Evaluation of the Effects of Microgravity on Activated Primary Human Hepatic Stellate Cells
Abstract
:1. Introduction
2. Results
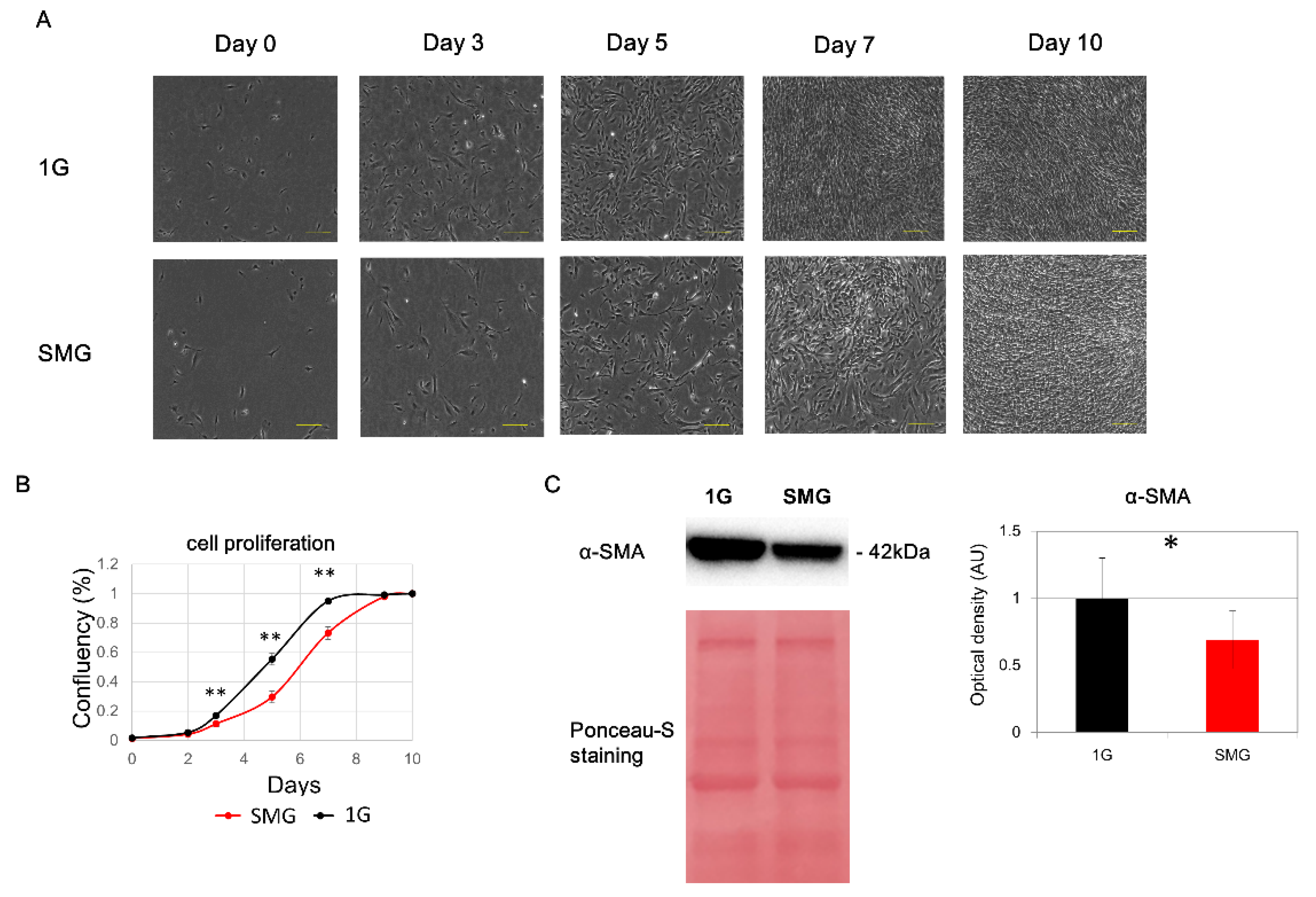
2.1. HHSteC Proliferation Is Reduced under SMG
2.2. Changes in Gene Expression Due to SMG Exposure
2.3. Mitochondrial Dysfunction Increases Oxidative Stress
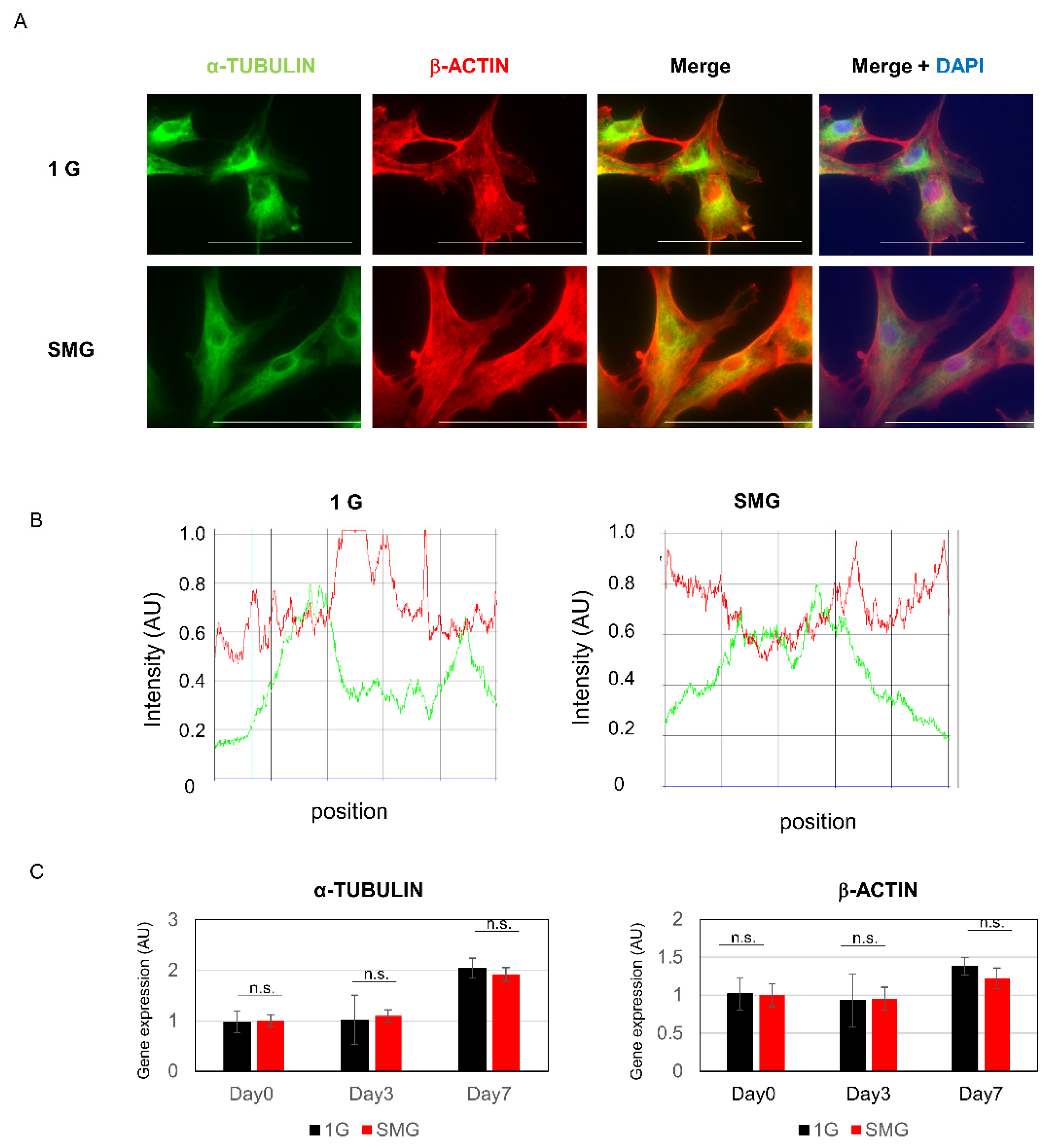
2.4. Effect of SMG on the Cytoskeleton
3. Discussion
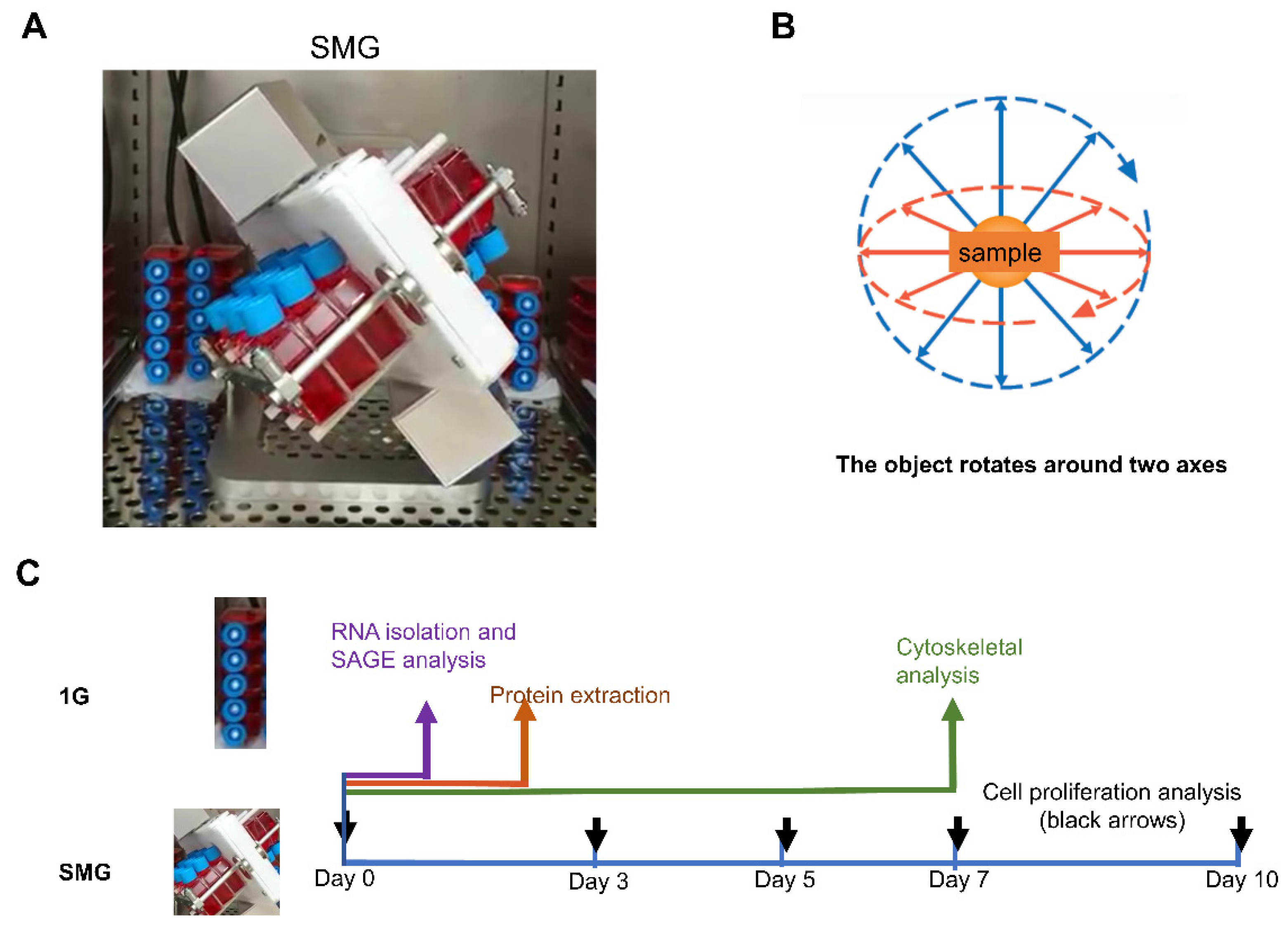
4. Materials and Methods
4.1. Cells and Cell Culturing
4.2. Cell Proliferation Assay
4.3. Total RNA Isolation
4.4. Serial Analysis of Gene Expression (SAGE)
4.5. Real-Time RT-PCR
4.6. Western Blot Analysis
4.7. ROS Activity Evaluation
4.8. Fluorescent Immunostaining
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawahara, Y.; Manabe, T.; Matsumoto, M.; Kajiume, T.; Matsumoto, M.; Yuge, L. LIF-free embryonic stem cell culture in simulated microgravity. PLoS ONE 2009, 4, e6343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monticone, M.; Liu, Y.; Pujic, N.; Cancedda, R. Activation of nervous system development genes in bone marrow derived mesenchymal stem cells following spaceflight exposure. J. Cell. Biochem. 2010, 111, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Mangala, L.S.; Zhang, Y.; He, Z.; Emami, K.; Ramesh, G.T.; Story, M.; Rohde, L.H.; Wu, H. Effects of simulated microgravity on expression profile of microRNA in human lymphoblastoid cells. J. Biol. Chem. 2011, 286, 32483–32490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Tanaka, S.; Sekine, K.; Kita, S.; Okamura, A.; Takebe, T.; Zheng, Y.W.; Ueno, Y.; Tanaka, J.; Taniguchi, H. The generation of pancreatic beta-cell spheroids in a simulated microgravity culture system. Biomaterials 2013, 34, 5785–5791. [Google Scholar] [CrossRef]
- Furukawa, T.; Tanimoto, K.; Fukazawa, T.; Imura, T.; Kawahara, Y.; Yuge, L. Simulated microgravity attenuates myogenic differentiation via epigenetic regulations. NPJ Microgravity 2018, 4, 11. [Google Scholar] [CrossRef]
- Jonscher, K.R.; Alfonso-Garcia, A.; Suhalim, J.L.; Orlicky, D.J.; Potma, E.O.; Ferguson, V.L.; Bouxsein, M.L.; Bateman, T.A.; Stodieck, L.S.; Levi, M.; et al. Spaceflight Activates Lipotoxic Pathways in Mouse Liver. PLoS ONE 2016, 11, e0152877. [Google Scholar]
- Ishikawa, M.; Sekine, K.; Okamura, A.; Zheng, Y.W.; Ueno, Y.; Koike, N.; Tanaka, J.; Taniguchi, H. Reconstitution of hepatic tissue architectures from fetal liver cells obtained from a three-dimensional culture with a rotating wall vessel bioreactor. J. Biosci. Bioeng. 2011, 111, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Dai, Z.Q.; Ling, S.K.; Zhang, H.Y.; Wan, Y.M.; Li, Y.H. Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells. J. Biomed. Sci. 2009, 16, 87. [Google Scholar] [CrossRef] [Green Version]
- Murata, Y.; Yasuda, T.; Watanabe-Asaka, T.; Oda, S.; Mantoku, A.; Takeyama, K.; Chatani, M.; Kudo, A.; Uchida, S.; Suzuki, H.; et al. Histological and Transcriptomic Analysis of Adult Japanese Medaka Sampled Onboard the International Space Station. PLoS ONE 2015, 10, e0138799. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Ishida, Y.; Keogh, A.; Strain, A. Evaluation of the function of primary human hepatocytes co-cultured with the human hepatic stellate cell (HSC) line LI90. Int. J. Artif. Organs 1998, 21, 353–359. [Google Scholar] [CrossRef]
- Chen, H.L.; Wu, H.L.; Fon, C.C.; Chen, P.J.; Lai, M.Y.; Chen, D.S. Long-term culture of hepatocytes from human adults. J. Biomed. Sci. 1998, 5, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Runge, D.; Michalopoulos, G.K.; Strom, S.C.; Runge, D.M. Recent advances in human hepatocyte culture systems. Biochem. Biophys. Res. Commun. 2000, 274, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gallud, A.; Kloditz, K.; Ytterberg, J.; Ostberg, N.; Katayama, S.; Skoog, T.; Gogvadze, V.; Chen, Y.Z.; Xue, D.; Moya, S.; et al. Cationic gold nanoparticles elicit mitochondrial dysfunction: A multi-omics study. Sci. Rep. 2019, 9, 4366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Li, Y.; Chen, J. Duration of simulated microgravity affects the differentiation of mesenchymal stem cells. Mol. Med. Rep. 2017, 15, 3011–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, J.L.; Boittin, F.X.; Halet, G.; Arnaudeau, S.; Mironneau, C.; Mironneau, J. Effect of a 14-day hindlimb suspension on cytosolic Ca2+ concentration in rat portal vein myocytes. Am. J. Physiol. 1997, 273, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Dabertrand, F.; Porte, Y.; Macrez, N.; Morel, J.L. Spaceflight regulates ryanodine receptor subtype 1 in portal vein myocytes in the opposite way of hypertension. J. Appl. Physiol. 2012, 112, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Liu, M.; Fan, Y.; Deng, X. A potential gravity-sensing role of vascular smooth muscle cell glycocalyx in altered gravitational stimulation. Astrobiology 2013, 7, 626–636. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chen, L.; Bai, Y.G.; Song, J.B.; Cheng, J.H.; Ma, H.Z.; Ma, J.; Xie, M.J. miR-137 and its target T-type CaV 3.1 channel modulate dedifferentiation and proliferation of cerebrovascular smooth muscle cells in simulated microgravity rats by regulating calcineurin/NFAT pathway. Cell Prolif. 2020, 53, e12774. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.F.; Wang, H.M.; Jiang, M.; Wang, L.; Lin, L.J.; Zhao, Y.Z.; Shao, J.J.; Zhou, J.J.; Xie, M.J.; Li, X.; et al. Mitochondrial Oxidative Stress Enhances Vasoconstriction by Altering Calcium Homeostasis in Cerebrovascular Smooth Muscle Cells under Simulated Microgravity. Biomed. Environ. Sci. 2021, 34, 203–212. [Google Scholar]
- Kang, H.; Fan, Y.; Sun, A.; Jia, X.; Deng, X. Simulated microgravity exposure modulates the phenotype of cultured vascular smooth muscle cells. Cell Biochem. Biophys. 2013, 66, 121–130. [Google Scholar] [CrossRef]
- Dai, Z.Q.; Wang, R.; Ling, S.K.; Wan, Y.M.; Li, Y.H. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif. 2007, 40, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.I.; Chien, K.H.; Lin, H.T.; Tai, M.C.; Chen, J.T.; Liang, C.M. Effect of microgravity on the mesenchymal stem cell characteristics of limbal fibroblasts. J. Chin. Med. Assoc. 2017, 80, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Quynh Chi, H.N.; Nghia Son, H.; Chinh Chung, D.; Huan, L.D.; Hong Diem, T.; Long, L.T. Simulated microgravity reduces proliferation and reorganizes the cytoskeleton of human umbilical cord mesenchymal stem cells. Physiol. Res. 2020, 69, 897–906. [Google Scholar] [PubMed]
- Yuge, L.; Kajiume, T.; Tahara, H.; Kawahara, Y.; Umeda, C.; Yoshimoto, R.; Wu, S.L.; Yamaoka, K.; Asashima, M.; Kataoka, K.; et al. Microgravity potentiates stem cell proliferation while sustaining the capability of differentiation. Stem Cells Dev. 2006, 15, 921–929. [Google Scholar] [CrossRef]
- Michaletti, A.; Gioia, M.; Tarantino, U.; Zolla, L. Effects of microgravity on osteoblast mitochondria: A proteomic and metabolomics profile. Sci. Rep. 2017, 7, 15376. [Google Scholar] [CrossRef] [Green Version]
- Becherini, P.; Caffa, I.; Piacente, F.; Damonte, P.; Vellone, V.G.; Passalacqua, M.; Benzi, A.; Bonfiglio, T.; Reverberi, D.; Khalifa, A.; et al. SIRT6 enhances oxidative phosphorylation in breast cancer and promotes mammary tumorigenesis in mice. Cancer Metab. 2021, 9, 6. [Google Scholar] [CrossRef]
- Piao, J.; Tsuji, K.; Ochi, H.; Iwata, M.; Koga, D.; Okawa, A.; Morita, S.; Takeda, S.; Asou, Y. Sirt6 regulates postnatal growth plate differentiation and proliferation via Ihh signaling. Sci. Rep. 2013, 3, 3022. [Google Scholar] [CrossRef] [Green Version]
- Guignandon, A.; Usson, Y.; Laroche, N.; Lafage-Proust, M.H.; Sabido, O.; Alexandre, C.; Vico, L. Effects of intermittent or continuous gravitational stresses on cell-matrix adhesion: Quantitative analysis of focal contacts in osteoblastic ROS 17/2.8 cells. Exp. Cell Res. 1997, 236, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Ma, X.; Yang, C.; Su, P.; Yin, C.; Qian, A.R. The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment. Int. J. Mol. Sci. 2017, 18, 2132. [Google Scholar] [CrossRef] [Green Version]
- Jeong, A.J.; Kim, Y.J.; Lim, M.H.; Lee, H.; Noh, K.; Kim, B.H.; Chung, J.W.; Cho, C.H.; Kim, S.; Ye, S.K. Microgravity induces autophagy via mitochondrial dysfunction in human Hodgkin’s lymphoma cells. Sci. Rep. 2018, 8, 14646. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.; Goswami, M.; Kalen, A.L.; Goswami, P.C.; Sarsour, E.H. N-acetyl-L-cysteine increases MnSOD activity and enhances the recruitment of quiescent human fibroblasts to the proliferation cycle during wound healing. Mol. Biol. Rep. 2016, 43, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Wang, Y.C.; Zhao, T.Z.; Zhang, S.; Du, T.Y.; Yang, C.B.; Li, Y.H.; Sun, X.Q. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3K-Akt-eNOS signal pathway. PLoS ONE 2012, 7, e40365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Shi, Y.; Qiu, C.; Zhao, J.; Gong, Y.; Nie, C.; Wu, B.; Yang, Y.; Wang, F.; Luo, L. Effects of Simulated Microgravity on Ultrastructure and Apoptosis of Choroidal Vascular Endothelial Cells. Front. Physiol. 2020, 11, 577325. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Juvekar, A.; Lyssiotis, C.A.; Lien, E.C.; Albeck, J.G.; Oh, D.; Varma, G.; Hung, Y.P.; Ullas, S.; Lauring, J.; et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 2016, 164, 433–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitano, M.; Bloomston, P.M. Hepatic Stellate Cells and microRNAs in Pathogenesis of Liver Fibrosis. J. Clin. Med. 2016, 5, 38. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Yang, C.; Zhang, H.; Wu, F.; Chen, J.; Li, K.; Wang, H.; Li, Y.; Li, Y.; et al. Upregulation of miR-223 in the rat liver inhibits proliferation of hepatocytes under simulated microgravity. Exp. Mol. Med. 2017, 49, e348. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lu, T.; Wong, M.; Wang, X.; Stodieck, L.; Karouia, F.; Story, M.; Wu, H. Transient gene and microRNA expression profile changes of confluent human fibroblast cells in spaceflight. FASEB J. 2016, 30, 2211–2224. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Zhao, J.; Tang, N.; Zeng, X.; Wu, K.; Ye, C.; Shi, J.; Lu, C.; Ning, B.; Zhang, J.; et al. MicroRNA-155 attenuates activation of hepatic stellate cell by simultaneously preventing EMT process and ERK1 signalling pathway. Liver Int. 2015, 35, 1234–1243. [Google Scholar] [CrossRef]
- McDaniel, K.; Herrera, L.; Zhou, T.; Francis, H.; Han, Y.; Levine, P.; Lin, E.; Glaser, S.; Alpini, G.; Meng, F. The functional role of microRNAs in alcoholic liver injury. J. Cell. Mol. Med. 2014, 18, 197–207. [Google Scholar] [CrossRef]
- Hong, Y.; He, H.; Jiang, G.; Zhang, H.; Tao, W.; Ding, Y.; Yuan, D.; Liu, J.; Fan, H.; Lin, F.; et al. miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell 2020, 19, e13128. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Wu, C.; Xu, Z.; Xia, P.; Dong, P.; Chen, B.; Yu, F. Hepatic stellate cell is activated by microRNA-181b via PTEN/Akt pathway. Mol. Cell Biochem. 2015, 398, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Swenson, E.S.; Gaca, M.D.; Giordano, F.J.; Reiss, M.; Wells, R.G. Smad2 and Smad3 play different roles in rat hepatic stellate cell function and alpha-smooth muscle actin organization. Mol. Biol. Cell 2005, 16, 4214–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyler, A. A model illustrating possible instability of cellular structure under conditions of weightlessness. J. Theor. Biol. 1966, 11, 59–62. [Google Scholar] [CrossRef]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafève, F.; Gasset, G.; Schoevaert, D. Weightlessness acts on human breast cancer cell line MCF-7. Adv. Space Res. 2003, 32, 1595–1603. [Google Scholar] [CrossRef]
- Rijken, P.J.; Boonstra, J.; Verkleij, A.J.; de Laat, S.W. Effects of gravity on the cellular response to epidermal growth factor. Adv. Space Biol. Med. 1994, 4, 159–188. [Google Scholar]
- Carlsson, S.I.; Bertilaccio, M.T.; Ballabio, E.; Maier, J.A. Endothelial stress by gravitational unloading: Effects on cell growth and cytoskeletal organization. Biochim. Biophys. Acta 2003, 1642, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Rosner, H.; Wassermann, T.; Moller, W.; Hanke, W. Effects of altered gravity on the actin and microtubule cytoskeleton of human SH-SY5Y neuroblastoma cells. Protoplasma 2006, 229, 225–234. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Romanov, Y.A. The role of cytoskeleton in cell changes under condition of simulated microgravity. Acta Astronaut. 2001, 48, 647–650. [Google Scholar] [CrossRef]
- Yang, F.; Dai, Z.; Tan, Y.; Li, Y. Effects of altered gravity on the cytoskeleton of neonatal rat cardiocytes. Microgravity Sci. Technol. 2010, 22, 45–52. [Google Scholar] [CrossRef]
- Vorselen, D.; Roos, W.H.; MacKintosh, F.C.; Wuite, G.J.; van Loon, J.J. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014, 28, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, A.; Pradhan-Sundd, T.; Singh, S.; Nagarajan, S.; Loizos, N.; Monga, S.P. Platelet-Derived Growth Factor Receptor alpha Contributes to Human Hepatic Stellate Cell Proliferation and Migration. Am. J. Pathol. 2017, 187, 2273–2287. [Google Scholar] [CrossRef] [PubMed]
- Hemmersbach, R.; Strauch, S.M.; Seibt, D.; Schuber, M. Comparative studies on gravisensitive protists on ground (2D and 3D clinostats) and in microgravity. Microgravity Sci. Technol. 2006, 18, 257–259. [Google Scholar] [CrossRef]
- Wei, L.; Diao, Y.; Qi, J.; Khokhlov, A.; Feng, H.; Yan, X.; Li, Y. Effect of change in spindle structure on proliferation inhibition of osteosarcoma cells and osteoblast under simulated microgravity during incubation in rotating bioreactor. PLoS ONE 2013, 8, e76710. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, K.; Terai, S.; Hirose, Y.; Takami, T.; Yamamoto, N.; Sakaida, I. Senescence marker protein 30 (SMP30)/regucalcin (RGN) expression decreases with aging, acute liver injuries and tumors in zebrafish. Biochem. Biophys. Res. Commun. 2011, 414, 331–336. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Nishimura, Y.; Sakuragi, A.; Duponselle, J.; Matsumoto, T.; Yamamoto, N.; Murata, T.; Sakaida, I.; Takami, T. Evaluation of the Effects of Microgravity on Activated Primary Human Hepatic Stellate Cells. Int. J. Mol. Sci. 2022, 23, 7429. https://doi.org/10.3390/ijms23137429
Fujisawa K, Nishimura Y, Sakuragi A, Duponselle J, Matsumoto T, Yamamoto N, Murata T, Sakaida I, Takami T. Evaluation of the Effects of Microgravity on Activated Primary Human Hepatic Stellate Cells. International Journal of Molecular Sciences. 2022; 23(13):7429. https://doi.org/10.3390/ijms23137429
Chicago/Turabian StyleFujisawa, Koichi, Yuto Nishimura, Akino Sakuragi, Jolien Duponselle, Toshihiko Matsumoto, Naoki Yamamoto, Tomoaki Murata, Isao Sakaida, and Taro Takami. 2022. "Evaluation of the Effects of Microgravity on Activated Primary Human Hepatic Stellate Cells" International Journal of Molecular Sciences 23, no. 13: 7429. https://doi.org/10.3390/ijms23137429
APA StyleFujisawa, K., Nishimura, Y., Sakuragi, A., Duponselle, J., Matsumoto, T., Yamamoto, N., Murata, T., Sakaida, I., & Takami, T. (2022). Evaluation of the Effects of Microgravity on Activated Primary Human Hepatic Stellate Cells. International Journal of Molecular Sciences, 23(13), 7429. https://doi.org/10.3390/ijms23137429





