wang, J.; Wang, T.; Zhao, W.; Chiu, K.Y.; et al. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. J. Bone Miner. Res. 2018, 33, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Zamli, Z.; Adams, M.A.; Tarlton, J.F.; Sharif, M. Increased Chondrocyte Apoptosis Is Associated with Progression of Osteoarthritis in Spontaneous Guinea Pig Models of the Disease. Int. J. Mol. Sci. 2013, 14, 17729–17743. [Google Scholar] [CrossRef] [Green Version]
- Zamli, Z.; Brown, K.R.; Sharif, M. Subchondral Bone Plate Changes More Rapidly than Trabecular Bone in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1496. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Scannel, B.P.; Honeycutt, P.R.; Mauerhan, D.R.; Norton, H.J.; Hanley, E.N., Jr. Cartilage Degeneration, Subchondral Mineral and Meniscal Mineral Densities in Hartley and Strain 13 Guinea Pigs. Open Rheumatol. J. 2015, 9, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Musci, R.V.; Walsh, M.A.; Konopka, A.R.; Wolff, C.A.; Peelor, F.F.; Reiser, R.F.; Santangelo, K.S.; Hamilton, K.L. The Dunkin Hartley Guinea Pig Is a Model of Primary Osteoarthritis That Also Exhibits Early Onset Myofiber Remodeling That Resembles Human Musculoskeletal Aging. Front. Physiol. 2020, 11, 571372. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Cheng, T.L.; Yang, C.D.; Chang, C.F.; Ho, C.J.; Chuang, S.C.; Li, J.Y.; Huang, S.H.; Lin, Y.S.; Shen, H.Y.; et al. Intra-Articular Injection of (−)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Antioxidants 2021, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Kanwat, H.; Singh, D.M.; Kumar, C.D.; Alka, B.; Biman, S.; Aman, H. The effect of intra-articular allogenic platelet rich plasma in Dunkin-Hartley guinea pig model of knee osteoarthritis. Muscles Ligaments Tendons J. 2018, 7, 426–434. [Google Scholar] [CrossRef]
- Chouhan, D.K.; Dhillon, M.S.; Patel, S.; Bansal, T.; Bhatia, A.; Kanwat, H. Multiple Platelet-Rich Plasma Injections versus Single Platelet-Rich Plasma Injection in Early Osteoarthritis of the Knee. An Experimental Study in a Guinea Pig Model of Early Knee Osteoarthritis. Am. J. Sports Med. 2019, 47, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Ringe, J.; Hemmati-Sadeghi, S.; Fröhlich, K.; Engels, A.; Reiter, K.; Dehne, T.; Sittinger, M. CCL25-Supplemented Hyaluronic Acid Attenuates Cartilage Degeneration in a Guinea Pig Model of Knee Osteoarthritis. J. Orthop. Res. 2019, 37, 1723–1729. [Google Scholar] [CrossRef]
- Sato, M.; Uchida, K.; Nakajima, H.; Miyazaki, T.; Guerrero, A.R.; Watanabe, S.; Roberts, S.; Baba, H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res. Ther. 2012, 14, R31. [Google Scholar] [CrossRef] [Green Version]
- Walczak, B.E.; Jiao, H.; Lee, M.S.; Li, W.J. Reprogrammed Synovial Fluid-Derived Mesenchymal Stem/Stromal Cells Acquire Enhanced Therapeutic Potential for Articular Cartilage Repair. Cartilage 2021, 13, 530S–543S. [Google Scholar] [CrossRef]
- Santangelo, K.S.; Bertone, A.L. Effective reduction of the interleukin-1β transcript in osteoarthritis-prone guinea pig chondrocytes via short hairpin RNA mediated RNA interference influences gene expression of mediators implicated in disease pathogenesis. Osteoarthr. Cartil. 2011, 19, 1449–1457. [Google Scholar] [CrossRef] [Green Version]
- Santangelo, K.S.; Nuovo, G.J.; Bertone, A.L. In vivo reduction or blockade of interleukin-1b in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthr. Cartil. 2012, 20, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Tian, W.; Xu, W.W.; Lu, X.; Zhang, Y.M.; Li, L.J.; Chang, F. Loss of Autophagy Causes Increased Apoptosis of Tibial Plateau Chondrocytes in Guinea Pigs with Spontaneous Osteoarthritis. Cartilage 2021, 13, 796S–807S. [Google Scholar] [CrossRef]
- Chen, C.H.; Kang, L.; Chang, L.H.; Cheng, T.L.; Lin, S.Y.; Wu, S.C.; Lin, Y.S.; Chuang, S.C.; Lee, T.C.; Chang, J.K.; et al. Intra-articular low-dose parathyroid hormone (1-34) improves mobility and articular cartilage quality in a preclinical age-related knee osteoarthritis model. Bone Joint Res. 2021, 10, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Y.; Tian, F.M.; Wang, W.Y.; Cheng, Y.; Song, H.P.; Zhang, Y.Z.; Zhang, L. Parathyroid hormone (1-34) prevents cartilage degradation and preserves subchondral bone micro-architecture in guinea pigs with spontaneous osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1869–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, L.H.; Afzali, M.F.; Radakovich, L.B.; Campbell, M.A.; Culver, L.A.; Olver, C.S.; Santangelo, K.S. Systemic administration of a pharmacologic iron chelator reduces cartilage lesion development in the Dunkin-Hartley model of primary osteoarthritis. Free Radic. Biol Med. 2022, 179, 47–58. [Google Scholar] [CrossRef]
- Thomsen, J.S.; Straarup, T.S.; Danielsen, C.C.; Oxlund, H.; Brüel, A. No effect of risedronate on articular cartilage damage in the Dunkin Hartley guinea pig model of Osteoarthritis. Scand. J. Rheumatol. 2013, 42, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Minton, D.M.; Elliehausen, C.J.; Javors, M.A.; Santangelo, K.S.; Konopka, A.R. Rapamycin-induced hyperglycemia is associated with exacerbated age-related osteoarthritis. Arthritis Res. Ther. 2022, 24, 20. [Google Scholar] [CrossRef]
- Radakovich, L.B.; Marolf, A.J.; Culver, L.A.; Santangelo, K.S. Calorie restriction with regular chow, but not a high-fat diet, delays onset of spontaneous osteoarthritis in the Hartley guinea pig model. Arthritis Res. Ther. 2019, 21, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horcajada, M.N.; Sanchez, C.; Membrez Scalfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Roberts, A.; Mauerhan, D.R.; Cox, M.; Hanley, E.N. Biological Effects and Osteoarthritic Disease-Modifying Activity of Small Molecule CM-01. J. Orthop. Res. 2018, 36, 309–317. [Google Scholar] [CrossRef]
- Taniguchi, S.; Ryu, J.; Seki, M.; Sumino, T.; Tokuhashi, Y.; Esumi, M. Long-Term Oral Administration of Glucosamine or Chondroitin Sulfate Reduces Destruction of Cartilage and Up-Regulation of MMP-3 mRNA in a Model of Spontaneous Osteoarthritis in Hartley Guinea Pigs. J. Orthop. Res. 2012, 30, 673–678. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Wang, B.; Kang, W.; Yu, H.; Li, X.; Dong, B.; Yuan, P. Electroacupuncture Alleviates Osteoarthritis by Suppressing NLRP3 Inflammasome Activation in Guinea Pigs. Evid.-Based Complement. Alternat. Med. 2020, 2020, 5476064. [Google Scholar]
- Spittler, A.P.; Afzali, M.F.; Martinez, R.B.; Culver, L.A.; Leavell, S.E.; Timkovich, A.E.; Sanford, J.L.; Story, M.R.; Santangelo, K.S. Evaluation of electroacupuncture for symptom modification in a rodent model of spontaneous osteoarthritis. Acupunct. Med. 2021, 39, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakamura, H.; Ozawa, H.; Hashimoto, S.; Iijima, N.; Higo, S.; Watanabe, H.; Mochizuki, Y.; Takai, S. Effectiveness of Radiofrequency Hyperthermia for Treating Cartilage in Guinea Pigs with Primary Osteoarthritis. Cartilage 2018, 9, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronesi, F.; Torricelli, P.; Giavaresi, G.; Sartori, M.; Cavani, F.; Setti, S.; Cadossi, M.; Ongaro, A.; Fini, M. In Vivo Effect of Two Different Pulsed Electromagnetic Field Frequencies on Osteoarthritis. J. Orthop. Res. 2014, 32, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kuo, S.M.; Tien, Y.C.; Shen, P.C.; Kuo, Y.W.; Huang, H.H. Steady Augmentation of Anti-Osteoarthritic Actions of Rapamycin by Liposome-Encapsulation in Collaboration with Low-Intensity Pulsed Ultrasound. Int. J. Nanomed. 2020, 15, 3771–3790. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Xing, L.; Tian, F. WNT signaling: A promising target for osteoarthritis therapy. Cell Commun. Signal. 2019, 17, 97. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, Y.; Han, R.; Cai, G.; He, C.; Wang, G.; Jia, D. T140 blocks the SDF-1/CXCR4 signaling pathway and prevents cartilage degeneration in an osteoarthritis disease model. PLoS ONE 2017, 12, e0176048. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Moore, D.C.; Li, Y.; Zhang, G.; Wei, X.; Lee, J.K.; Wei, L. Attenuation of osteoarthritis via blockade of the SDF-1/CXCR4 signaling pathway. Arthritis Res. Ther. 2012, 14, R177. [Google Scholar] [CrossRef] [Green Version]
- Wallace, I.J.; Bendele, A.M.; Riew, G.; Frank, E.H.; Hung, H.H.; Holowka, N.B.; Bolze, A.S.; Venable, E.M.; Yegian, A.K.; Dingwall, H.L.; et al. Physical inactivity and knee osteoarthritis in guinea pigs. Osteoarthr. Cartil. 2019, 27, 1721–1728. [Google Scholar] [CrossRef]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 3–6. [Google Scholar]
- Yu, D.; Xu, J.; Liu, F.; Wang, X.; Mao, Y.; Zhu, Z. Subchondral bone changes and the impacts on joint pain and articular cartilage degeneration in osteoarthritis. Clin. Exp. Rheumatol. 2016, 34, 929–934. [Google Scholar]
- Burrage, P.S.; Brinckerhoff, C.E. Molecular targets in osteoarthritis: Metalloproteinases and their inhibitors. Curr. Drug Targets 2007, 8, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 2004, 427, S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Giavaresi, G.; Maglio, M.; Scotto d’Abusco, A.; Politi, L.; Scandurra, R.; Olivotto, E.; Grigolo, B.; Borzì, R.M.; Fini, M. Chondroprotective activity of N-acetyl phenylalanine glucosamine derivative on knee joint structure and inflammation in a murine model of osteoarthritis. Osteoarthr. Cartil. 2017, 25, 589–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassallo, V.; Stellavato, A.; Cimini, D.; Pirozzi, A.V.A.; Alfano, A.; Cammarota, M.; Balato, G.; D’Addona, A.; Ruosi, C.; Schiraldi, C. Unsulfated biotechnological chondroitin by itself as well as in combination with high molecular weight hyaluronan improves the inflammation profile in osteoarthritis in vitro model. J. Cell. Biochem. 2021, 122, 1021–1036. [Google Scholar] [CrossRef]
- Denayer, T.; Stöhr, T.; Van Roy, M. Animal models in translational medicine: Validation and prediction. New Horiz. Transl. Med. 2014, 2, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Foster, N.C.; Hall, N.M.; Haj, A.J.E. Two-Dimensional and Three-Dimensional Cartilage Model Platforms for Drug Evaluation and High-Throughput Screening Assays. Tissue Eng. Part B Rev. 2022, 28, 421–436. [Google Scholar] [CrossRef]
- Morey-Holton, E.; Globus, R.K.; Kaplansky, A.; Durnova, G. The hindlimb unloading rat model: Literature overview, technique update and comparison with space flight data. Adv. Space Biol. Med. 2005, 10, 7–40. [Google Scholar] [PubMed]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Contartese, D.; Tschon, M.; De Mattei, M.; Fini, M. Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 3696. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar] [PubMed]
- Murphy, M.P.; Koepke, L.S.; Lopez, M.T.; Tong, X.; Ambrosi, T.H.; Gulati, G.S.; Marecic, O.; Wang, Y.; Ransom, R.C.; Hoover, M.Y.; et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020, 26, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Saaiq, M.; Ashraf, B. Modifying “Pico” Question into “Picos” Model for More Robust and Reproducible Presentation of the Methodology Employed in A Scientific Study. World J. Plast. Surg. 2017, 6, 390–392. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Salamanna, F.; Martini, L.; Fini, M. Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model. Int. J. Mol. Sci. 2022, 23, 7309. https://doi.org/10.3390/ijms23137309
Veronesi F, Salamanna F, Martini L, Fini M. Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model. International Journal of Molecular Sciences. 2022; 23(13):7309. https://doi.org/10.3390/ijms23137309
Chicago/Turabian StyleVeronesi, Francesca, Francesca Salamanna, Lucia Martini, and Milena Fini. 2022. "Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model" International Journal of Molecular Sciences 23, no. 13: 7309. https://doi.org/10.3390/ijms23137309
APA StyleVeronesi, F., Salamanna, F., Martini, L., & Fini, M. (2022). Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model. International Journal of Molecular Sciences, 23(13), 7309. https://doi.org/10.3390/ijms23137309







