Interleukin 15 in Cell-Based Cancer Immunotherapy
Abstract
:1. Introduction
2. Biology of IL-15 and Its Receptor
3. Functionality of IL-15
4. Administration of IL-15 and Its Derivatives for Cancer Immunotherapy
4.1. hetIL-15 (NIZ985)
4.2. hetIL-15Fc
4.3. N-803 (ALT-803)
4.4. RLI
4.5. NKTR-255
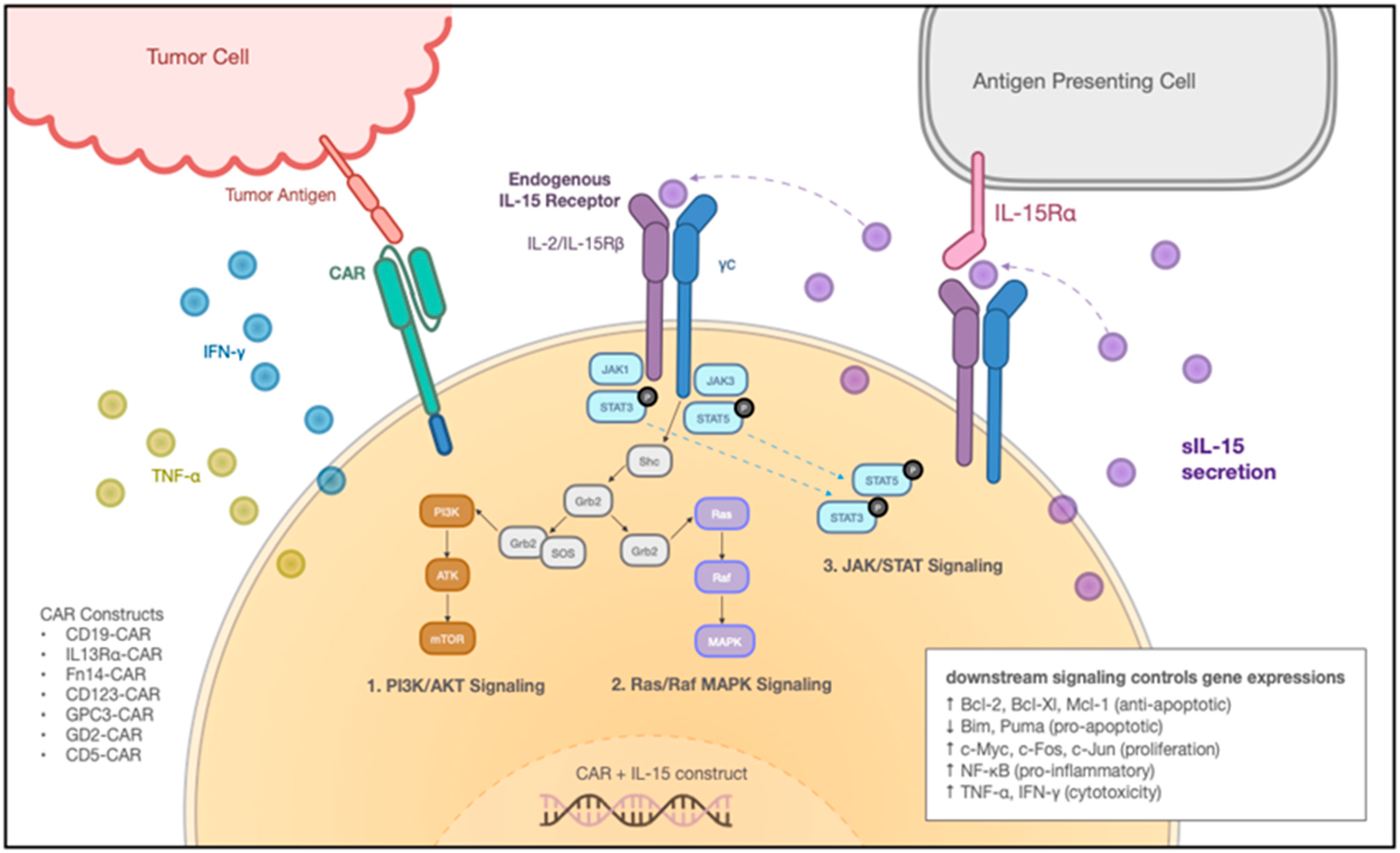
5. Adoptive Transfer of IL-15-Armored Immune Cells for Cancer Immunotherapy
5.1. IL-15-Armored CAR T Cells
5.2. IL-15-Armed NK Cells
5.3. IL-15 Armored Unconventional T Cells
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Rohaan, M.W.; Wilgenhof, S.; Haanen, J. Adoptive cellular therapies: The current landscape. Virchows Arch. 2019, 474, 449–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leick, M.B.; Maus, M.V.; Frigault, M.J. Clinical Perspective: Treatment of Aggressive B Cell Lymphomas with FDA-Approved CAR-T Cell Therapies. Mol. Ther. 2021, 29, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lundqvist, A. Immunomodulatory Effects of IL-2 and IL-15; Implications for Cancer Immunotherapy. Cancers 2020, 12, 3586. [Google Scholar] [CrossRef] [PubMed]
- Sim, G.C.; Radvanyi, L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Budagian, V.; Bulanova, E.; Paus, R.; Bulfone-Paus, S. IL-15/IL-15 receptor biology: A guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006, 17, 259–280. [Google Scholar] [CrossRef]
- Read, K.A.; Powell, M.D.; McDonald, P.W.; Oestreich, K.J. IL-2, IL-7, and IL-15: Multistage regulators of CD4(+) T helper cell differentiation. Exp. Hematol. 2016, 44, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Jin, L.; Wang, F.; Zhang, Y.; Liu, B.; Zhao, T. Chimeric antigen receptor T (CAR-T) cells expanded with IL-7/IL-15 mediate superior antitumor effects. Protein Cell 2019, 10, 764–769. [Google Scholar] [CrossRef] [Green Version]
- Cieri, N.; Camisa, B.; Cocchiarella, F.; Forcato, M.; Oliveira, G.; Provasi, E.; Bondanza, A.; Bordignon, C.; Peccatori, J.; Ciceri, F.; et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013, 121, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Ikemizu, S.; Chirifu, M.; Davis, S.J. IL-2 and IL-15 signaling complexes: Different but the same. Nat. Immunol. 2012, 13, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A. Twelve immunotherapy drugs that could cure cancers. Immunol. Rev. 2008, 222, 357–368. [Google Scholar] [CrossRef]
- Van den Bergh, J.M.J.; Smits, E.L.J.M.; Versteven, M.; De Reu, H.; Berneman, Z.N.; Van Tendeloo, V.F.I.; Lion, E. Characterization of Interleukin-15-Transpresenting Dendritic Cells for Clinical Use. J. Immunol. Res. 2017, 2017, 1975902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldmann, T.A.; Dubois, S.; Miljkovic, M.D.; Conlon, K.C. IL-15 in the Combination Immunotherapy of Cancer. Front. Immunol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Stravokefalou, V.; Stellas, D.; Karaliota, S.; Felber, B.K.; Pavlakis, G.N. Heterodimeric IL-15 in Cancer Immunotherapy. Cancers 2021, 13, 837. [Google Scholar] [CrossRef]
- Hurton, L.V.; Singh, H.; Najjar, A.M.; Switzer, K.C.; Mi, T.; Maiti, S.; Olivares, S.; Rabinovich, B.; Huls, H.; Forget, M.A.; et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. USA 2016, 113, E7788–E7797. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Grabstein, K.H.; Eisenman, J.; Shanebeck, K.; Rauch, C.; Srinivasan, S.; Fung, V.; Beers, C.; Richardson, J.; Schoenborn, M.A.; Ahdieh, M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994, 264, 965–968. [Google Scholar] [CrossRef]
- Carson, W.E.; Giri, J.G.; Lindemann, M.J.; Linett, M.L.; Ahdieh, M.; Paxton, R.; Anderson, D.; Eisenmann, J.; Grabstein, K.; Caligiuri, M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994, 180, 1395–1403. [Google Scholar] [CrossRef]
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.M.; Johnson, L.; Glaccum, M.B.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Valentine, V.; Kirstein, M.N.; Shapiro, D.N.; Morris, S.W. Chromosomal assignment and genomic structure of Il15. Genomics 1995, 25, 701–706. [Google Scholar] [CrossRef]
- Leonard, W.J. Cytokines and immunodeficiency diseases. Nat. Rev. Immunol. 2001, 1, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochman, Y.; Spolski, R.; Leonard, W.J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.G.; Ahdieh, M.; Eisenman, J.; Shanebeck, K.; Grabstein, K.; Kumaki, S.; Namen, A.; Park, L.S.; Cosman, D.; Anderson, D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994, 13, 2822–2830. [Google Scholar] [CrossRef]
- Lorenzen, I.; Dingley, A.J.; Jacques, Y.; Grotzinger, J. The structure of the interleukin-15 alpha receptor and its implications for ligand binding. J. Biol. Chem. 2006, 281, 6642–6647. [Google Scholar] [CrossRef] [Green Version]
- Giri, J.G.; Kumaki, S.; Ahdieh, M.; Friend, D.J.; Loomis, A.; Shanebeck, K.; DuBose, R.; Cosman, D.; Park, L.S.; Anderson, D.M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995, 14, 3654–3663. [Google Scholar] [CrossRef]
- Dubois, S.; Mariner, J.; Waldmann, T.A.; Tagaya, Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 2002, 17, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Mortier, E.; Bernard, J.; Plet, A.; Jacques, Y. Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. J. Immunol. 2004, 173, 1681–1688. [Google Scholar] [CrossRef] [Green Version]
- Desbois, M.; Béal, C.; Charrier, M.; Besse, B.; Meurice, G.; Cagnard, N.; Jacques, Y.; Béchard, D.; Cassard, L.; Chaput, N. IL-15 superagonist RLI has potent immunostimulatory properties on NK cells: Implications for antimetastatic treatment. J. Immunother. Cancer 2020, 8, e000632. [Google Scholar] [CrossRef]
- Mortier, E.; Bernard, J.; Plet, A.; Jacques, Y. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J. Biol. Chem. 2006, 281, 1612–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chertova, E.; Bergammaschi, C.; Chertov, O.; Sowder, R.; Bear, J.; Roser, J.D.; Beach, R.K.; Lifson, J.D.; Felber, B.K.; Pavlakis, G.N. Characterization and favorable in vivo properties of heterodimeric soluble IL-15.IL-15Ralpha cytokine compared to IL-15 monomer. J. Biol. Chem. 2013, 288, 18093–18103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Usiskin, I.M.; Bergamaschi, C.; Hanlon, D.J.; Edelson, R.L.; Justesen, S.; Pavlakis, G.N.; Flavell, R.A.; Fahmy, T.M. Configuration-dependent Presentation of Multivalent IL-15:IL-15Ralpha Enhances the Antigen-specific T Cell Response and Anti-tumor Immunity. J. Biol. Chem. 2016, 291, 8931–8950. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Xu, Y. IL-15R alpha-IgG1-Fc enhances IL-2 and IL-15 anti-tumor action through NK and CD8+ T cells proliferation and activation. J. Mol. Cell Biol. 2010, 2, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Bentebibel, S.E.; Diab, A. Cytokines in the Treatment of Melanoma. Curr. Oncol. Rep. 2021, 23, 83. [Google Scholar] [CrossRef]
- Han, K.P.; Zhu, X.; Liu, B.; Jeng, E.; Kong, L.; Yovandich, J.L.; Vyas, V.V.; Marcus, W.D.; Chavaillaz, P.A.; Romero, C.A.; et al. IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 2011, 56, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Marcus, W.D.; Xu, W.; Lee, H.I.; Han, K.; Egan, J.O.; Yovandich, J.L.; Rhode, P.R.; Wong, H.C. Novel human interleukin-15 agonists. J. Immunol. 2009, 183, 3598–3607. [Google Scholar] [CrossRef] [Green Version]
- Rhode, P.R.; Egan, J.O.; Xu, W.; Hong, H.; Webb, G.M.; Chen, X.; Liu, B.; Zhu, X.; Wen, J.; You, L.; et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol. Res. 2016, 4, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Maiti, M.; Hennessy, M.; Chang, T.; Kuo, P.; Addepalli, M.; Obalapur, P.; Sheibani, S.; Wilczek, J.; Pena, R.; et al. NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy. J. Immunother. Cancer 2021, 9, e002024. [Google Scholar] [CrossRef]
- Fiore, P.F.; Di Matteo, S.; Tumino, N.; Mariotti, F.R.; Pietra, G.; Ottonello, S.; Negrini, S.; Bottazzi, B.; Moretta, L.; Mortier, E.; et al. Interleukin-15 and cancer: Some solved and many unsolved questions. J. Immunother. Cancer 2020, 8, e001428. [Google Scholar] [CrossRef] [PubMed]
- Bouchaud, G.; Garrigue-Antar, L.; Sole, V.; Quemener, A.; Boublik, Y.; Mortier, E.; Perdreau, H.; Jacques, Y.; Plet, A. The exon-3-encoded domain of IL-15ralpha contributes to IL-15 high-affinity binding and is crucial for the IL-15 antagonistic effect of soluble IL-15Ralpha. J. Mol. Biol. 2008, 382, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.R.; Waldmann, T.A.; Kruhlak, M.J.; Dubois, S. Paracrine and transpresentation functions of IL-15 are mediated by diverse splice versions of IL-15Ralpha in human monocytes and dendritic cells. J. Biol. Chem. 2012, 287, 40328–40338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Hu, B.; Zhang, Y.; Song, Y.; Lin, D.; Liu, Y.; Mei, Y.; Sandikin, D.; Sun, W.; Zhang, M.; et al. An activation-induced IL-15 isoform is a natural antagonist for IL-15 function. Sci. Rep. 2016, 6, 25822. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, R.N.; Stover, L.; Dutcher, J.P. Managing toxicities of high-dose interleukin-2. Oncology 2002, 16 (Suppl. 13), 11–20. [Google Scholar]
- Buchbinder, E.I.; Gunturi, A.; Perritt, J.; Dutcher, J.; Aung, S.; Kaufman, H.L.; Ernstoff, M.S.; Miletello, G.P.; Curti, B.D.; Daniels, G.A.; et al. A retrospective analysis of High-Dose Interleukin-2 (HD IL-2) following Ipilimumab in metastatic melanoma. J. Immunother. Cancer 2016, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Conlon, K.; Watson, D.C.; Waldmann, T.A.; Valentin, A.; Bergamaschi, C.; Felber, B.K.; Peer, C.J.; Figg, W.D.; Potter, E.L.; Roederer, M.; et al. Phase I study of single agent NIZ985, a recombinant heterodimeric IL-15 agonist, in adult patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2021, 9, e003388. [Google Scholar] [CrossRef]
- Liquitaya-Montiel, A.J.; Mendoza, L. Dynamical Analysis of the Regulatory Network Controlling Natural Killer Cells Differentiation. Front. Physiol. 2018, 9, 1029. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.Y. Transcription Factors Associated With IL-15 Cytokine Signaling During NK Cell Development. Front. Immunol. 2021, 12, 610789. [Google Scholar] [CrossRef]
- Huntington, N.D.; Legrand, N.; Alves, N.L.; Jaron, B.; Weijer, K.; Plet, A.; Corcuff, E.; Mortier, E.; Jacques, Y.; Spits, H.; et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 2009, 206, 25–34. [Google Scholar] [CrossRef]
- Perera, P.Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The role of interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect. 2012, 14, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, R.J.; Macduff, B.M.; Eisenman, J.; Paxton, R.; Grabstein, K.H. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J. Immunol. 1995, 154, 483–490. [Google Scholar] [PubMed]
- Cassatella, M.A.; McDonald, P.P. Interleukin-15 and its impact on neutrophil function. Curr. Opin. Hematol. 2000, 7, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.; Lavastre, V.; Savoie, A.; Ratthe, C.; Saller, R.; Hostanska, K.; Girard, D. Modulation of interleukin-15-induced human neutrophil responses by the plant lectin Viscum album agglutinin-I. Clin. Immunol. 2001, 101, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, Y.; Burton, J.D.; Miyamoto, Y.; Waldmann, T.A. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J. 1996, 15, 4928–4939. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Smits, E.L.; Cools, N.; Goossens, H.; Berneman, Z.N.; Van Tendeloo, V.F. Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J. Transl. Med. 2009, 7, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, V.V.; Esposito, D.; Sumpter, T.L.; Broadt, T.L.; Hartley, J.; Knapp, G.C., 4th; Cheng, W.; Jiang, M.S.; Roach, J.M.; Yang, X.; et al. Clinical manufacturing of recombinant human interleukin 15. I. Production cell line development and protein expression in E. coli with stop codon optimization. Biotechnol. Prog. 2012, 28, 497–507. [Google Scholar] [CrossRef]
- Tang, F.; Zhao, L.T.; Jiang, Y.; Ba, D.; Cui, L.X.; He, W. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cell Mol. Immunol. 2008, 5, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Sneller, M.C.; Kopp, W.C.; Engelke, K.J.; Yovandich, J.L.; Creekmore, S.P.; Waldmann, T.A.; Lane, H.C. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood 2011, 118, 6845–6848. [Google Scholar] [CrossRef] [Green Version]
- Lugli, E.; Goldman, C.K.; Perera, L.P.; Smedley, J.; Pung, R.; Yovandich, J.L.; Creekmore, S.P.; Waldmann, T.A.; Roederer, M. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood 2010, 116, 3238–3248. [Google Scholar] [CrossRef]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C.; et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1525–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hangasky, J.A.; Waldmann, T.A.; Santi, D.V. Interleukin 15 Pharmacokinetics and Consumption by a Dynamic Cytokine Sink. Front. Immunol. 2020, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Hangasky, J.A.; Chen, W.; Dubois, S.P.; Daenthanasanmak, A.; Muller, J.R.; Reid, R.; Wladmann, T.A.; Santi, D.V. A very long-acting IL-15: Implications for the immunotherapy of cancer. J. Immunother. Cancer 2022, 10, e004104. [Google Scholar] [CrossRef]
- Isvoranu, G.; Surcel, M.; Munteanu, A.N.; Bratu, O.G.; Ionita-Radu, F.; Neagu, M.T.; Chiritoiu-Butnaru, M. Therapeutic potential of interleukin-15 in cancer (Review). Exp. Ther. Med. 2021, 22, 675. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Pandit, H.; Nagy, B.A.; Stellas, D.; Jensen, S.M.; Bear, J.; Cam, M.; Valentin, A.; Fox, B.A.; Felber, B.K.; et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-gamma, CXCL9 and CXCL10. J. Immunother. Cancer 2020, 8, e000599. [Google Scholar] [CrossRef]
- Watson, D.C.; Moysi, E.; Valentin, A.; Bergamaschi, C.; Devasundaram, S.; Fortis, S.P.; Bear, J.; Chertova, E.; Bess, J., Jr.; Sowder, R.; et al. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog. 2018, 14, e1006902. [Google Scholar]
- Zhang, M.; Yao, Z.; Dubois, S.; Ju, W.; Muller, J.R.; Waldmann, T.A. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 7513–7518. [Google Scholar] [CrossRef] [Green Version]
- Dubois, S.; Patel, H.J.; Zhang, M.; Waldmann, T.A.; Muller, J.R. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 2008, 180, 2099–2106. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.S.; Kwilas, A.R.; Xu, W.; Alter, S.; Jeng, E.K.; Wong, H.C.; Schlom, J.; Hodge, J.W. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 2016, 7, 16130–16145. [Google Scholar] [CrossRef]
- Felices, M.; Chu, S.; Kodal, B.; Bendzick, L.; Ryan, C.; Lenvik, A.J.; Boylan, K.; Wong, H.C.; Skubitz, A.; Miller, J.S.; et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol. Oncol. 2017, 145, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Morishima, C.; Velcheti, V.; Miller, J.S.; Lee, S.M.; Silk, A.W.; Holtan, S.G.; Lacroix, A.M.; Fling, S.P.; Kaiser, J.C.; et al. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 5552–5561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; DeFor, T.E.; et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Bessard, A.; Sole, V.; Bouchaud, G.; Quemener, A.; Jacques, Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol. Cancer Ther. 2009, 8, 2736–2745. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Perales, M.A.; Turtle, C.J.; Cairo, M.S.; Cowan, A.J.; Saeed, H.; Budde, L.E.; Tan, A.; Lee, Z.; Kai, K.; et al. Phase I study protocol: NKTR-255 as monotherapy or combined with daratumumab or rituximab in hematologic malignancies. Future Oncol. 2021, 17, 3549–3560. [Google Scholar] [CrossRef]
- Robinson, T.O.; Hegde, S.M.; Chang, A.; Gangadharan, A.; Rivas, S.; Madakamutil, L.; Zalevsky, J.; Miyazaki, T.; Schluns, K.S. NKTR-255 is a polymer-conjugated IL-15 with unique mechanisms of action on T and natural killer cells. J. Clin. Investig. 2021, 131, e144365. [Google Scholar] [CrossRef]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Geyer, M.B.; Brentjens, R.J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: Interpreting clinical outcomes to date. Blood 2016, 127, 3312–3320. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [Green Version]
- Hoyos, V.; Savoldo, B.; Quintarelli, C.; Mahendravada, A.; Zhang, M.; Vera, J.; Heslop, H.E.; Rooney, C.M.; Brenner, M.K.; Dotti, G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010, 24, 1160–1170. [Google Scholar] [CrossRef] [Green Version]
- Krenciute, G.; Prinzing, B.L.; Yi, Z.; Wu, M.F.; Liu, H.; Dotti, G.; Balyasnikova, I.V.; Gottschalk, S. Transgenic Expression of IL15 Improves Antiglioma Activity of IL13Ralpha2-CAR T Cells but Results in Antigen Loss Variants. Cancer Immunol. Res. 2017, 5, 571–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Zhang, Z.; Cai, L.; Tang, X.; Huang, J.; Yu, L.; Wang, G.; Zhong, K.; Cao, Y.; Liu, C.; et al. Fn14-targeted BiTE and CAR-T cells demonstrate potent preclinical activity against glioblastoma. Oncoimmunology 2021, 10, 1983306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, C.; Landoni, E.; Metelitsa, L.; Dotti, G.; Savoldo, B. Eradication of Neuroblastoma by T Cells Redirected with an Optimized GD2-Specific Chimeric Antigen Receptor and Interleukin-15. Clin. Cancer Res. 2019, 25, 2915–2924. [Google Scholar] [CrossRef] [Green Version]
- Batra, S.A.; Rathi, P.; Guo, L.; Courtney, A.N.; Fleurence, J.; Balzeau, J.; Shaik, R.S.; Nguyen, T.P.; Wu, M.F.; Bulsara, S.; et al. Glypican-3-Specific CAR T Cells Coexpressing IL15 and IL21 Have Superior Expansion and Antitumor Activity against Hepatocellular Carcinoma. Cancer Immunol. Res. 2020, 8, 309–320. [Google Scholar] [CrossRef]
- Sun, Y.; Su, Y.; Wang, Y.; Liu, N.; Li, Y.; Chen, J.; Qiao, Z.; Niu, J.; Hu, J.; Zhang, B.; et al. CD19 CAR-T Cells with Membrane-Bound IL-15 for B-Cell Acute Lymphoblastic Leukemia after Failure of CD19 and CD22 CAR-T Cells: Case Report. Front. Immunol. 2021, 12, 728962. [Google Scholar] [CrossRef]
- Feng, J.; Xu, H.; Cinquina, A.; Wu, Z.; Chen, Q.; Zhang, P.; Wang, X.; Shan, H.; Xu, L.; Zhang, Q.; et al. Treatment of Aggressive T Cell Lymphoblastic Lymphoma/leukemia Using Anti-CD5 CAR T Cells. Stem. Cell Rev. Rep. 2021, 17, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Ho, W.J.; Marple, A.; Ravich, J.W.; Tam, A.; Rahnama, R.; Fearnow, A.; Rietberg, C.; Yanik, S.; Solomou, E.E.; et al. Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities. J. Immunother. Cancer 2021, 9, e003894. [Google Scholar] [CrossRef]
- Christodoulou, I.; Ho, W.J.; Marple, A.; Ravich, J.W.; Tam, A.; Rahnama, R.; Fearnow, A.; Rietberg, C.; Yanik, S.; Solomou, E.E.; et al. Engineered Interleukin-15 Autocrine Signaling Invigorates Anti-CD123 CAR-NK Cells. Blood 2021, 138 (Suppl. 1), 2806. [Google Scholar] [CrossRef]
- Du, Z.; Ng, Y.Y.; Zha, S.; Wang, S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol. Ther. Methods Clin. Dev. 2021, 23, 582–596. [Google Scholar] [CrossRef]
- Makkouk, A.; Yang, X.C.; Barca, T.; Lucas, A.; Turkoz, M.; Wong, J.; Nishimoto, K.P.; Brodey, M.M.; Tabrizizad, M.; Gundurao, S.; et al. Off-the-shelf Vdelta1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J. Immunother. Cancer 2021, 9, e003441. [Google Scholar] [CrossRef]
- Heczey, A.; Courtney, A.N.; Montalbano, A.; Robinson, S.; Liu, K.; Li, M.; Ghatwai, N.; Dakhova, O.; Liu, B.; Raveh-Sadka, T.; et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: An interim analysis. Nat. Med. 2020, 26, 1686–1690. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Steel, J.C.; Zhang, M.; Morris, J.C.; Waldmann, T.A. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin. Cancer Res. 2010, 16, 6019–6028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.E.; Curtsinger, J.M.; Robertson, P.; Grzywacz, B.; Conlon, K.C.; Waldmann, T.A.; et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Petrus, M.; Bamford, R.; Shih, J.H.; Morris, J.C.; Janik, J.E.; Waldmann, T.A. Increased serum soluble IL-15Ralpha levels in T-cell large granular lymphocyte leukemia. Blood 2012, 119, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Badoual, C.; Bouchaud, G.; Agueznay, N.; Mortier, E.; Hans, S.; Gey, A.; Fernani, F.; Peyrard, S.; Puig, P.L.; Bruneval, P.; et al. The soluble alpha chain of interleukin-15 receptor: A proinflammatory molecule associated with tumor progression in head and neck cancer. Cancer Res. 2008, 68, 3907–3914. [Google Scholar] [CrossRef] [Green Version]
- Elpek, K.G.; Rubinstein, M.P.; Bellemare-Pelletier, A.; Goldrath, A.W.; Turley, S.J. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc. Natl. Acad. Sci. USA 2010, 107, 21647–21652. [Google Scholar] [CrossRef] [Green Version]
- Felices, M.; Lenvik, A.J.; McElmurry, R.; Chu, S.; Hinderlie, P.; Bendzick, L.; Geller, M.A.; Tolar, J.; Blazar, B.R.; Miller, J.S. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018, 3, e96219. [Google Scholar] [CrossRef]
- Romee, R.; Schneider, S.E.; Leong, J.W.; Chase, J.M.; Keppel, C.R.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Cytokine activation induces human memory-like NK cells. Blood 2012, 120, 4751–4760. [Google Scholar] [CrossRef] [Green Version]

| IL-15 Isoforms | Construct | Binding Targets/Affinity | Cell Specificity | References |
|---|---|---|---|---|
| sch rhIL-15 | Escherichia coli-derived IL-15 monomer | IL-15Rα, IL-2/IL-15Rβ/γc | Mature NK cells, NKT cells | [16,23,25] |
| Receptor-Linker-IL-15 (RLI) | Receptor-Linker-IL-15 fusion protein consisting of IL-15 linked to the sushi domain of IL-15Rα | IL-2/IL-15Rβ/γc | CD8+ T cells, NK cells, neutrophils | [16,30,31] |
| hetIL-15 (NIZ985) | IL-15 heterodimer with soluble IL-15Rα | IL-2/IL-15Rβ/γc | NK cells, CD8+ T cells | [32] |
| hetIL-15FC of human IgG1 (hetIL-15Fc) | A fully glycosylated form of hetIL-15 where the C-terminus of IL-15Rα is covalently linked to the Fc region of human IgG1 | IL-2/IL-15Rβ/γc, FcγRs | NK and memory-like CD8+ T | [16,33,34,35,36] |
| N-803 (ALT-803) | An IL-15 super-agonist comprised of an IL-15 variant with N72D mutant complexed with a human IL-15Rα sushi domain-Fc fusion protein | IL-2/IL-15Rβ/γc, FcγRs | NK, CD4+ and CD8+ memory T-cells | [37,38,39] |
| NKTR-255 | A polyethylene glycol-conjugate of rhIL-15 | IL-15Rα, IL-2/IL-15Rβ/γc | Mature NK, CD8+ T cells | [40] |
| Others | ||||
| IL-15RαΔ2 | Deletion of sushi domain; found on cell membrane despite not binding IL-15 | not binding to IL-15 | [41] | |
| IL-15RαΔ3 | Deletion of linker/hinge region | Moderate affinity to IL-15 | [42] | |
| IL-15RαIC3 | Directed to secretory pathway to function as a soluble secreted cytokine | High for IL-15 | [43] | |
| IL-15Δ6 | Induced during immune activation; interferes with IL-15/IL-15Rα super-agonist generation; no effect on T cell proliferation when complexed with IL-15Rα | Lower for IL-15Rα; complex does not bind IL-15Rβ/γc | [44] | |
| IL-15LSP | Directed to secretory pathway by association with golgi apparatus and endoplasmic reticulum | High for IL-15Rα | [8] | |
| IL-2 | IL-15 |
|---|---|
| Stimulation of Tregs due to IL2-Ra presence on Tregs | Lack of stimulation on Treg due to lack of interaction between Treg |
| Promotes AICD | Suppresses AICD |
| Increased apoptosis | Reduced apoptosis |
| Reduced T-cell persistence | Increased T-cell persistence |
| Higher toxicity profile | Lower toxicity profile |
| Correlated with CLS | Unassociated with CLS |
| Displays grade 3 and 4 AEs | Displays grade 1 and grade 2 AEs |
| Clinical Trial | Agent | Description | Malignancies | Institution |
|---|---|---|---|---|
| NCT01385423 | rhIL-15 | i.v. IL-15 in combination with haploidentical donor NK cells | AML; Myelodysplastic Syndrome | Masonic Cancer Center, University of Minnesota |
| NCT02395822 | rhIL-15 | s.c. IL-15 in combination with donor IL-15 activated NK cells | AML | Masonic Cancer Center, University of Minnesota |
| NCT04150562 | rhIL-15 | i.v. IL-15 in combination with avelumab | ccRCC | National Cancer Institute |
| NCT01727076 | rhIL-15 | Efficacy and dose escalation study of IL-15 administered s.c. | Advanced Melanoma; Kidney Cancer; NSCLC; SCHNC | National Cancer Institute |
| NCT01021059 | rhIL-15 | Safety and dose escalation study of Il-15 (i.v.) | Metastatic Malignant Melanoma; mRCC | National Cancer Institute |
| NCT01369888 | rhIL-15 | Safety and dosage study of IL-15 administered (ivb) following lymphodepleting chemotherapy and adoptive cell transfer of TILs | Metastatic Melanoma | National Cancer Institute |
| NCT01572493 | rhIL-15 | Continuous intravenous infusion (CIV) of IL-15 | Lymphoma; Carcinoma | National Cancer Institute |
| NCT03759184 | rhIL-15 | CIV of IL-15 in combination with obinutuzumab | CLL | National Cancer Institute |
| NCT04185220 | rhIL-15 | CIV of IL-15 in combination with mogamulizmab | Adult T-cell Lymphoma/Leukemia; Sezary Syndrome; Mycosis Fungoides | National Cancer Institute |
| NCT03388632 | rhIL-15 | Safety and dosage study of IL-15 (s.c.) in combination with checkpoint inhibitors nivolumab or ipilimumab or both | Metastatic Solid Tumors; Treatment-Refractory Cancers | National Cancer Institute |
| NCT02689453 | rhIL-15 | Dosage, safety, and efficacy study of IL-15 (s.c.) in combination with alemtuzumab | Relapsed T-cell Lymphoma; ATL; PTCL; CTCL; T-cell Prolymphocytic Leukemia | National Cancer Institute |
| NCT03905135 | rhIL-15 | CIV of IL-15 in combination with avelumab (Bacenico) | Peripheral T-cell Lymphoma; Mycosis Fungoides; Sezary Syndrome; ALCL | National Cancer Institute |
| NCT01875601 | rhIL-15 | Toxicity and dose escalation study of IL-15 in combination with NK cell infusion following lymphodepletion, analysis of pharmacokinetics in pediatric patients, and anti-tumor efficacy | Solid Tumors; Brain Tumors; Sarcoma; Pediatric Cancers; Neuroblastoma | National Cancer Institute |
| NCT02452268 | NIZ985 | Dose escalation and expansion study oNIZ985 and NIZ985 (s.c.) in combination with PDR001 | Metastatic and Advanced Solid Tumors | Novartis Pharmaceuticals |
| NCT02452268 | NIZ985 | Dose escalation and expansion study of NIZ985 and NIZ985 (s.c.) in combination with spartalizumab | In escalation: solid tumors and lymphoma In expansion: melanoma | Novartis Pharmaceuticals |
| NCT01885897 | N-803 | Dose escalation and extended study of ALT-803 (i.v.) | AML; ALL; MDS; Lymphoma;CLL; CML | Masonic Cancer Center, University of Minnesota |
| NCT02099539 | N-803 | Dose escalation study of N-803 (i.v. vs. s.c.) | Relapsed or Refractory MM | Altor BioScience |
| NCT03853317 | N-803 | N-803 in combination with off-the-shelf CD16 targeted NK cells (haNK) and avelumab | Merkel Cell Carcinoma | ImmunityBio Incorporated |
| NCT02989844 | N-803 | N-803 for the maintenance after allogeneic hematopoietic cell transplant (alloHCT) | AML; MDS | Masonic Cancer Center, University of Minnesota |
| NCT04847466 | N-803 | Efficacy study of irradiated PD-L1 CAR-NK cells combined with pembrolizumab and N-803 (s.c.) | GEJ; HNSCC | National Cancer Institute |
| NCT03022825 NCT02138734 | N-803 | BCG in combination with N-803 or BCG alone or N-803 alone administered via intravesical instillation | NMIBC | ImmunityBio Incorporated |
| NCT04247282 | N-803 | TriAd vaccine in combination with N-803 (s.c.) | Head and Neck Cancer Head and Neck Neoplasms | National Cancer Institute |
| NCT03493945 | N-803 | 2-. 3-, or 4 4-drug combinations of M7824, BN-brachyury vaccine, N-803 and Epacodstat | mPC; Prostate Cancer; Prostate Neoplasm; Solid Tumors | National Cancer Institute |
| NCT03520686 | N-803 | N-803 (s.c.) in combination with either pembrolizumab, carboplatin + nab-paclitaxel + pembrolizumab, or cisplatin + carboplatin + nab-paclitaxel + pembrolizumab | NSCLC | ImmunityBio Incorporated |
| NCT05096663 | N-803 | N-803 (s.c.) in combination with pembrolizumab in comparison to standard care therapy a | Advanced NSCCLC | Southwest Oncology Group |
| NCT04491955 | N-803 | N-803 (s.c.) in combination with CV301 vaccine, M7824, and NHS-IL12 | Small Bowel Cancers Colorectal Cancers | National Cancer Institute |
| NCT04927884 | N-803 | N-803 in combination with sacituzumab | Advanced Triple Negative Breast Cancer | ImmunityBio Incorporated |
| NCT04898543 | N-803 | N-803 (s.c.) in combination with memory-cytokine enriched NK (m-ceNK) cells | Metastatic Solid Tumors | ImmunityBio Incorporated |
| NCT04290546 | N-803 | N-803 (s.c.) in combination with cytokine-induced memory-like (CIML) NK-enriched cells | HNSCC | Dana-Farber Cancer Institute |
| NCT03003728 | N-803 | N-803 (s.c.) in combination with elotuzumab, melphalan, and expanded NK cell autologous stem cell transplantation | MM | University of Arkansas |
| NCT02559674 | N-803 | Dose escalation study of N-803 (s.c.) in combination with gemcitabine and nab-paclitaxel | Advanced Pancreatic Cancer | Altor BioScience |
| NCT03228667 | N-803 | PD-1/PD-L1 checkpoint inhibitors in combination with N-803 and subsequently combined with PD-L1t-haNK cell therapy in patients with prior treatment of PD-1/PD-L1 checkpoint inhibitors | NSCLC; SCLC; Urothelial Carcinoma; HNSCC; MCC; Melanoma; RCC; Gastric Cancer; Cervical Cancer; and others | ImmunityBio Incorporated |
| NCT04659629 | NL-201 | Safety study of NL-201 (i.v.) alone or in combination with pembrolizumab | Solid Tumor Cancers | Neoleukin Therapeutics Incorporated |
| NCT04136756 | NKTR-255 | NKTR-255 (i.v.) IL-15 receptor agonist alone or in combination with daratumumab or rituximab | MM; NHL; Indolent Non-Hodgkin Lymphoma | Nektar Theraputics |
| NCT05327530 | NKTR-255 | NKTR-255 (i.v.) IL-15 receptor agonist in combination with avelumab | Locally Advanced or Metastatic Urothelial Carcinoma | EMD Serono Research and Development Institute Incorporated |
| NCT04616196 | NKTR-255 | NKTR-255 (i.v.) IL-15 receptor agonist alone or in combination with cetuximab | HNSCC; CRC; cSCC; ASCC; Cerivcal Cancer | Nektar Theraputics |
| NCT05359211 | NKTR-255 | NKTR-255 (i.v.) IL-15 receptor agonist in combination with autologous CD19-CAR T cells | B-Cell Lymphoma | Fred Hutchinson Cancer Center |
| Clinical Trial | Agent | Description | Malignancies | Institution |
|---|---|---|---|---|
| NCT04377932 NCT05103631 | GPC3-CAR IL-15 armored T cells | GPC3-CAR T cells armored with IL-15 | Liver Cancer; RMS; Malignant Rhabdoid Tumor; Liposarcoma; Wilms Tumor; Yolk Sac Tumor | Baylor College of Medicine |
| NCT04715191 | GPC3-CAR IL-15 and IL-21 armored T cells | GPC3-CAR T cells armored with IL-15 and IL-21 | Liver Cancer; RMS; Malignant Rhabdoid Tumor; Liposarcoma; Wilms Tumor; Yolk Sac Tumor | Baylor College of Medicine |
| NCT04093648 | GPC3-CAR IL-15 and IL-15/IL-21 armored T cells (TEGAR) | Safety and dosage study of GPC3-CAR T cells co-expressing IL-15 or both IL-15 and IL-21 | HCC HBL | Baylor College of Medicine |
| NCT04844086 | CD19-mbIL15-CAR T cells | Safety, efficacy, and dosage study of RPM CD19 mbIL-15 CAR T cells | B-ALL; Relapsed/Refractory NHL; PMBCL; DLBCL; FL; MCL; High-grade B-Cell Lymphoma | Eden BioCell Ltd. |
| NCT05110742 | CD5 CAR IL-15 Transduced NK Cells | Dosage and efficacy study for CAR5/IL-15-transduced cord blood-derived NK cells | T-cell malignancies; MCL; CLL | M.D. Anderson Cancer Center |
| NCT05092451 | CAR.70/IL-15 transduced NK cells | Dosage and efficacy study for CAR70/IL-15-transduced cord blood-derived NK cells | B-cell lymphoma; MDS; AML | M.D. Anderson Cancer Center |
| NCT03774654 | CD19.CAR NKT (ANCHOR) cells | Safety, efficacy, and dose escalation study of allogeneic CD19 CAR NKT cells co-expressing IL-15 (ANCHOR) | Refractory B-NHL; Refractory B-Cell SLL; Relapsed Adult ALL; Relapsed CLL; Relapsed NHL | Baylor College of Medicine |
| NCT02652910 | IL-7/IL-15 armored CD19-CAR T cells | Efficacy and persistence study of IL-7/IL-15 armored CD19-CAR T cells | Recurrent Adult DLBCL; Recurrent FL; Recurrent MCL; Stage III & IV Adult DLBCL; Stage III & IV FL; Stage III & IV MCL | Xinqiao Hospital of Chongqing |
| NCT03721068 | iC9.GD2.CAR.IL-15 T cells | Safety and dosage study of GD2-CAR T cells co-expressing IL-15 and iC9 | NB; OS | University of North Carolina Lineberger Comprehensive Cancer Center |
| NCT04814004 | hCD19.IL15.CAR iNKT cells | Safety and efficacy study of hCD19.CAR invariant natural killer T cells | ALL; B-cell Lymphoma; CLL | Xuzhou Medical University |
| NCT03579927 | CD19-CD28-zeta-2A-iCasp9-IL15 NK cells | Toxicity and dosage study of CD19-CD28-zeta-2A-iC9-IL-15 NK cells | MCL; Recurrent/refractory DLBCL; Recurrent/refractory FL; Recurrent/refractory B-Cell NHL | M.D. Anderson Cancer Center |
| NCT03056339 | iC9/CAR.19/IL15-Transduced CB NK Cells | Efficacy and dosage study of umbilical and cord blood (CB) derived iC9/CAR.19/IL-15 transduced NK cells | B-Lymphoid Malignancies; ALL; CLL; NHL | M.D. Anderson Cancer Center |
| NCT05334329 | NK cells co-expressing PD-L1 and IL-15 | Safety, dosage, and persistence study of COH06 umbilical cord blood-derived NK cells co-expressing PD-L1 and IL-15 alone or in combination with atezolizumab | Advanced/metastatic/refractory NSCLC; Stage III, IIIA, IIIB, IIIC, IV, IVA, and IVB Lung Cancer | City of Hope Medical Center |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Husman, T.; Cen, X.; Tsao, T.; Brown, J.; Bajpai, A.; Li, M.; Zhou, K.; Yang, L. Interleukin 15 in Cell-Based Cancer Immunotherapy. Int. J. Mol. Sci. 2022, 23, 7311. https://doi.org/10.3390/ijms23137311
Zhou Y, Husman T, Cen X, Tsao T, Brown J, Bajpai A, Li M, Zhou K, Yang L. Interleukin 15 in Cell-Based Cancer Immunotherapy. International Journal of Molecular Sciences. 2022; 23(13):7311. https://doi.org/10.3390/ijms23137311
Chicago/Turabian StyleZhou, Yang, Tiffany Husman, Xinjian Cen, Tasha Tsao, James Brown, Aarushi Bajpai, Miao Li, Kuangyi Zhou, and Lili Yang. 2022. "Interleukin 15 in Cell-Based Cancer Immunotherapy" International Journal of Molecular Sciences 23, no. 13: 7311. https://doi.org/10.3390/ijms23137311
APA StyleZhou, Y., Husman, T., Cen, X., Tsao, T., Brown, J., Bajpai, A., Li, M., Zhou, K., & Yang, L. (2022). Interleukin 15 in Cell-Based Cancer Immunotherapy. International Journal of Molecular Sciences, 23(13), 7311. https://doi.org/10.3390/ijms23137311








