Effects of Dietary n-3 LCPUFA Supplementation on the Hippocampus of Aging Female Mice: Impact on Memory, Lipid Raft-Associated Glutamatergic Receptors and Neuroinflammation
Abstract
:1. Introduction
2. Results
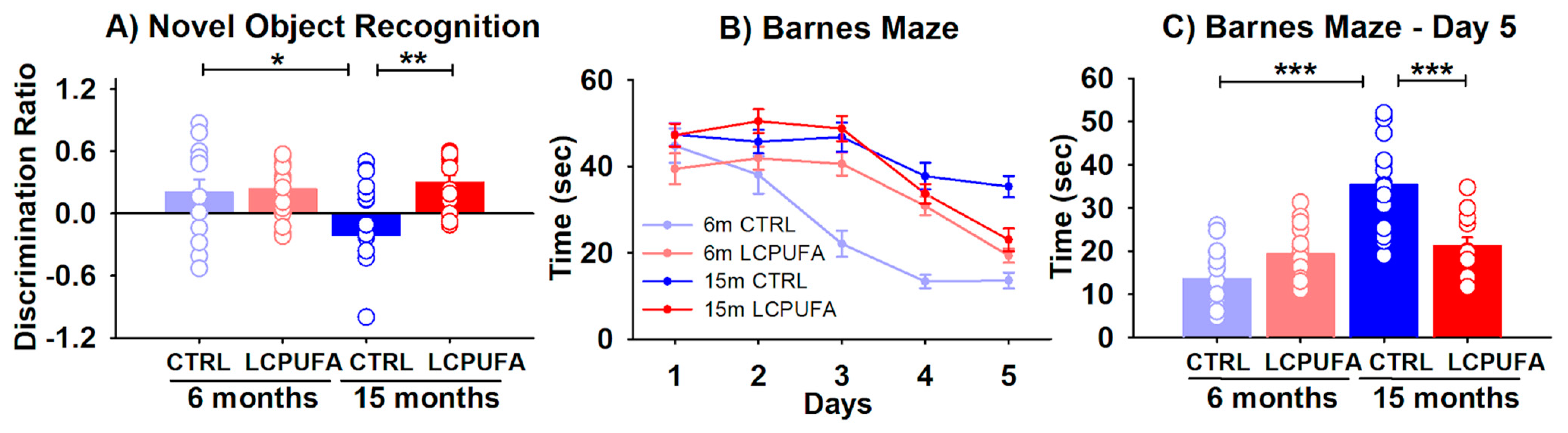
2.1. A n-3 LCPUFA-Enriched Diet Improves Spatial and Object Recognition Memory Detriment during Aging
2.2. A n-3 LCPUFA-Enriched Diet Modulates the Glutamatergic Receptors in Hippocampal Lipid Rafts
2.3. ERα Expression Is Enhanced by n-3 LCPUFA in Lipid Rafts of Aged Females
2.4. Changes in Lipid Composition of Hippocampal Lipid Rafts with Age Are Partly Reverted by the n-3 LCPUFA-Enriched Diet
2.5. Hippocampal Neuroinflammation during Aging Is Neutralized by a n-3 LCPUFA-Enriched Diet
2.6. Hippocampal Content of Proinflammatory Cytokines Is Modulated by Aging and an n-3 LCPUFA-Enriched Diet
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Nutritional Enrichment
4.3. Behavioral Tests
4.3.1. Novel Object Recognition
4.3.2. Barnes Maze
4.4. Lipid Raft Extraction from Total Hippocampal Lysate
4.5. Slot-Blot Analysis
4.6. Lipid Analyses
4.7. Immunohistochemistry
4.8. Microglia and Astrocytes Analysis
4.9. ELISA Immunoassay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazan, N.G. Synaptic Lipid Signaling: Significance of Polyunsaturated Fatty Acids and Platelet-Activating Factor. J. Lipid Res. 2003, 44, 2221–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Morales, V.; Montero, F.; González-Forero, D.; Rodríguez-Bey, G.; Gómez-Pérez, L.; Medialdea-Wandossell, M.J.; Domínguez-Vías, G.; García-Verdugo, J.M.; Moreno-López, B. Membrane-Derived Phospholipids Control Synaptic Neurotransmission and Plasticity. PLoS Biol. 2015, 13, e1002153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaner, A.; da Silva Santana, T.T.; Schroeder, T.; Einicker-Lamas, M.; Girardini, J.; Costa, M.R.; Banchio, C. Author Correction: Specific Phospholipids Regulate the Acquisition of Neuronal and Astroglial Identities in Post-Mitotic Cells. Sci. Rep. 2019, 9, 20222. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C. Problems with Essential Fatty Acids: Time for a New Paradigm? Prog. Lipid Res. 2003, 42, 544–568. [Google Scholar] [CrossRef]
- Echeverría, F.; Valenzuela, R.; Catalina Hernandez-Rodas, M.; Valenzuela, A. Docosahexaenoic Acid (DHA), a Fundamental Fatty Acid for the Brain: New Dietary Sources. Prostaglandins Leukot. Essent. Fat. Acids 2017, 124, 1–10. [Google Scholar] [CrossRef]
- Custers; Emma, E.M.; Kiliaan; Amanda, J. Dietary Lipids from Body to Brain. Prog. Lipid Res. 2022, 85, 101144. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.-D. Marine Omega-3 (n-3) Phospholipids: A Comprehensive Review of Their Properties, Sources, Bioavailability, and Relation to Brain Health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef] [Green Version]
- Taha, A.Y. Linoleic Acid–Good or Bad for the Brain? NPJ Sci. Food 2020, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Zárate, R.; el Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of Long Chain Polyunsaturated Fatty Acids in Human Health. Clin. Transl. Med. 2017, 6, e25. [Google Scholar] [CrossRef] [Green Version]
- Janssen, C.I.F.; Kiliaan, A.J. Long-Chain Polyunsaturated Fatty Acids (LCPUFA) from Genesis to Senescence: The Influence of LCPUFA on Neural Development, Aging, and Neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Skowronska-Krawczyk, D.; Budin, I. Aging Membranes: Unexplored Functions for Lipids in the Lifespan of the Central Nervous System. Exp. Gerontol. 2020, 131, 110817. [Google Scholar] [CrossRef] [PubMed]
- Tsui-Pierchala, B.A.; Encinas, M.; Milbrandt, J.; Johnson, E.M. Lipid Rafts in Neuronal Signaling and Function. Trends Neurosci. 2002, 25, 412–417. [Google Scholar] [CrossRef]
- Díaz, M.; Fabelo, N.; Martín, V.; Ferrer, I.; Gómez, T.; Marín, R. Biophysical Alterations in Lipid Rafts from Human Cerebral Cortex Associate with Increased BACE1/AβPP Interaction in Early Stages of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 43, 1185–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, M.O.W.; Kuchenbecker, J.; Grösgen, S.; Burg, V.K.; Hundsdörfer, B.; Rothhaar, T.L.; Friess, P.; de Wilde, M.C.; Broersen, L.M.; Penke, B.; et al. Docosahexaenoic Acid Reduces Amyloid Beta Production via Multiple Pleiotropic Mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar] [CrossRef] [Green Version]
- Georgieva, R.; Mircheva, K.; Vitkova, V.; Balashev, K.; Ivanova, T.; Tessier, C.; Koumanov, K.; Nuss, P.; Momchilova, A.; Staneva, G. Phospholipase A2-Induced Remodeling Processes on Liquid-Ordered/Liquid-Disordered Membranes Containing Docosahexaenoic or Oleic Acid: A Comparison Study. Langmuir 2016, 32, 1756–1770. [Google Scholar] [CrossRef]
- Jacobs, M.L.; Faizi, H.A.; Peruzzi, J.A.; Vlahovska, P.M.; Kamat, N.P. EPA and DHA Differentially Modulate Membrane Elasticity in the Presence of Cholesterol. Biophys. J. 2021, 120, 2317–2329. [Google Scholar] [CrossRef]
- Fabelo, N.; Martín, V.; Santpere, G.; Marín, R.; Torrent, L.; Ferrer, I.; Díaz, M. Severe Alterations in Lipid Composition of Frontal Cortex Lipid Rafts from Parkinson’s Disease and Incidental Parkinson’s Disease. Mol. Med. 2011, 17, 1107–1118. [Google Scholar] [CrossRef]
- Díaz, M.; Marín, R. Lipid Rafts and Development of Alzheimer’s Disease. In Cerebral and Cerebellar Cortex—Interaction and Dynamics in Health and Disease; IntechOpen Limited: London, UK, 2021; Volume 25. [Google Scholar]
- Marin, R.; Díaz, M.; Alonso, R.; Sanz, A.; Arévalo, M.A.; Garcia-Segura, L.M. Role of Estrogen Receptor Alpha in Membrane-Initiated Signaling in Neural Cells: Interaction with IGF-1 Receptor. J. Steroid Biochem. Mol. Biol. 2009, 114, 2–7. [Google Scholar] [CrossRef]
- Marin, R.; Marrero-Alonso, J.; Fernandez, C.; Cury, D.; Diaz, M. Estrogen Receptors in Lipid Raft Signalling Complexes for Neuroprotection. Front. Biosci. 2012, 4, 1420–1433. [Google Scholar] [CrossRef]
- Marin, R. Signalosomes in the Brain: Relevance in the Development of Certain Neuropathologies Such as Alzheimer’s Disease. Front. Physiol. 2011, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Marin, R.; Diaz, M. Estrogen Interactions With Lipid Rafts Related to Neuroprotection. Impact of Brain Ageing and Menopause. Front. Neurosci. 2018, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Ramírez, C.; Morales, A.; González, M.; Alonso, R.; Díaz, M. Modulation of Abeta-Induced Neurotoxicity by Estrogen Receptor Alpha and Other Associated Proteins in Lipid Rafts. Steroids 2008, 73, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.M.; González, M.; Díaz, M.; Alonso, R.; Ferrer, I.; Santpere, G.; Puig, B.; Meyer, G.; Marin, R. VDAC and ERalpha Interaction in Caveolae from Human Cortex Is Altered in Alzheimer’s Disease. Mol. Cell. Neurosci. 2009, 42, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Gonzalez, C. Neuroprotective Role of Estrogens: Relationship with Insulin/IGF-1 Signaling. Front. Biosci. 2012, 4, 607–619. [Google Scholar] [CrossRef]
- Yaqoob, P.; Shaikh, S.R. The Nutritional and Clinical Significance of Lipid Rafts. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 156–166. [Google Scholar] [CrossRef]
- Petursdottir, A.L.; Farr, S.A.; Morley, J.E.; Banks, W.A.; Skuladottir, G.V. Effect of Dietary N-3 Polyunsaturated Fatty Acids on Brain Lipid Fatty Acid Composition, Learning Ability, and Memory of Senescence-Accelerated Mouse. J. Gerontol. Ser. A 2008, 63, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Ueda, Y.; Wang, M.-F.; Irei, A.V.; Sarukura, N.; Sakai, T.; Hsu, T.-F. Effect of Dietary Lipids on Longevity and Memory in the SAMP8 Mice. J. Nutr. Sci. Vitaminol. 2011, 57, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Taga, C.; Nishiga, M.; Fujiwara, M.; Konishi, F.; Tanaka, K.; Kamei, C. Effect of Docosahexaenoic Acid-Fortified Chlorella vulgaris Strain CK22 on the Radial Maze Performance in Aged Mice. Biol. Pharm. Bull. 2002, 25, 1090–1092. [Google Scholar] [CrossRef] [Green Version]
- Che, H.; Zhou, M.; Zhang, T.; Zhang, L.; Ding, L.; Yanagita, T.; Xu, J.; Xue, C.; Wang, Y. Comparative Study of the Effects of Phosphatidylcholine Rich in DHA and EPA on Alzheimer’s Disease and the Possible Mechanisms in CHO-APP/PS1 Cells and SAMP8 Mice. Food Funct. 2018, 9, 643–654. [Google Scholar] [CrossRef]
- Butler, M.J.; Deems, N.P.; Muscat, S.; Butt, C.M.; Belury, M.A.; Barrientos, R.M. Dietary DHA Prevents Cognitive Impairment and Inflammatory Gene Expression in Aged Male Rats Fed a Diet Enriched with Refined Carbohydrates. Brain Behav. Immun. 2021, 98, 198–209. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentivoglio, M.; Mariotti, R.; Bertini, G. Neuroinflammation and Brain Infections: Historical Context and Current Perspectives. Brain Res. Rev. 2011, 66, 152–173. [Google Scholar] [CrossRef] [PubMed]
- Von Bernhardi, R.; Eugenin-von Bernhardi, L.; Eugenin, J. Microglial Cell Dysregulation in Brain Aging and Neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viviani, B.; Gardoni, F.; Bartesaghi, S.; Corsini, E.; Facchi, A.; Galli, C.L.; Di Luca, M.; Marinovich, M. Interleukin-1 Beta Released by Gp120 Drives Neural Death through Tyrosine Phosphorylation and Trafficking of NMDA Receptors. J. Biol. Chem. 2006, 281, 30212–30222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, A.Y.; Swayze, R.D.; El-Husseini, A.; Song, C. Interleukin-1 Beta Modulates AMPA Receptor Expression and Phosphorylation in Hippocampal Neurons. J. Neuroimmunol. 2006, 175, 97–106. [Google Scholar] [CrossRef]
- Taoro-González, L.; Cabrera-Pastor, A.; Sancho-Alonso, M.; Arenas, Y.M.; Meseguer-Estornell, F.; Balzano, T.; Elmlili, N.; Felipo, V. Differential Role of Interleukin-1β in Neuroinflammation-Induced Impairment of Spatial and Nonspatial Memory in Hyperammonemic Rats. FASEB J. 2019, 33, 9913–9928. [Google Scholar] [CrossRef]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Ha, B.K.; Zastrow, M.V.; Beattie, M.S.; Malenka, R.C. Control of Synaptic Strength by Glial TNFα. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef]
- Stellwagen, D.; Beattie, E.C.; Seo, J.Y.; Malenka, R.C. Differential Regulation of AMPA Receptor and GABA Receptor Trafficking by Tumor Necrosis Factor-α. J. Neurosci. 2005, 25, 3219–3228. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, D.; Zhu, A.; Gong, C.; Kil, K.-E.; Kura, S.; Choi, J.-K.; Brownell, A.-L. Hypo-Anxious Phenotype of Adolescent Offspring Prenatally Exposed to LPS Is Associated with Reduced MGluR5 Expression in Hippocampus. Open J. Med. Psychol. 2014, 3, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Recchiuti, A.; Krishnamoorthy, S.; Fredman, G.; Chiang, N.; Serhan, C.N. MicroRNAs in Resolution of Acute Inflammation: Identification of Novel Resolvin Dl-MiRNA Circuits. FASEB J. 2011, 25, 544–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisicchia, E.; Sasso, V.; Catanzaro, G.; Leuti, A.; Besharat, Z.M.; Chiacchiarini, M.; Molinari, M.; Ferretti, E.; Viscomi, M.T.; Chiurchiù, V. Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage Via ALX/FPR2 Receptor-Regulated MicroRNAs. Mol. Neurobiol. 2018, 55, 6894–6905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Zhang, J.; Chen, F.; Lin, Y. Neuroprotectin D1 Attenuates Brain Damage Induced by Transient Middle Cerebral Artery Occlusion in Rats through TRPC6/CREB Pathways. Mol. Med. Rep. 2013, 8, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, C.; Nadjar, A.; Buaud, B.; Vaysse, C.; Aubert, A.; Pallet, V.; Layé, S.; Joffre, C. Resolvin D1 and E1 Promote Resolution of Inflammation in Microglial Cells in Vitro. Brain Behav. Immun. 2016, 55, 249–259. [Google Scholar] [CrossRef]
- Famenini, S.; Rigali, E.A.; Olivera-Perez, H.M.; Dang, J.; Chang, M.T.; Halder, R.; Rao, R.V.; Pellegrini, M.; Porter, V.; Bredesen, D.; et al. Increased Intermediate M1-M2 Macrophage Polarization and Improved Cognition in Mild Cognitive Impairment Patients on ω-3 Supplementation. FASEB J. 2017, 31, 148–160. [Google Scholar] [CrossRef]
- Orr, S.K.; Palumbo, S.; Bosetti, F.; Mount, H.T.; Kang, J.X.; Greenwood, C.E.; Ma, D.W.L.; Serhan, C.N.; Bazinet, R.P. Unesterified Docosahexaenoic Acid Is Protective in Neuroinflammation. J. Neurochem. 2013, 127, 378–393. [Google Scholar] [CrossRef] [Green Version]
- Delpech, J.-C.; Madore, C.; Joffre, C.; Aubert, A.; Kang, J.X.; Nadjar, A.; Layé, S. Transgenic Increase in N-3/n-6 Fatty Acid Ratio Protects Against Cognitive Deficits Induced by an Immune Challenge through Decrease of Neuroinflammation. Neuropsychopharmacology 2015, 40, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Beery, A.K. Inclusion of Females Does Not Increase Variability in Rodent Research Studies. Curr. Opin. Behav. Sci. 2018, 23, 143–149. [Google Scholar] [CrossRef]
- Perez, S.E.; Berg, B.M.; Moore, K.A.; He, B.; Counts, S.E.; Fritz, J.J.; Hu, Y.-S.; Lazarov, O.; Lah, J.J.; Mufson, E.J. DHA Diet Reduces AD Pathology in Young APPswe/PS1ΔE9 Transgenic Mice: Possible Gender Effects. J. Neurosci. Res. 2010, 88, 1026–1040. [Google Scholar] [CrossRef] [Green Version]
- da Costa, A.E.M.; Gomes, N.S.; Gadelha Filho, C.V.J.; Oliveira e Silva Linhares, M.G.; da Costa, R.O.; Chaves Filho, A.J.M.; Cordeiro, R.C.; Vasconcelos, G.S.; da Silva, F.E.R.; da Silva Araujo, T.; et al. Sex Influences in the Preventive Effects of Peripubertal Supplementation with N-3 Polyunsaturated Fatty Acids in Mice Exposed to the Two-Hit Model of Schizophrenia. Eur. J. Pharmacol. 2021, 897, 173949. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, N.; Nadjar, A.; Sierra, A.; Valero, J. Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. Int. J. Mol. Sci. 2022, 23, 3399. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, B.L.; Barnes, C.A.; Meltzer, J.; Sutherland, R.J. Hippocampal Granule Cells Are Necessary for Normal Spatial Learning but Not for Spatially-Selective Pyramidal Cell Discharge. Exp. Brain Res. 1989, 76, 485–496. [Google Scholar] [CrossRef]
- Broadbent, N.J.; Squire, L.R.; Clark, R.E. Spatial Memory, Recognition Memory, and the Hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 14515–14520. [Google Scholar] [CrossRef] [Green Version]
- Lüscher, C.; Malenka, R.C. NMDA Receptor-Dependent Long-Term Potentiation and Long-Term Depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Zhang, J.; Liu, Y.-S.; Li, L.; He, Y.-L. Research Advances on Flotillins. Virol. J. 2011, 8, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, G.P.; Nichols, B.J. The Roles of Flotillin Microdomains–Endocytosis and Beyond. J. Cell Sci. 2011, 124, 3933–3940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canerina-Amaro, A.; Hernandez-Abad, L.G.; Ferrer, I.; Quinto-Alemany, D.; Mesa-Herrera, F.; Ferri, C.; Puertas-Avendaño, R.A.; Diaz, M.; Marin, R. Lipid Raft ER Signalosome Malfunctions in Menopause and Alzheimer’s Disease. Front. Biosci.-Sch. 2017, 9, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Fabelo, N.; Martín, V.; Marín, R.; Santpere, G.; Aso, E.; Ferrer, I.; Díaz, M. Evidence for Premature Lipid Raft Aging in APP/PS1 Double-Transgenic Mice, a Model of Familial Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2012, 71, 868–881. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.; Fabelo, N.; Marin, R. Genotype-Induced Changes in Biophysical Properties of Frontal Cortex Lipid Raft from APP/PS1 Transgenic Mice. Front. Physiol. 2012, 3, 454. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, J.P.; Church, D.F.; Pryor, W.A. The Kinetics of the Autoxidation of Polyunsaturated Fatty Acids. Lipids 1987, 22, 299–304. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes Are Active Players in Cerebral Innate Immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef]
- Tardy, M.; Fages, C.; Le Prince, G.; Rolland, B.; Nunez, J. Regulation of the Glial Fibrillary Acidic Protein (GFAP) and of Its Encoding MRNA in the Developing Brain and in Cultured Astrocytes. In Molecular Aspects of Development and Aging of the Nervous System; Lauder, J.M., Privat, A., Giacobini, E., Timiras, P.S., Vernadakis, A., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1989; pp. 41–52. ISBN 978-1-4757-5876-4. [Google Scholar]
- Probert, L. TNF and Its Receptors in the CNS: The Essential, the Desirable and the Deleterious Effects. Neuroscience 2015, 302, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Cardona, A.E. The IL-1β Phenomena in Neuroinflammatory Diseases. J. Neural Transm. 2018, 125, 781–795. [Google Scholar] [CrossRef]
- Benice, T.S.; Rizk, A.; Kohama, S.; Pfankuch, T.; Raber, J. Sex-Differences in Age-Related Cognitive Decline in C57BL/6J Mice Associated with Increased Brain Microtubule-Associated Protein 2 and Synaptophysin Immunoreactivity. Neuroscience 2006, 137, 413–423. [Google Scholar] [CrossRef]
- Pereda, D.; Pardo, M.R.; Morales, Y.; Dominguez, N.; Arnau, M.R.; Borges, R. Mice Lacking Chromogranins Exhibit Increased Aggressive and Depression-like Behaviour. Behav. Brain Res. 2015, 278, 98–106. [Google Scholar] [CrossRef]
- Pereda, D.; Al-Osta, I.; Okorocha, A.E.; Easton, A.; Hartell, N.A. Changes in Presynaptic Calcium Signalling Accompany Age-Related Deficits in Hippocampal LTP and Cognitive Impairment. Aging Cell 2019, 18, e13008. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, A.R.; Truckenbrod, L.M.; Campos, K.T.; Williams, S.A.; Burke, S.N. Sex Differences in Age-Related Impairments Vary across Cognitive and Physical Assessments in Rats. Behav. Neurosci. 2020, 134, 69–81. [Google Scholar] [CrossRef]
- Labrousse, V.F.; Nadjar, A.; Joffre, C.; Costes, L.; Aubert, A.; Grégoire, S.; Bretillon, L.; Layé, S. Short-Term Long Chain Omega3 Diet Protects from Neuroinflammatory Processes and Memory Impairment in Aged Mice. PLoS ONE 2012, 7, e36861. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Cutuli, D.; De Bartolo, P.; Caporali, P.; Laricchiuta, D.; Foti, F.; Ronci, M.; Rossi, C.; Neri, C.; Spalletta, G.; Caltagirone, C.; et al. N-3 Polyunsaturated Fatty Acids Supplementation Enhances Hippocampal Functionality in Aged Mice. Front. Aging Neurosci. 2014, 6, 220. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-B.; Zhang, L.; Li, W.-T.; Yang, Y.; Zhao, J.-M. Artesunate Restores Spatial Learning of Rats with Hepatic Encephalopathy by Inhibiting Ammonia-Induced Oxidative Damage in Neurons and Dysfunction of Glutamate Signaling in Astroglial Cells. Biomed. Pharmacother. 2016, 84, 972–978. [Google Scholar] [CrossRef]
- Zhou, M.; Ding, L.; Wen, M.; Che, H.; Huang, J.; Zhang, T.; Xue, C.; Mao, X.; Wang, Y. Mechanisms of DHA-Enriched Phospholipids in Improving Cognitive Deficits in Aged SAMP8 Mice with High-Fat Diet. J. Nutr. Biochem. 2018, 59, 64–75. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Vandal, M.; Léveillé, P.; Pinçon, A.; Calon, F.; Plourde, M. Docosahexaenoic Acid Prevents Cognitive Deficits in Human Apolipoprotein E Epsilon 4-Targeted Replacement Mice. Neurobiol. Aging 2017, 57, 28–35. [Google Scholar] [CrossRef]
- Jiang, L.; Shi, Y.; Wang, L.; Yang, Z. The Influence of Orally Administered Docosahexaenoic Acid on Cognitive Ability in Aged Mice. J. Nutr. Biochem. 2009, 20, 735–741. [Google Scholar] [CrossRef]
- Carrié, I.; Guesnet, P.; Bourre, J.-M.; Francès, H. Diets Containing Long-Chain n-3 Polyunsaturated Fatty Acids Affect Behaviour Differently during Development than Ageing in Mice. Br. J. Nutr. 2000, 83, 439–447. [Google Scholar] [CrossRef]
- Hosono, T.; Mouri, A.; Nishitsuji, K.; Jung, C.-G.; Kontani, M.; Tokuda, H.; Kawashima, H.; Shibata, H.; Suzuki, T.; Nabehsima, T.; et al. Arachidonic or Docosahexaenoic Acid Diet Prevents Memory Impairment in Tg2576 Mice. J. Alzheimer’s Dis. 2015, 48, 149–162. [Google Scholar] [CrossRef]
- Martin, D.S.D.; Spencer, P.; Horrobin, D.F.; Lynch, M.A. Long-Term Potentiation in Aged Rats Is Restored When the Age-Related Decrease in Polyunsaturated Fatty Acid Concentration Is Reversed. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 121–130. [Google Scholar] [CrossRef]
- Babayan, A.H.; Kramár, E.A. Rapid Effects of Oestrogen on Synaptic Plasticity: Interactions with Actin and Its Signalling Proteins. J. Neuroendocrinol. 2013, 25, 1163–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manahan-Vaughan, D. Group 1 and 2 Metabotropic Glutamate Receptors Play Differential Roles in Hippocampal Long-Term Depression and Long-Term Potentiation in Freely Moving Rats. J. Neurosci. 1997, 17, 3303–3311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manahan-Vaughan, D.; Reymann, K.G. Group 1 Metabotropic Glutamate Receptors Contribute to Slow-Onset Potentiation in the Rat CA1 Region in Vivo. Neuropharmacology 1997, 36, 1533–1538. [Google Scholar] [CrossRef]
- Latif-Hernandez, A.; Faldini, E.; Ahmed, T.; Balschun, D. Separate Ionotropic and Metabotropic Glutamate Receptor Functions in Depotentiation vs. LTP: A Distinct Role for Group1 MGluR Subtypes and NMDARs. Front. Cell. Neurosci. 2016, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Manahan-Vaughan, D.; Ngomba, R.T.; Storto, M.; Kulla, A.; Catania, M.V.; Chiechio, S.; Rampello, L.; Passarelli, F.; Capece, A.; Reymann, K.G.; et al. An Increased Expression of the MGlu5 Receptor Protein Following LTP Induction at the Perforant Path–Dentate Gyrus Synapse in Freely Moving Rats. Neuropharmacology 2003, 44, 17–25. [Google Scholar] [CrossRef]
- Privitera, L.; Hogg, E.L.; Gaestel, M.; Wall, M.J.; Corrêa, S.A.L. The MK2 Cascade Regulates MGluR-Dependent Synaptic Plasticity and Reversal Learning. Neuropharmacology 2019, 155, 121–130. [Google Scholar] [CrossRef]
- Hering, H.; Lin, C.-C.; Sheng, M. Lipid Rafts in the Maintenance of Synapses, Dendritic Spines, and Surface AMPA Receptor Stability. J. Neurosci. 2003, 23, 3262–3271. [Google Scholar] [CrossRef] [Green Version]
- Delint-Ramírez, I.; Salcedo-Tello, P.; Bermudez-Rattoni, F. Spatial Memory Formation Induces Recruitment of NMDA Receptor and PSD-95 to Synaptic Lipid Rafts. J. Neurochem. 2008, 106, 1658–1668. [Google Scholar] [CrossRef]
- Roh, S.-E.; Hong, Y.H.; Jang, D.C.; Kim, J.; Kim, S.J. Lipid Rafts Serve as Signaling Platforms for MGlu1 Receptor-Mediated Calcium Signaling in Association with Caveolin. Mol. Brain 2014, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.; Fabelo, N.; Ferrer, I.; Marín, R. “Lipid Raft Aging” in the Human Frontal Cortex during Nonpathological Aging: Gender Influences and Potential Implications in Alzheimer’s Disease. Neurobiol. Aging 2018, 67, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Aryal, S.; Hussain, S.; Drevon, C.A.; Nagelhus, E.; Hvalby, Ø.; Jensen, V.; Walaas, S.I.; Davanger, S. Omega-3 Fatty Acids Regulate Plasticity in Distinct Hippocampal Glutamatergic Synapses. Eur. J. Neurosci. 2019, 49, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Katakura, M.; Mamun, A.A.; Shido, O. Docosahexaenoic Acid Helps to Lessen Extinction Memory in Rats. Molecules 2018, 23, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Kevala, K.; Kim, J.; Moon, H.-S.; Jun, S.B.; Lovinger, D.; Kim, H.-Y. Docosahexaenoic Acid Promotes Hippocampal Neuronal Development and Synaptic Function. J. Neurochem. 2009, 111, 510–521. [Google Scholar] [CrossRef] [Green Version]
- Bean, L.A.; Ianov, L.; Foster, T.C. Estrogen Receptors, the Hippocampus, and Memory. Neuroscientist 2014, 20, 534–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingwood, D.; Simons, K. Lipid Rafts As a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Nickels, J.D.; Smith, M.D.; Alsop, R.J.; Himbert, S.; Yahya, A.; Cordner, D.; Zolnierczuk, P.; Stanley, C.B.; Katsaras, J.; Cheng, X.; et al. Lipid Rafts: Buffers of Cell Membrane Physical Properties. J. Phys. Chem. B 2019, 123, 2050–2056. [Google Scholar] [CrossRef]
- Kwon, O.-H.; Cho, Y.Y.; Lee, J.H.; Chung, S. O-GlcNAcylation Inhibits Endocytosis of Amyloid Precursor Protein by Decreasing Its Localization in Lipid Raft Microdomains. Membranes 2021, 11, 909. [Google Scholar] [CrossRef]
- van der Wurff, I.S.M.; Meyer, B.J.; de Groot, R.H.M. Effect of Omega-3 Long Chain Polyunsaturated Fatty Acids (n-3 LCPUFA) Supplementation on Cognition in Children and Adolescents: A Systematic Literature Review with a Focus on n-3 LCPUFA Blood Values and Dose of DHA and EPA. Nutrients 2020, 12, 3115. [Google Scholar] [CrossRef]
- Ojo, J.O.; Rezaie, P.; Gabbott, P.L.; Stewart, M.G. Impact of Age-Related Neuroglial Cell Responses on Hippocampal Deterioration. Front. Aging Neurosci. 2015, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef] [Green Version]
- Crain, J.M.; Nikodemova, M.; Watters, J.J. Microglia Express Distinct M1 and M2 Phenotypic Markers in the Postnatal and Adult Central Nervous System in Male and Female Mice. J. Neurosci. Res. 2013, 91, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala Frigerio, C.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.-T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep. 2019, 27, 1293–1306.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, M.; Haque, A.; Banik, N.L.; Nagarkatti, P.; Nagarkatti, M.; Ray, S.K. Estrogen Receptor Agonists for Attenuation of Neuroinflammation and Neurodegeneration. Brain Res. Bull. 2014, 109, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Cutuli, D.; Landolfo, E.; Nobili, A.; De Bartolo, P.; Sacchetti, S.; Chirico, D.; Marini, F.; Pieroni, L.; Ronci, M.; D’Amelio, M.; et al. Behavioral, Neuromorphological, and Neurobiochemical Effects Induced by Omega-3 Fatty Acids Following Basal Forebrain Cholinergic Depletion in Aged Mice. Alzheimer’s Res. Ther. 2020, 12, 150. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Kalueff, A.V.; Song, C. Dietary Eicosapentaenoic Acid Normalizes Hippocampal Omega-3 and 6 Polyunsaturated Fatty Acid Profile, Attenuates Glial Activation and Regulates BDNF Function in a Rodent Model of Neuroinflammation Induced by Central Interleukin-1β Administration. Eur. J. Nutr. 2018, 57, 1781–1791. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The Impact of Inflammation on Cognitive Function in Older Adults: Implications for Health Care Practice and Research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome Signalling in Brain Function and Neurodegenerative Disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef]
- Delpech, J.-C.; Herron, S.; Botros, M.B.; Ikezu, T. Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci. 2019, 42, 361–372. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The Opposing Effects of N−3 and N−6 Fatty Acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-Y.; Tsao, Y.-Y.; Leung, Y.-M.; Su, K.-P. Docosahexaenoic Acid Suppresses Neuroinflammatory Responses and Induces Heme Oxygenase-1 Expression in BV-2 Microglia: Implications of Antidepressant Effects for Omega-3 Fatty Acids. Neuropsychopharmacology 2010, 35, 2238–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic Acid-Containing Choline Phospholipid Modulates LPS-Induced Neuroinflammation in Vivo and in Microglia in Vitro. J. Neuroinflamm. 2017, 14, 170. [Google Scholar] [CrossRef]
- Moranis, A.; Delpech, J.-C.; De Smedt-Peyrusse, V.; Aubert, A.; Guesnet, P.; Lavialle, M.; Joffre, C.; Layé, S. Long Term Adequate N-3 Polyunsaturated Fatty Acid Diet Protects from Depressive-like Behavior but Not from Working Memory Disruption and Brain Cytokine Expression in Aged Mice. Brain Behav. Immun. 2012, 26, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Jensen, M.T.; Salem, N.; Hussein, N.; Cracchiolo, J.; Dickson, A.; Leighty, R.; Potter, H. A Diet High in Omega-3 Fatty Acids Does Not Improve or Protect Cognitive Performance in Alzheimer’s Transgenic Mice. Neuroscience 2007, 149, 286–302. [Google Scholar] [CrossRef]
- Ameen-Ali, K.E.; Eacott, M.J.; Easton, A. A New Behavioural Apparatus to Reduce Animal Numbers in Multiple Types of Spontaneous Object Recognition Paradigms in Rats. J. Neurosci. Methods 2012, 211, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Ennaceur, A. Effects of lesions of the substantia innominata/ventral pallidum, globus pallidus and medial septum on rat’s performance in object-recognition and radial-maze tasks: Physostigmine and amphetamine treatments. Pharmacol. Res. 1998, 38, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Sunyer, B.; Patil, S.; Höger, H.; Lubec, G. Barnes Maze, a Useful Task to Assess Spatial Reference Memory in the Mice. Available online: https://www.researchsquare.com (accessed on 9 May 2022).
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of Spatial Learning and Memory in the Barnes Maze Task in Rodents—Methodological Consideration. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Martín, V.; Fabelo, N.; Santpere, G.; Puig, B.; Marín, R.; Ferrer, I.; Díaz, M. Lipid Alterations in Lipid Rafts from Alzheimer’s Disease Human Brain Cortex. J. Alzheimer’s Dis. 2010, 19, 489–502. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]





| 6 Months | 15 Months | |||
|---|---|---|---|---|
| CTRL | n-3 LCPUFA | CTRL | n-3 LCPUFA | |
| C16:0 (Palmitic acid) | 26.91 ± 0.97 | 27.65 ± 1.93 | 29.78 ± 1.64 | 29.99 ± 0.99 |
| C18:0 (Stearic acid) | 18.45 ± 0.54 | 19.97 ± 1.44 | 22.36 ± 1.27 | 19.31 ± 0.73 |
| C18:1n9 (Oleic acid) | 14.87 ± 0.51 | 13.74 ± 0.35 | 12.45 ± 0.89 | 13.24 ± 0.75 |
| C18:2n6 (Linoleic acid) | 1.22 ± 0.36 | 0.69 ± 0.39 | 0.81 ± 0.34 | 0.13 ± 0.13 |
| C18:3n3 (Linolenic acid) | 0.23 ± 0.10 | 0.11 ± 0.11 | 0.14 ± 0.09 | 0.00 ± 0.00 |
| C20:4n6 (AA) | 5.85 ± 0.16 a | 4.97 ± 0.40 ab | 5.00 ± 0.18 ab | 4.76 ± 0.24 b |
| C20:5n3 (EPA) | 1.14 ± 0.44 | 0.65 ± 0.42 | 0.76 ± 0.27 | 0.79 ± 0.27 |
| C22:5n-3 (DPA) | 0.21 ± 0.07 ab | 0.36 ± 0.06 a | 0.14 ± 0.05 b | 0.42 ± 0.06 a |
| C22:6n3 (DHA) | 8.64 ± 0.64 a | 9.22 ± 0.77 a | 6.33 ± 0.25 b | 10.10 ± 0.48 a |
| C24:1n9 (Nervonic Acid) | 1.15 ± 0.25 | 0.91 ± 0.23 | 0.77 ± 0.15 | 1.50 ± 0.41 |
| Indexes and Totals | ||||
| n-3 series | 10.17 ± 1.12 a | 10.45 ± 0.68 a | 7.33 ± 0.31 b | 11.40 ± 0.47 a |
| n-6 series | 8.71 ± 0.66 | 7.47 ± 0.40 | 8.08 ± 0.43 | 6.91 ± 0.27 |
| Monoenes series | 17.32 ± 0.33 a | 15.90 ± 0.72 ab | 14.28 ± 1.08 b | 16.24 ± 0.63 b |
| n-6/n-3 ratio | 0.92 ± 0.14 ab | 0.72 ± 0.02 b | 1.10 ± 0.05 a | 0.61 ± 0.03 b |
| Saturates | 47.87 ± 1.30 | 49.65 ± 3.04 | 54.39 ± 2.80 | 50.99 ± 1.58 |
| Monoenes | 22.64 ± 0.54 | 21.02 ± 1.30 | 19.71 ± 1.39 | 21.55 ± 0.91 |
| PUFA | 18.89 ± 0.90 a | 17.92 ± 1.06 ab | 15.41 ± 0.67 b | 18.31 ± 0.61 ab |
| n-3 LCPUFA | 10.03 ± 1.00 a | 10.27 ± 0.62 ab | 7.40 ± 0.22 b | 11.26 ± 0.48 a |
| PUFA/Saturates | 0.40 ± 0.02 a | 0.37 ± 0.03 ab | 0.29 ± 0.02 b | 0.36 ± 0.03 ab |
| UI (Unsaturation Index) | 114.76 ± 5.68 a | 111.11 ± 4.96 a | 92.09 ± 2.95 b | 114.41 ± 3.53 a |
| PI (Peroxidation Index) | 109.64 ± 7.18 a | 108.14 ± 6.85 a | 84.60 ± 3.39 b | 113.63 ± 4.53 a |
| Nutritional Additives | Standard Diet (per kg) | n-3 LCPUFA-Enriched Diet (per kg) |
|---|---|---|
| Vitamin A | 21,000 (UI) | 21,000 (UI) |
| Vitamin D3 | 1100 (UI) | 1100 (UI) |
| Iron | 50 mg | 50 mg |
| Magnesium | 40 mg | 40 mg |
| Zinc | 31 mg | 31 mg |
| Copper | 7 mg | 7 mg |
| Iodine | 6.2 mg | 6.2 mg |
| Technological additives | Standard diet (per kg) | n-3 LCPUFA-enriched diet (per kg) |
| Sepiolite | 760 mg | 760 mg |
| Analytical constituents | Standard diet (%) | n-3 LCPUFA-enriched diet (per kg) |
| Moisture | 12.00 | 12.00 |
| Crude protein | 14.50 | 14.50 |
| Crude fibers | 4.50 | 4.50 |
| Crude ash | 4.70 | 4.70 |
| Crude oil and fats | 4.00 | 4.82 |
| Of which fatty acids | Standard diet (%) | n-3 LCPUFA-enriched diet (%) |
| C16:0 (Palmitic acid) | 0.5 | 0.5 |
| C18:0 (Stearic acid) | 0.1 | 0.1 |
| C18:1n9 (Oleic acid) | 0.7 | 0.7 |
| C18:2n6 (Linoleic acid) | 2.0 | 2.0 |
| C18:3n3 (Linolenic acid) | 0.1 | 0.1 |
| C20:5n3 (EPA) | — | 0.56 |
| C22:6n3 (DHA) | — | 0.26 |
| Total fatty acids classes | Standard diet (%) | n-3 LCPUFA-enriched diet (%) |
| Total Saturated | 0.6 | 0.6 |
| Total Monounsaturated | 0.7 | 0.7 |
| Total Polyunsaturated | 2.1 | 2.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taoro-González, L.; Pereda, D.; Valdés-Baizabal, C.; González-Gómez, M.; Pérez, J.A.; Mesa-Herrera, F.; Canerina-Amaro, A.; Pérez-González, H.; Rodríguez, C.; Díaz, M.; et al. Effects of Dietary n-3 LCPUFA Supplementation on the Hippocampus of Aging Female Mice: Impact on Memory, Lipid Raft-Associated Glutamatergic Receptors and Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 7430. https://doi.org/10.3390/ijms23137430
Taoro-González L, Pereda D, Valdés-Baizabal C, González-Gómez M, Pérez JA, Mesa-Herrera F, Canerina-Amaro A, Pérez-González H, Rodríguez C, Díaz M, et al. Effects of Dietary n-3 LCPUFA Supplementation on the Hippocampus of Aging Female Mice: Impact on Memory, Lipid Raft-Associated Glutamatergic Receptors and Neuroinflammation. International Journal of Molecular Sciences. 2022; 23(13):7430. https://doi.org/10.3390/ijms23137430
Chicago/Turabian StyleTaoro-González, Lucas, Daniel Pereda, Catalina Valdés-Baizabal, Miriam González-Gómez, José A. Pérez, Fátima Mesa-Herrera, Ana Canerina-Amaro, Herminia Pérez-González, Covadonga Rodríguez, Mario Díaz, and et al. 2022. "Effects of Dietary n-3 LCPUFA Supplementation on the Hippocampus of Aging Female Mice: Impact on Memory, Lipid Raft-Associated Glutamatergic Receptors and Neuroinflammation" International Journal of Molecular Sciences 23, no. 13: 7430. https://doi.org/10.3390/ijms23137430
APA StyleTaoro-González, L., Pereda, D., Valdés-Baizabal, C., González-Gómez, M., Pérez, J. A., Mesa-Herrera, F., Canerina-Amaro, A., Pérez-González, H., Rodríguez, C., Díaz, M., & Marin, R. (2022). Effects of Dietary n-3 LCPUFA Supplementation on the Hippocampus of Aging Female Mice: Impact on Memory, Lipid Raft-Associated Glutamatergic Receptors and Neuroinflammation. International Journal of Molecular Sciences, 23(13), 7430. https://doi.org/10.3390/ijms23137430









