Effects of Combined Gentamicin and Furosemide Treatment on Cochlear Macrophages
Abstract
1. Introduction
2. Methods
2.1. Mice and Gentamicin/Furosemide (G/F) Treatment
2.2. ABR Measurement
2.3. Tissue-Processing, Image Acquisition and Processing
3. Results
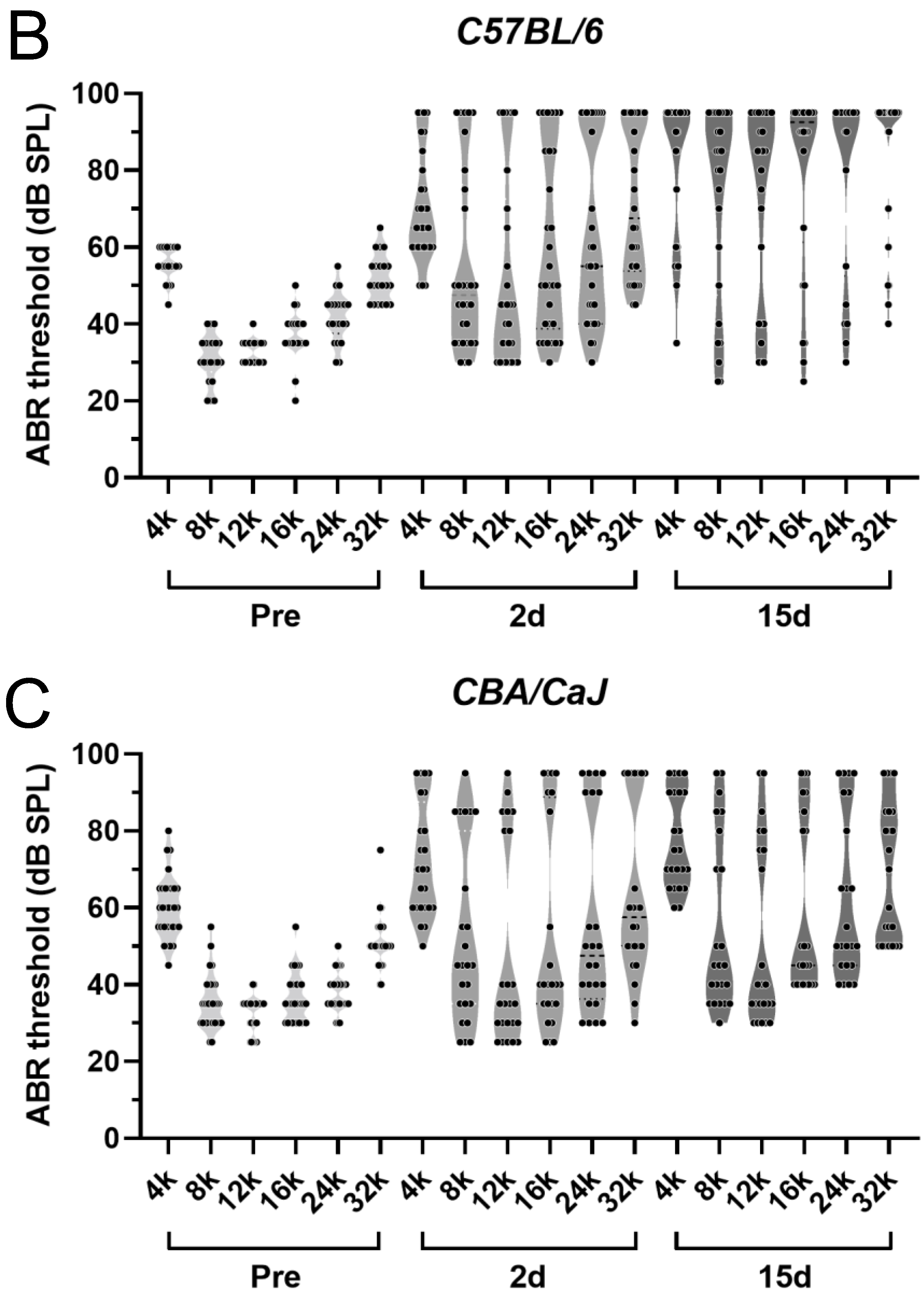
3.1. G/F Treatment Induced More ABR Threshold Shift in C57 BL/6 Mice
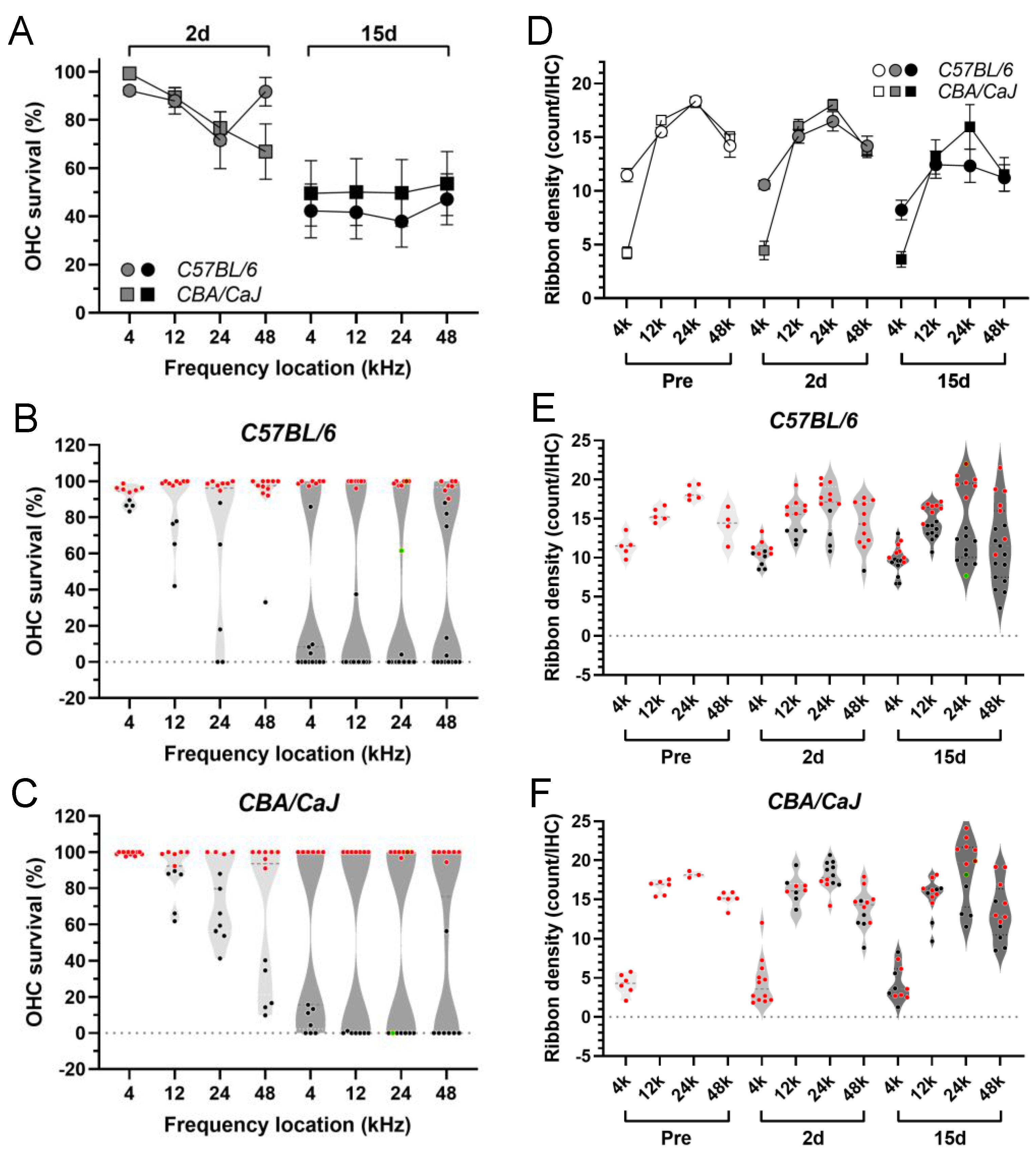
3.2. Morphological Analysis of ABR Correlates in the Cochlea
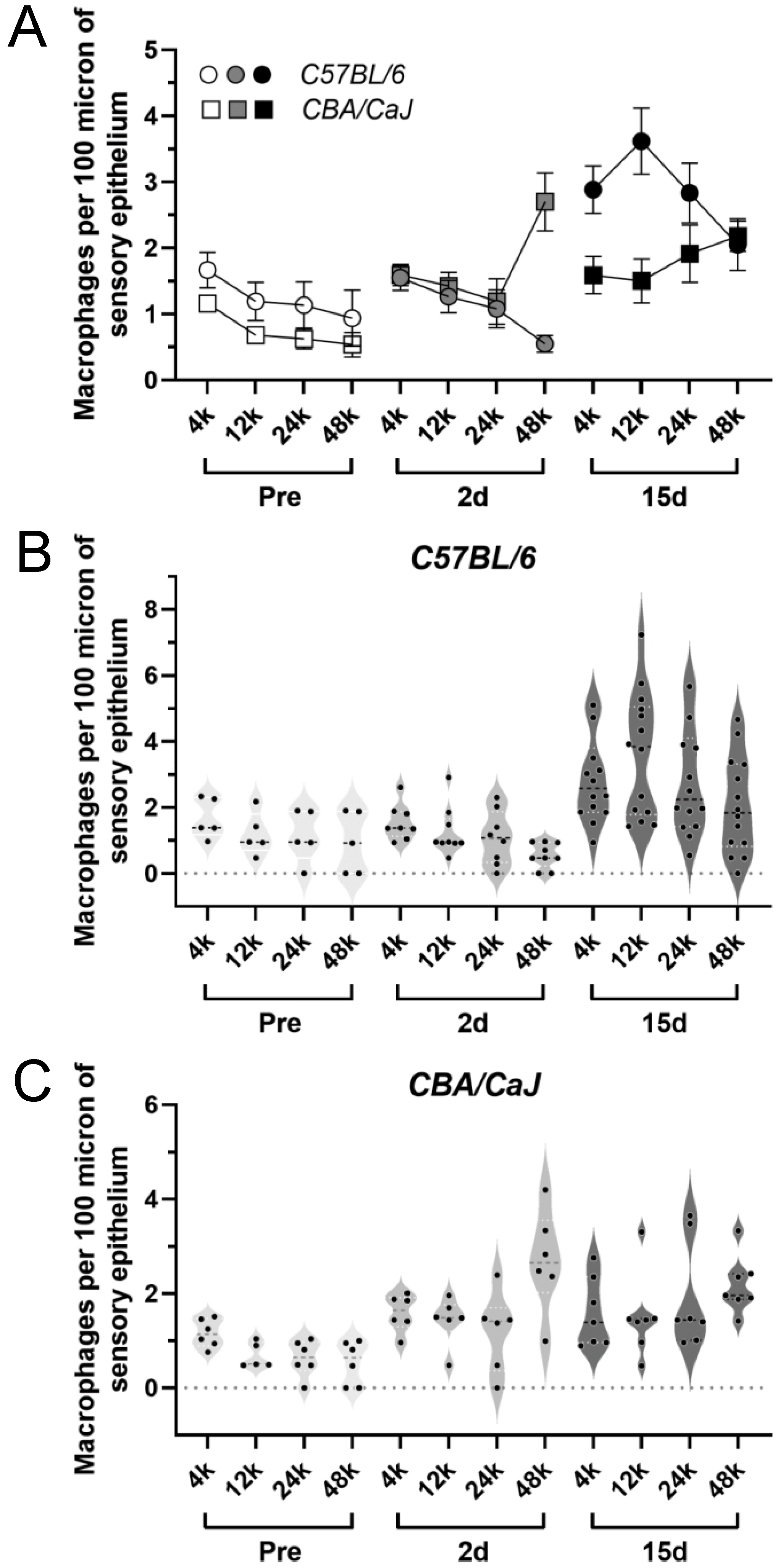
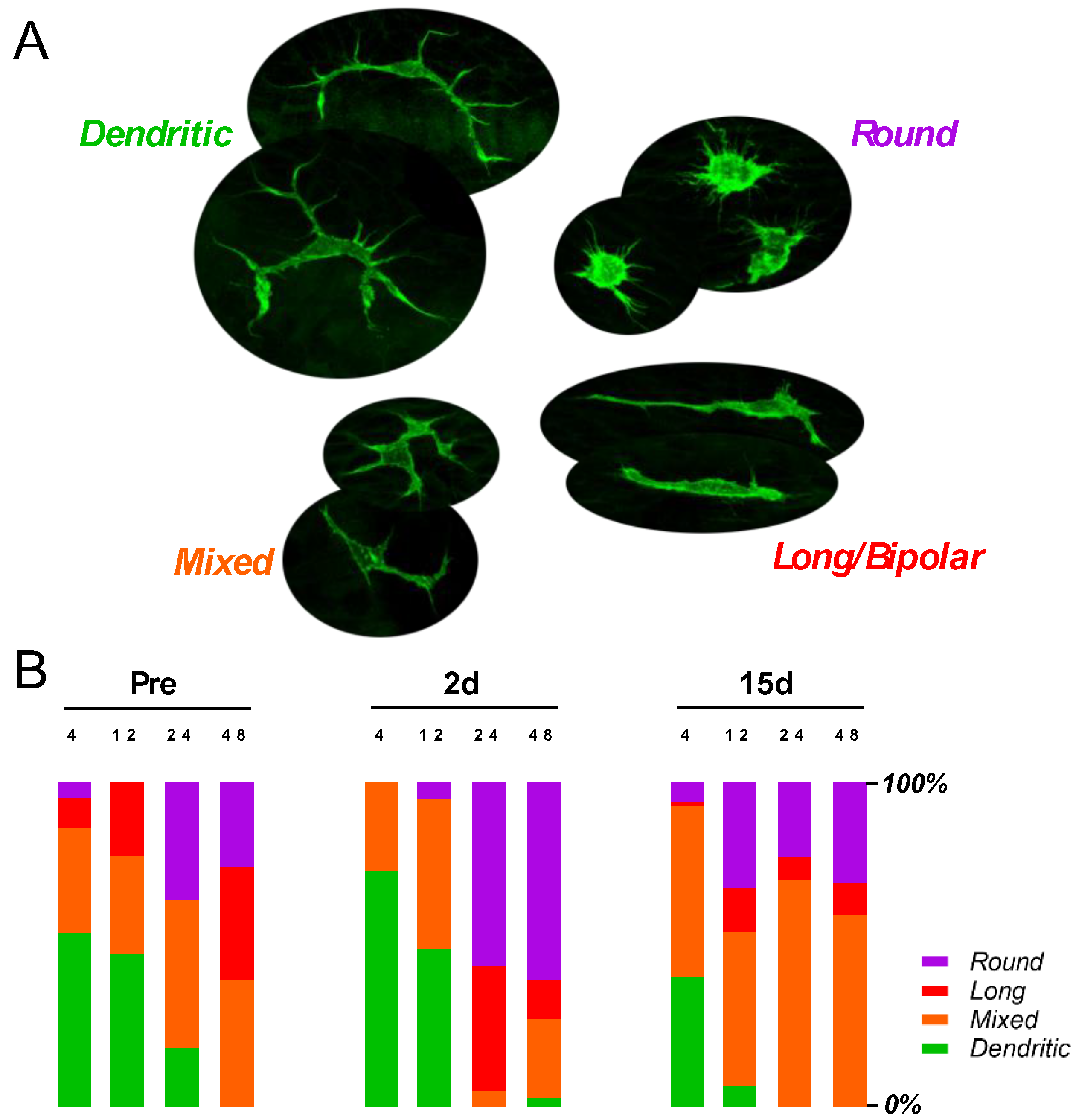
3.3. Elevated Macrophage Activity after G/F Treatment
3.4. Elevated Macrophage Activity Associated with Cochlear Damage in B6 Mice
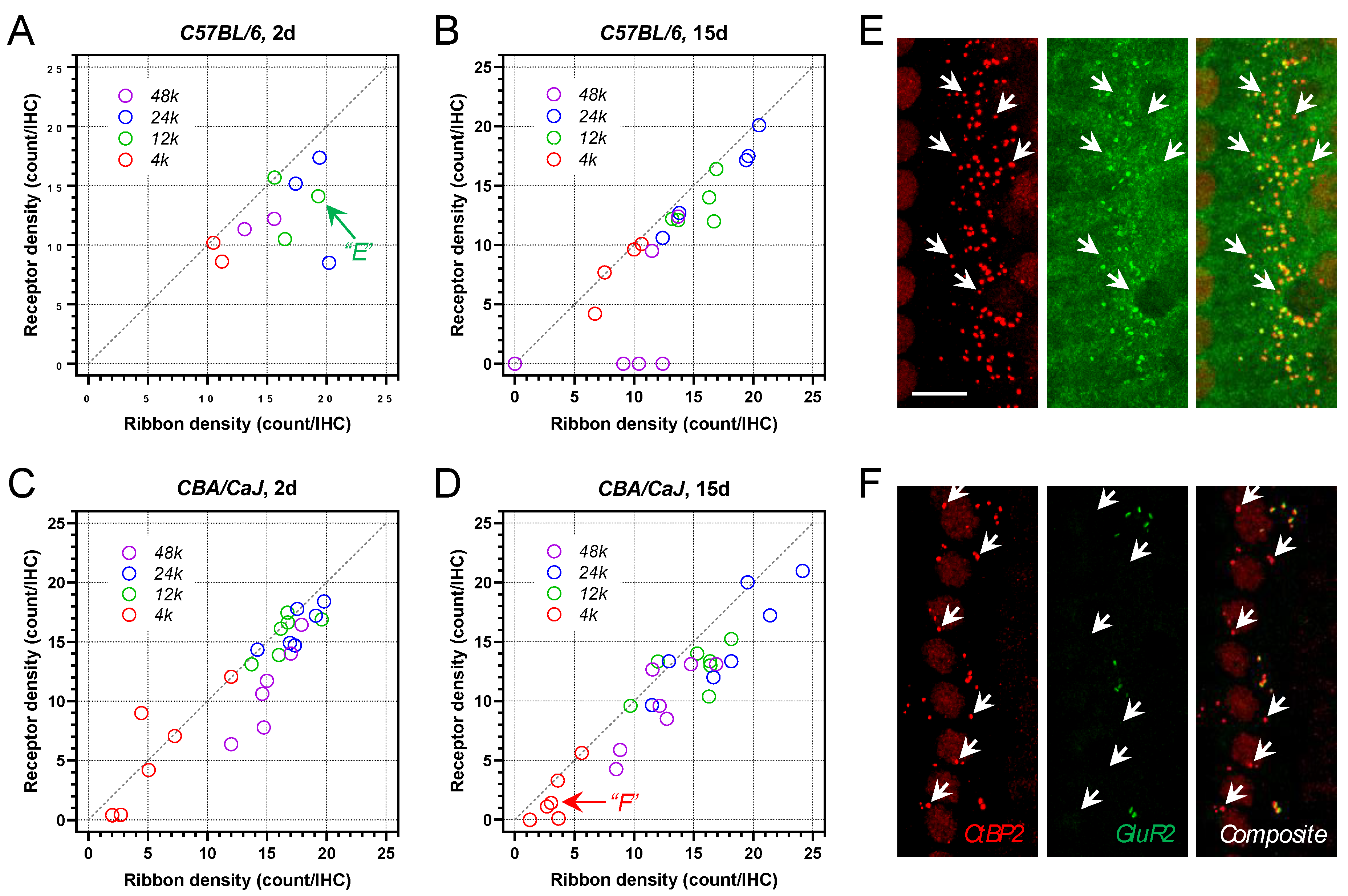
3.5. Postsynaptic AMPA Receptor Density
4. Discussion
4.1. G/F Dosing Protocol and Cochlear Damage
4.2. Cochlear Inflammation and Macrophage Activation
4.3. Differential Damage between CBA and B6
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Prayle, A.; Watson, A.; Fortnum, H.; Smyth, A. Side effects of aminoglycosides on the kidney, ear and balance in cystic fibrosis. Thorax 2010, 65, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; He, W.; Yang, W.; Hetrick, A.P.; Gonzalez, J.G.; Sargsyan, L.; Wu, H.; Jung, T.T.K.; Li, H. Intratympanic Lipopolysaccharide Elevates Systemic Fluorescent Gentamicin Uptake in the Cochlea. Laryngoscope 2021, 131, E2573–E2582. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Quintanilla-Dieck, L.; Jiang, M.; Liu, J.; Urdang, Z.D.; Allensworth, J.J.; Cross, C.P.; Li, H.; Steyger, P.S. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci. Transl. Med. 2015, 7, 298ra118. [Google Scholar] [CrossRef] [PubMed]
- Kraft, S.; Hsu, C.; Brough, D.E.; Staecker, H. Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. Laryngoscope 2013, 123, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, P.J.; Wise, A.K.; Flynn, B.O.; Nayagam, B.A.; Richardson, R.T. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS ONE 2014, 9, e102077. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, Z.; Li, X.; Zhou, Y.; Hu, Z. Generation of new hair cells by DNA methyltransferase (Dnmt) inhibitor 5-azacytidine in a chemically-deafened mouse model. Sci. Rep. 2019, 9, 7997. [Google Scholar] [CrossRef]
- Su, G.L.; Colesa, D.J.; Pfingst, B.E. Effects of deafening and cochlear implantation procedures on postimplantation psychophysical electrical detection thresholds. Hear. Res. 2008, 241, 64–72. [Google Scholar] [CrossRef][Green Version]
- Sunwoo, W.; Delgutte, B.; Chung, Y. Chronic Bilateral Cochlear Implant Stimulation Partially Restores Neural Binaural Sensitivity in Neonatally-Deaf Rabbits. J. Neurosci. 2021, 41, 3651–3664. [Google Scholar] [CrossRef]
- Taylor, R.R.; Nevill, G.; Forge, A. Rapid hair cell loss: A mouse model for cochlear lesions. J. Assoc. Res. Otolaryngol. 2008, 9, 44–64. [Google Scholar] [CrossRef]
- Ding, D.; Liu, H.; Qi, W.; Jiang, H.; Li, Y.; Wu, X.; Sun, H.; Gross, K.; Salvi, R. Ototoxic effects and mechanisms of loop diuretics. J. Otol. 2016, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Mukherjea, D.; Sheehan, K.; Jajoo, S.; Rybak, L.P.; Ramkumar, V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell. Death Dis. 2011, 2, e180. [Google Scholar] [CrossRef] [PubMed]
- Forge, A.; Schacht, J. Aminoglycoside antibiotics. Audiol. Neurootol. 2020, 5, 3–22. [Google Scholar] [CrossRef]
- Hirose, K.; Sato, E. Comparative analysis of combination kanamycin-furosemide versus kanamycin alone in the mouse cochlea. Hear. Res. 2011, 272, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.D.; Zhang, C.; Hu, B.H. Lower level noise exposure that produces only TTS modulates the immune homeostasis of cochlear macrophages. J. Neuroimmunol. 2018, 323, 152–166. [Google Scholar] [CrossRef]
- Ladrech, S.; Wang, J.; Simonneau, L.; Puel, J.L.; Lenoir, M. Macrophage contribution to the response of the rat organ of Corti to amikacin. J. Neurosci. Res. 2007, 85, 1970–1979. [Google Scholar] [CrossRef]
- Sato, E.; Shick, H.E.; Ransohoff, R.M.; Hirose, K. Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury. J. Assoc. Res. Otolaryngol. 2010, 11, 223–234. [Google Scholar] [CrossRef]
- Frye, M.D.; Ryan, A.F.; Kurabi, A. Inflammation associated with noise-induced hearing loss. J. Acoust. Soc. Am. 2019, 146, 4020. [Google Scholar] [CrossRef]
- Kaur, T.; Zamani, D.; Tong, L.; Rubel, E.W.; Ohlemiller, K.K.; Hirose, K.; Warchol, M.E. Fractalkine Signaling Regulates Macrophage Recruitment into the Cochlea and Promotes the Survival of Spiral Ganglion Neurons after Selective Hair Cell Lesion. J. Neurosci. 2015, 35, 15050–15061. [Google Scholar] [CrossRef]
- Kaur, T.; Ohlemiller, K.K.; Warchol, M.E. Genetic disruption of fractalkine signaling leads to enhanced loss of cochlear afferents following ototoxic or acoustic injury. J. Comp. Neurol. 2018, 526, 824–835. [Google Scholar] [CrossRef]
- Tan, W.J.; Thorne, P.R.; Vlajkovic, S.M. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem. Cell. Biol. 2016, 146, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Santamaria, V.; Alvarado, J.C.; Melgar-Rojas, P.; Gabaldon-Ull, M.C.; Miller, J.M.; Juiz, J.M. The Role of Glia in the Peripheral and Central Auditory System Following Noise Overexposure: Contribution of TNF-alpha and IL-1beta to the Pathogenesis of Hearing Loss. Front. Neuroanat. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Edderkaoui, B.; Sargsyan, L.; Hetrick, A.; Li, H. Deficiency of Duffy Antigen Receptor for Chemokines Ameliorated Cochlear Damage From Noise Exposure. Front. Mol. Neurosci. 2018, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.B.; Zuo, J. The Contribution of Immune Infiltrates to Ototoxicity and Cochlear Hair Cell Loss. Front. Cell. Neurosci. 2017, 11, 106. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Lomberk, G.; Urrutia, R.A.; Kalinec, F. Resolution of Cochlear Inflammation: Novel Target for Preventing or Ameliorating Drug-, Noise- and Age-related Hearing Loss. Front. Cell Neurosci. 2017, 11, 192. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’amico, R.; Evangelista, M.; Peli, A.; Peritore, A.F.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef]
- Sha, S.H.; Qiu, J.H.; Schacht, J. Aspirin to prevent gentamicin-induced hearing loss. N. Engl. J. Med. 2006, 354, 1856–1857. [Google Scholar] [CrossRef]
- Behnoud, F.; Davoudpur, K.; Goodarzi, M.T. Can aspirin protect or at least attenuate gentamicin ototoxicity in humans? Saudi Med. J. 2009, 30, 1165–1169. [Google Scholar]
- Harrop-Jones, A.; Wang, X.; Fernandez, R.; Dellamary, L.; Ryan, A.F.; Lebel, C.; Piu, F. The Sustained-Exposure Dexamethasone Formulation OTO-104 Offers Effective Protection against Noise-Induced Hearing Loss. Audiol. Neurootol. 2016, 21, 12–21. [Google Scholar] [CrossRef]
- Sargsyan, L.; Hetrick, A.P.; Gonzalez, J.G.; Leek, M.R.; Martin, G.K.; Li, H. Effects of combined gentamicin and furosemide treatment on cochlear ribbon synapses. Neurotoxicology 2021, 84, 73–83. [Google Scholar] [CrossRef]
- Hong, J.; Chen, Y.; Zhang, Y.; Li, J.; Ren, L.; Yang, L.; Shi, L.; Li, A.; Zhang, T.; Li, H.; et al. N-Methyl-D-Aspartate Receptors Involvement in the Gentamicin-Induced Hearing Loss and Pathological Changes of Ribbon Synapse in the Mouse Cochlear Inner Hair Cells. Neural Plast. 2018, 2018, 3989201. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Sha, S.H.; Mclaren, J.D.; Kawamoto, K.; Raphael, Y.; Schacht, J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear. Res. 2001, 158, 165–178. [Google Scholar] [CrossRef]
- Reiss, L.A.; Stark, G.; Nguyen-Huynh, A.T.; Spear, K.A.; Zhang, H.; Tanaka, C.; Li, H. Morphological correlates of hearing loss after cochlear implantation and electro-acoustic stimulation in a hearing-impaired Guinea pig model. Hear. Res. 2015, 327, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Sheth, S.; Sheehan, K.; Dhukhwa, A.; Al Aameri, R.F.H.; Mamillapalli, C.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Oral Administration of Caffeine Exacerbates Cisplatin-Induced Hearing Loss. Sci. Rep. 2019, 9, 9571. [Google Scholar] [CrossRef]
- Bako, P.; Gerlinger, I.; Wolpert, S.; Muller, M.; Lowenheim, H. The ototoxic effect of locally applied kanamycin and furosemide in guinea pigs. J. Neurosci. Methods 2022, 372, 109527. [Google Scholar] [CrossRef]
- Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Trovato Salinaro, A.; Maiolino, L.; Calabrese, V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2019, 21, 284. [Google Scholar] [CrossRef]
- Hough, K.; Verschuur, C.A.; Cunningham, C.; Newman, T.A. Macrophages in the cochlea; an immunological link between risk factors and progressive hearing loss. Glia 2022, 70, 219–238. [Google Scholar] [CrossRef]
- Okano, T.; Nakagawa, T.; Kita, T.; Kada, S.; Yoshimoto, M.; Nakahata, T.; Ito, J. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J. Neurosci. Res. 2008, 86, 1758–1767. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, C.; Frye, M.; Yang, W.; Ding, D.; Sharma, A.; Guo, W.; Hu, B.H. Differential fates of tissue macrophages in the cochlea during postnatal development. Hear. Res. 2018, 365, 110–126. [Google Scholar] [CrossRef]
- Biber, K.; Laurie, D.J.; Berthele, A.; Sommer, B.; Tolle, T.R.; Gebicke-Harter, P.J.; Van Calker, D.; Boddeke, H.W. Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J. Neurochem. 1999, 72, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Komune, S.; Tono, T.; Yamasaki, M.; Haruta, A.; Kato, E. A role of glutamate in drug-induced ototoxicity: In vivo microdialysis study combined with on-line enzyme fluorometric detection of glutamate in the guinea pig cochlea. Brain Res. 2000, 852, 492–495. [Google Scholar] [CrossRef]
- Hickox, A.E.; Larsen, E.; Heinz, M.G.; Shinobu, L.; Whitton, J.P. Translational issues in cochlear synaptopathy. Hear. Res. 2017, 349, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hickman, T.T.; Hashimoto, K.; Liberman, L.D.; Liberman, M.C. Synaptic migration and reorganization after noise exposure suggests regeneration in a mature mammalian cochlea. Sci. Rep. 2020, 10, 19945. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, K.A.; Guo, D.; Micucci, S.; De Gruttola, V.; Liberman, M.C.; Kujawa, S.G. Noise-induced Cochlear Synaptopathy with and Without Sensory Cell Loss. Neuroscience 2020, 427, 43–57. [Google Scholar] [CrossRef]



| Comparison | p Value | p Value Summary | F (DFn, DFd) | n |
|---|---|---|---|---|
| Pre-treatment, B6 vs CBA | 0.5456 | n.s. | F (1, 300) = 0.3661 | 21 vs 31 |
| 2 d posttreatment, B6 vs CBA | 0.0462 | * | F (1, 312) = 4.006 | 30 vs 24 |
| 15 d posttreatment, B6 vs CBA | <0.0001 | **** | F (1, 282) = 39.49 | 26 vs 23 |
| B6, pre- vs 2 d posttreatment | <0.0001 | **** | F (1, 294) = 105.6 | 21 vs 30 |
| CBA, pre- vs 2 d posttreatment | <0.0001 | **** | F (1, 318) = 72.56 | 31 vs 24 |
| B6, 2 d vs 15 d posttreatment | <0.0001 | **** | F (1, 324) = 43.97 | 30 vs 26 |
| CBA, 2 d vs 15 d posttreatment | 0.0453 | * | F (1, 270) = 4.046 | 24 vs 23 |
| Comparison | p Value | p Value Summary | F (DFn, DFd) | n |
|---|---|---|---|---|
| B6, pre- vs 2 d posttreatment | 0.0497 | * | F (1, 57) = 4.020 | 5 vs 12 |
| B6, pre- vs 15 d posttreatment | 0.0048 | ** | F (1, 90) = 8.358 | 5 vs 20 |
| B6, 2 d vs 15 d posttreatment | 0.0027 | ** | F (1, 117) = 9.384 | 12 vs 20 |
| CBA, pre- vs 2 d posttreatment | 0.44 | n.s. | F (1, 61) = 0.6041 | 6 vs 12 |
| CBA, pre- vs 15 d posttreatment | 0.0451 | * | F (1, 65) = 4.175 | 6 vs 14 |
| CBA, 2 d vs 15 d posttreatment | 0.0123 | * | F (1, 92) = 6.528 | 12 vs 14 |
| Comparison | p Value | p Value Summary | F (DFn, DFd) | n |
|---|---|---|---|---|
| Pre-treatment, B6 vs CBA | 0.0101 | * | F (1, 35) = 7.396 | 5 vs 6 |
| 2 d posttreatment, B6 vs CBA | 0.0015 | ** | F (1, 50) = 11.36 | 9 vs 6 |
| 15 d posttreatment, B6 vs CBA | 0.0019 | ** | F (1, 76) = 10.30 | 14 vs 7 |
| B6, pre- vs 2 d posttreatment | 0.5306 | n.s. | F (1, 46) = 0.3992 | 5 vs 9 |
| B6, pre- vs 15 d posttreatment | <0.0001 | **** | F (1, 68) = 18.27 | 5 vs 14 |
| B6, 2 d vs 15 d posttreatment | <0.0001 | **** | F (1, 82) = 35.76 | 9 vs 14 |
| CBA, pre- vs 2 d posttreatment | <0.0001 | **** | F (1, 39) = 31.38 | 6 vs 6 |
| CBA, pre- vs 15 d posttreatment | <0.0001 | **** | F (1, 43) = 28.85 | 6 vs 7 |
| CBA, 2 d vs 15 d posttreatment | 0.7597 | n.s. | F (1, 44) = 0.09477 | 6 vs 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargsyan, L.; Swisher, A.R.; Hetrick, A.P.; Li, H. Effects of Combined Gentamicin and Furosemide Treatment on Cochlear Macrophages. Int. J. Mol. Sci. 2022, 23, 7343. https://doi.org/10.3390/ijms23137343
Sargsyan L, Swisher AR, Hetrick AP, Li H. Effects of Combined Gentamicin and Furosemide Treatment on Cochlear Macrophages. International Journal of Molecular Sciences. 2022; 23(13):7343. https://doi.org/10.3390/ijms23137343
Chicago/Turabian StyleSargsyan, Liana, Austin R. Swisher, Alisa P. Hetrick, and Hongzhe Li. 2022. "Effects of Combined Gentamicin and Furosemide Treatment on Cochlear Macrophages" International Journal of Molecular Sciences 23, no. 13: 7343. https://doi.org/10.3390/ijms23137343
APA StyleSargsyan, L., Swisher, A. R., Hetrick, A. P., & Li, H. (2022). Effects of Combined Gentamicin and Furosemide Treatment on Cochlear Macrophages. International Journal of Molecular Sciences, 23(13), 7343. https://doi.org/10.3390/ijms23137343






