Injectable Platelet-Rich Fibrin as a Drug Carrier Increases the Antibacterial Susceptibility of Antibiotic—Clindamycin Phosphate
Abstract
:1. Introduction
2. Results
2.1. Structural Changes in PRF and PRF_CLP Samples at 37 °C
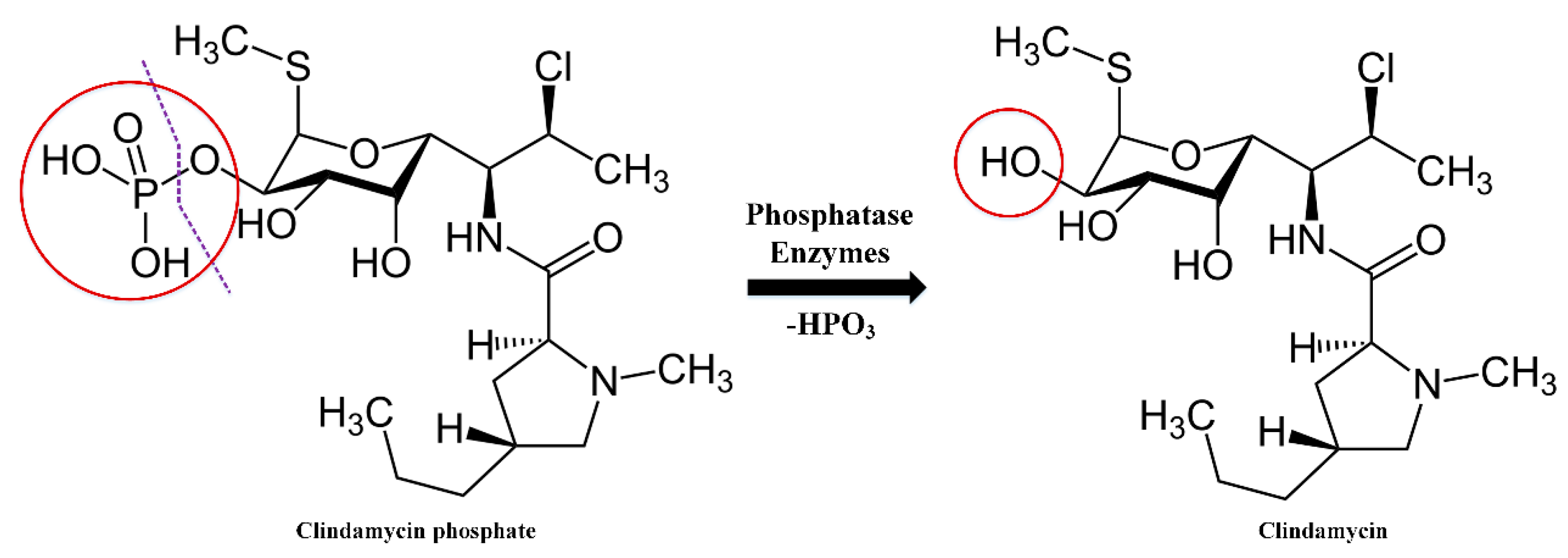
2.2. Drug Release Kinetics
2.3. Effect of PRF_CLP on Antibacterial Properties
2.4. Cell Viability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Blood Collection and Platelet-Rich Fibrin Production
4.3. Characterization of Prepared Samples
4.3.1. Chemical Structure
4.3.2. Morphology
4.3.3. Evaluation of CLP Kinetics
4.4. Preparation of Bacterial Suspension
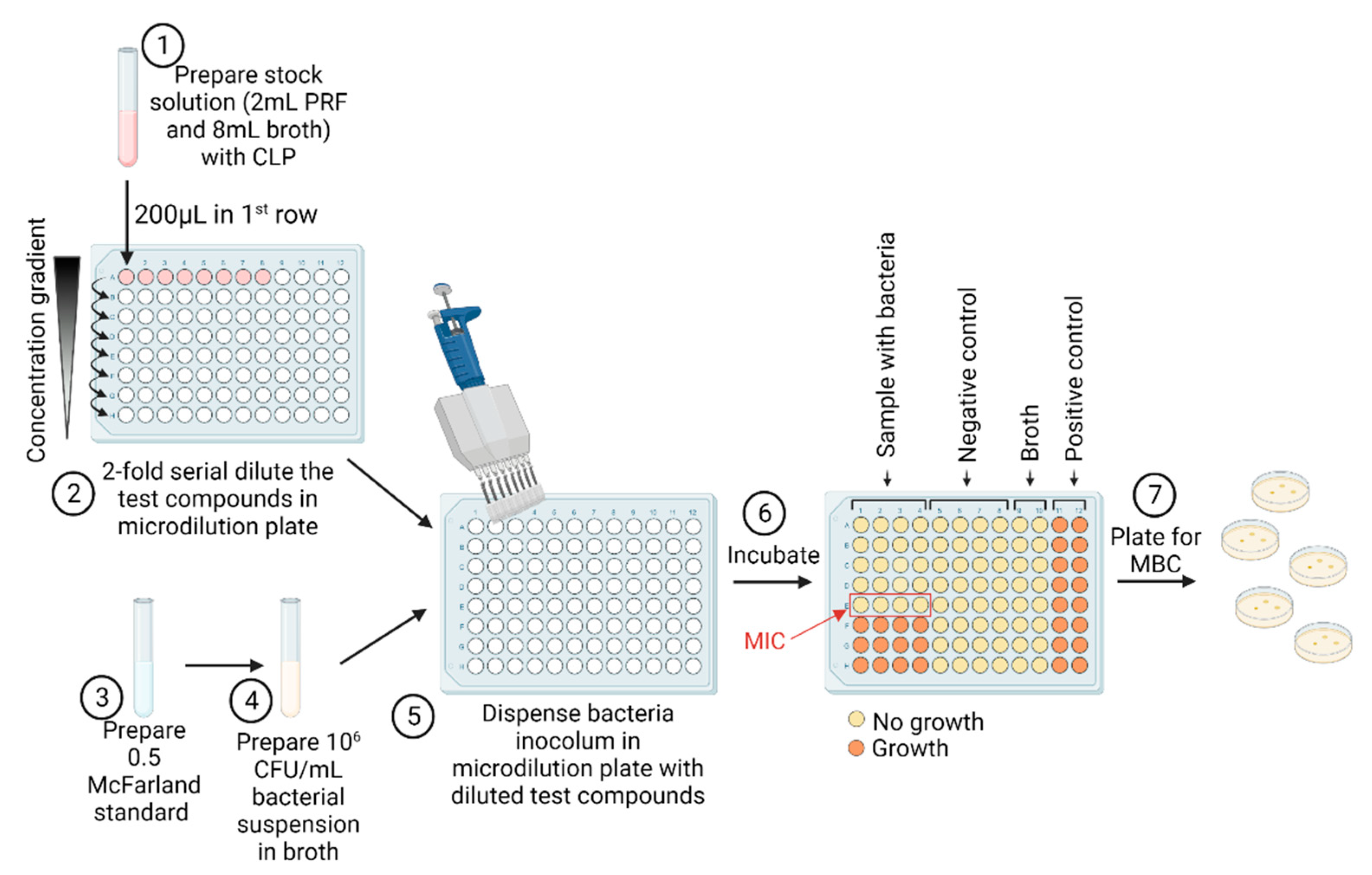
4.5. Determination of Antibacterial Properties
4.5.1. Determination of Minimal Inhibitory Concentration
4.5.2. Determination of Minimal Bactericidal Concentration
4.6. Cell Viability Experiments
4.7. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chou, T.; Chang, H.; Wang, J. Autologous platelet concentrates in maxillofacial regenerative therapy. Kaohsiung J. Med. Sci. 2020, 36, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent. Clin. N. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Jasmine, S.; Thangavelu, A.; Janarthanan, K.; Krishnamoorthy, R.; Alshatwi, A.A. Antimicrobial and antibiofilm potential of injectable platelet rich fibrin—a second-generation platelet concentrate—against biofilm producing oral staphylococcus isolates. Saudi J. Biol. Sci. 2020, 27, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kour, P.; Pudakalkatti, P.; Vas, A.; Das, S.; Padmanabhan, S. Comparative evaluation of antimicrobial efficacy of platelet-rich plasma, platelet-rich fibrin, and injectable platelet-rich fibrin on the standard strains of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Contemp. Clin. Dent. 2018, 9, S325. [Google Scholar] [CrossRef]
- Heal, C.; Buettner, P.; Browning, S. Risk factors for wound infection after minor surgery in general practice. Med. J. Aust. 2006, 185, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, Q.; Huang, C.; Mo, A.; Li, J.; Zuo, Y. Biological properties of an anti-bacterial membrane for guided bone regeneration: An experimental study in rats. Clin. Oral Implant. Res. 2010, 21, 321–327. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef] [Green Version]
- DeLeo, F.R.; Diep, B.A.; Otto, M. Host Defense and Pathogenesis in Staphylococcus aureus Infections. Infect. Dis. Clin. N. Am. 2009, 23, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Aguilar, G.; Hammerman, W.A.; Mason, E.O.; Kaplan, S.L. Clindamycin treatment of invasive infections caused by community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus in children. Pediatr. Infect. Dis. J. 2003, 22, 593–599. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Nasr, M.; Elkhatib, W.F.; Eltayeb, W.N.; Elshamy, A.A.; El-Sayyad, G.S. Nanobiotic formulations as promising advances for combating MRSA resistance: Susceptibilities and post-antibiotic effects of clindamycin, doxycycline, and linezolid. RSC Adv. 2021, 11, 39696–39706. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Cranio-Maxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Vera, J.R. Treatment options in odontogenic infection. Med. Oral Patol. Oral Cir. Bucal 2004, 9 (Suppl. S25–S31), 19–24. [Google Scholar]
- Li, H.; Deng, J.; Yue, Z.; Zhang, Y.; Sun, H.; Ren, X. Clindamycin hydrochloride and clindamycin phosphate: Two drugs or one? A retrospective analysis of a spontaneous reporting system. Eur. J. Clin. Pharmacol. 2017, 73, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Aloysius, H.; Inoyama, D.; Chen, Y.; Hu, L. Enzyme-mediated hydrolytic activation of prodrugs. Acta Pharm. Sin. B 2011, 1, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Stanković, M.; Savić, V.; Marinković, V. Determination of Clindamycin Phosphate in Different Vaginal Gel Formulations by Reverse Phase High Performance Liquid Chromatography. Acta Fac. Med. Naissensis 2013, 30, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Morozowich, W.; Karnes, H.A. Case Study: Clindamycin 2-Phosphate, A Prodrug of Clindamycin. In Prodrugs; Springer: New York, NY, USA, 2007; pp. 1207–1219. [Google Scholar]
- Brown, L.W. High-Pressure Liquid Chromatographic Assays for Clindamycin, Clindamycin Phosphate, and Clindamycin Palmitate. J. Pharm. Sci. 1978, 67, 1254–1257. [Google Scholar] [CrossRef]
- Borin, M.T.; Ryan, K.K.; Hopkins, N.K. Systemic Absorption of Clindamycin after Intravaginal Administration of Clindamycin Phosphate Ovule or Cream. J. Clin. Pharmacol. 1999, 39, 805–810. [Google Scholar] [CrossRef]
- Borin, M.T.; Powley, G.W.; Tackwell, K.R.; Batts, D.H. Absorption of clindamycin after intravaginal application of clindamycin phosphate 2% cream. J. Antimicrob. Chemother. 1995, 35, 833–841. [Google Scholar] [CrossRef]
- Amr, S.; Brown, M.B.; Martin, G.P.; Forbes, B. Activation of clindamycin phosphate by human skin. J. Appl. Microbiol. 2001, 90, 550–554. [Google Scholar] [CrossRef]
- Mostafavi, S.; Karkhane, R.; Riazi-Esfahani, M.; Dorkoosh, F.; Rafiee-Tehrani, M.; Tamaddon, L. Thermoanalytical characterization of clindamycin-loaded intravitreal implants prepared by hot melt extrusion. Adv. Biomed. Res. 2015, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 2018, 123, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Desai, T.A. Simultaneous bactericidal and osteogenic effect of nanoparticulate calcium phosphate powders loaded with clindamycin on osteoblasts infected with Staphylococcus aureus. Mater. Sci. Eng. C 2014, 37, 210–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, S.; Singh, B.R. A Distinct Utility of the Amide III Infrared Band for Secondary Structure Estimation of Aqueous Protein Solutions Using Partial Least Squares Methods. Biochemistry 2004, 43, 2541–2549. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Faizullin, D.A.; Zuev, Y.F.; Weisel, J.W. The α-Helix to β-Sheet Transition in Stretched and Compressed Hydrated Fibrin Clots. Biophys. J. 2012, 103, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Vukomanović, M.; Zavašnik-Bergant, T.; Bračko, I.; Škapin, S.D.; Ignjatović, N.; Radmilović, V.; Uskoković, D. Poly(d,l-lactide-co-glycolide)/hydroxyapatite core–shell nanospheres. Part 3: Properties of hydroxyapatite nano-rods and investigation of a distribution of the drug within the composite. Colloids Surf. B Biointerfaces 2011, 87, 226–235. [Google Scholar] [CrossRef]
- Wang, S.-M.; Bu, S.-S.; Liu, H.-M.; Li, H.-Y.; Liu, W.; Wang, Y.-D. Separation and characterization of clindamycin phosphate and related impurities in injection by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 899–906. [Google Scholar] [CrossRef]
- Mohamed, A.; Abd-Motagaly, A.; Ahmed, O.; Amin, S.; Mohamed Ali, A. Investigation of Drug–Polymer Compatibility Using Chemometric-Assisted UV-Spectrophotometry. Pharmaceutics 2017, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Pradid, J.; Keawwatana, W.; Boonyang, U.; Tangbunsuk, S. Biological properties and enzymatic degradation studies of clindamycin-loaded PLA/HAp microspheres prepared from crocodile bones. Polym. Bull. 2017, 74, 5181–5194. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health 2020, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Mardashev, S.R.; Nikolaev Ya, A.; Sokolov, N.N. Isolation and properties of a homogenous L asparaginase preparation from Pseudomonas fluorescens AG (Russian). Biokhimiya 1975, 40, 984–989. [Google Scholar]
- Wang, S.; Li, Y.; Li, S.; Yang, J.; Tang, R.; Li, X.; Li, L.; Fei, J. Platelet-rich plasma loaded with antibiotics as an affiliated treatment for infected bone defect by combining wound healing property and antibacterial activity. Platelets 2021, 32, 479–491. [Google Scholar] [CrossRef]
- Aleksandra, A.; Misic, M.; Mira, Z.; Violeta, N.; Dragana, I.; Zoran, B.; Dejan, V.; Milanko, S.; Dejan, B. Prevalence of inducible clindamycin resistance among community-associated staphylococcal isolates in central Serbia. Indian J. Med. Microbiol. 2014, 32, 49–52. [Google Scholar] [CrossRef]
- Indrawattana, N.; Sungkhachat, O.; Sookrung, N.; Chongsa-nguan, M.; Tungtrongchitr, A.; Voravuthikunchai, S.P.; Kong-ngoen, T.; Kurazono, H.; Chaicumpa, W. Staphylococcus aureus Clinical Isolates: Antibiotic Susceptibility, Molecular Characteristics, and Ability to Form Biofilm. Biomed Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Daum, R.S. Skin and Soft-Tissue Infections Caused by Methicillin-Resistant Staphylococcus aureus. N. Engl. J. Med. 2007, 357, 380–390. [Google Scholar] [CrossRef]
- Naimi, T.S. Comparison of Community- and Health Care–Associated Methicillin-Resistant Staphylococcus aureus Infection. JAMA 2003, 290, 2976–2984. [Google Scholar] [CrossRef] [Green Version]
- Schilcher, K.; Andreoni, F.; Dengler Haunreiter, V.; Seidl, K.; Hasse, B.; Zinkernagel, A.S. Modulation of Staphylococcus aureus Biofilm Matrix by Subinhibitory Concentrations of Clindamycin. Antimicrob. Agents Chemother. 2016, 60, 5957–5967. [Google Scholar] [CrossRef] [Green Version]
- Schilcher, K.; Andreoni, F.; Uchiyama, S.; Ogawa, T.; Schuepbach, R.A.; Zinkernagel, A.S. Increased Neutrophil Extracellular Trap–Mediated Staphylococcus aureus Clearance Through Inhibition of Nuclease Activity by Clindamycin and Immunoglobulin. J. Infect. Dis. 2014, 210, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Kuriyama, T.; Karasawa, T.; Nakagawa, K.; Saiki, Y.; Yamamoto, E.; Nakamura, S. Bacteriologic features and antimicrobial susceptibility in isolates from orofacial odontogenic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2000, 90, 600–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, F.-J.; Nasirzade, J.; Kargarpoor, Z.; Stähli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Navarro, L.B.; Barchiki, F.; Navarro Junior, W.; Carneiro, E.; da Silva Neto, U.X.; Westphalen, V.P.D. Assessment of platelet-rich fibrin in the maintenance and recovery of cell viability of the periodontal ligament. Sci. Rep. 2019, 9, 19476. [Google Scholar] [CrossRef] [PubMed]
- Bucur, M.; Constantin, C.; Neagu, M.; Zurac, S.; Dinca, O.; Vladan, C.; Cioplea, M.; Popp, C.; Nichita, L.; Ionescu, E. Alveolar blood clots and platelet-rich fibrin induce in vitro fibroblast proliferation and migration. Exp. Ther. Med. 2018, 17, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Vella, J.; Busuttil, F.; Bartolo, N.S.; Sammut, C.; Ferrito, V.; Serracino-Inglott, A.; Azzopardi, L.M.; LaFerla, G. A simple HPLC-UV method for the determination of ciprofloxacin in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 989, 80–85. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Yang, T.; Hsieh, S.; Hung, M.; Lin, E.T. High-performance liquid chromatographic determination of clindamycin in human plasma or serum: Application to the bioequivalency study of clindamycin phosphate injections. J. Chromatogr. B Biomed. Sci. Appl. 1997, 696, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egle, K.; Skadins, I.; Grava, A.; Micko, L.; Dubniks, V.; Salma, I.; Dubnika, A. Injectable Platelet-Rich Fibrin as a Drug Carrier Increases the Antibacterial Susceptibility of Antibiotic—Clindamycin Phosphate. Int. J. Mol. Sci. 2022, 23, 7407. https://doi.org/10.3390/ijms23137407
Egle K, Skadins I, Grava A, Micko L, Dubniks V, Salma I, Dubnika A. Injectable Platelet-Rich Fibrin as a Drug Carrier Increases the Antibacterial Susceptibility of Antibiotic—Clindamycin Phosphate. International Journal of Molecular Sciences. 2022; 23(13):7407. https://doi.org/10.3390/ijms23137407
Chicago/Turabian StyleEgle, Karina, Ingus Skadins, Andra Grava, Lana Micko, Viktors Dubniks, Ilze Salma, and Arita Dubnika. 2022. "Injectable Platelet-Rich Fibrin as a Drug Carrier Increases the Antibacterial Susceptibility of Antibiotic—Clindamycin Phosphate" International Journal of Molecular Sciences 23, no. 13: 7407. https://doi.org/10.3390/ijms23137407
APA StyleEgle, K., Skadins, I., Grava, A., Micko, L., Dubniks, V., Salma, I., & Dubnika, A. (2022). Injectable Platelet-Rich Fibrin as a Drug Carrier Increases the Antibacterial Susceptibility of Antibiotic—Clindamycin Phosphate. International Journal of Molecular Sciences, 23(13), 7407. https://doi.org/10.3390/ijms23137407





