Hypercholesterolemia in Cancer and in Anorexia Nervosa: A Hypothesis for a Crosstalk
Abstract
1. Introduction
2. Cholesterol: An Intriguing Molecule
3. Cholesterol Disorder in Cancer
4. Hypercholesterolemia in Anorexia Nervosa
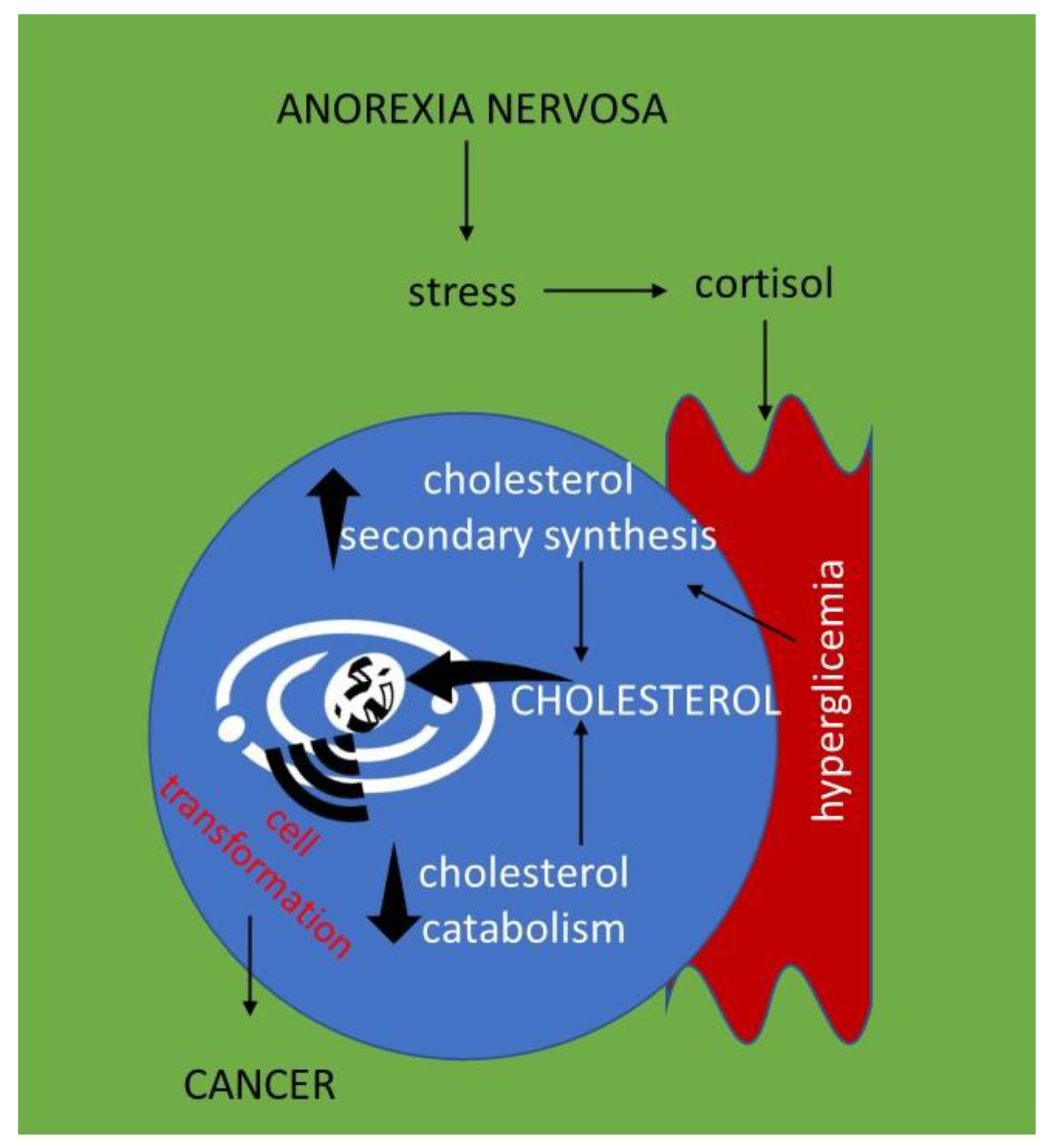
5. Hypercholesterolemia in Anorexia Nervosa and Susceptibility to Cancer: Hypothesis for a Crosstalk
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Chol | cholesterol |
| EDS | eating disorders |
| AN | anorexia nervosa |
References
- Barreira, J.V. The Role of Nutrition in Cancer Patients. Nutr. Cancer 2021, 73, 2849–2850. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.C.; Barr, R.D. The relevance of nutrition to pediatric oncology: A cancer control perspective. Pediatr. Blood Cancer 2020, 67, e28213. [Google Scholar] [CrossRef] [PubMed]
- Imoberdorf, R.; Rühlin, M.; Ballmer, P.E. Cancer and nutrition—A paradigma shift. Laryngo-Rhino-Otologie 2017, 96, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Damasco-Ávila, E.; Velasco-Hidalgo, L.; Zapata-Tarrés, M.; Cárdenas-Cardos, R.; Rivera-Luna, R. Feeding difficulties and eating disorders in pediatric patients with cancer. Bol. Med. Hosp. Infant. Mex. 2019, 76, 113–119. [Google Scholar] [CrossRef]
- Karamanis, G.; Skalkidou, A.; Tsakonas, G.; Brandt, L.; Ekbom, A.; Ekselius, L.; Papadopoulos, F.C. Cancer incidence and mortality patterns in women with anorexia nervosa. Int. J. Cancer 2014, 134, 1751–1757. [Google Scholar] [CrossRef]
- White, A.J.; Nichols, H.B.; Bradshaw, P.T.; Sandler, D.P. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer 2015, 121, 3700–3708. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Whelan, D.R.; Sandler, D.P.; Weinberg, C.R. Eating Disorders and Breast Cancer. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 206–211. [Google Scholar] [CrossRef]
- Papadopoulos, F.C.; Pantziaras, I.; Lagiou, P.; Brandt, L.; Ekselius, L.; Ekbom, A. Age at onset of anorexia nervosa and breast cancer risk. Eur. J. Cancer Prev. 2009, 18, 207–211. [Google Scholar] [CrossRef]
- Yeh, H.W.; Chien, W.C.; Chung, C.H.; Chang, H.A.; Kao, Y.C.; Tzeng, N.S. Eating disorders and the risk of esophageal and stomach cancers-A nationwide, population-based cohort study in Taiwan. Int. J. Eat. Disord. 2021, 54, 959–968. [Google Scholar] [CrossRef]
- Olson, R.E. Discovery of the lipoproteins, their role in fat transport and their significance as risk factors. J. Nutr. 1998, 128, 439S–443S. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Dong, M.; Han, X. Effect of cholesterol on the fluidity of supported lipid bilayers. Colloids Surf. B Biointerfaces 2020, 196, 111353. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Rose, J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533–544. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Villani, M. Nuclear lipid microdomains regulate cell function. Commun. Integr. Biol. 2009, 2, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Cascianelli, G.; Villani, M.; Tosti, M.; Marini, F.; Bartoccini, E.; Magni, M.V.; Albi, E. Lipid microdomains in cell nucleus. Mol. Biol. Cell 2008, 19, 5289–5295. [Google Scholar] [CrossRef] [PubMed]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.M.F.S.; Sousa, S.F. An Atomic-Level Perspective of HMG-CoA-Reductase: The Target Enzyme to Treat Hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Asuka, E.; Jialal, I. Hypercholesterolemia; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cortes, V.A.; Busso, D.; Maiz, A.; Arteaga, A.; Nervi, F.; Rigotti, A. Front Physiological and pathological implications of cholesterol. Front. Biosci.-Landmark 2014, 19, 416–428. [Google Scholar] [CrossRef]
- Kang, C.; LeRoith, D.; Gallagher, E.J. Diabetes, Obesity, and Breast Cancer. Endocrinology 2018, 159, 3801–3812. [Google Scholar] [CrossRef]
- Shakartalla, S.B.; Alhumaidi, R.B.; Shammout, O.D.A.; Al Shareef, Z.M.; Ashmawy, N.S.; Soliman, S.S.M. Dyslipidemia in breast cancer patients increases the risk of SAR-CoV-2 infection. Infect. Genet. Evol. 2021, 92, 104883. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.R.; Koch, M.O.; Cheng, L.; Masterson, T.A. Dyslipidemia, statins and prostate cancer. Expert Rev. Anticancer Ther. 2012, 12, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Yu, M.; Sang, C.; Bi, B.; Chen, J. Dyslipidemia and non-small cell lung cancer risk in Chinese population: A case-control study. Lipids Health Dis. 2018, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tian, Z. Dyslipidemia and colorectal cancer risk: A meta-analysis of prospective studies. Cancer Causes Control 2015, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Kim, E.; Han, M.; Jung, I.; Lee, J.; Jo, Y.S. Impact of Dyslipidemia on the Risk of Second Cancer in Thyroid Cancer Patients: A Korean National Cohort Study. Ann. Surg. Oncol. 2021, 28, 4373–4384. [Google Scholar] [CrossRef]
- Roberts, M.C.; Mensah, G.A.; Khoury, M.J. Leveraging Implementation Science to Address Health Disparities in Genomic Medicine: Examples from the Field. Ethn. Dis. 2019, 29, 187–192. [Google Scholar] [CrossRef]
- Neil, A.; Cooper, J.; Betteridge, J.; Capps, N.; McDowell, I.; Durrington, P.; Seed, M.; Humphries, S.E. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: A prospective registry study. Eur. Heart J. 2008, 29, 2625–2633. [Google Scholar] [CrossRef]
- Vaseghi, G.; Javanmard, S.H.; Heshmat-Ghahdarijani, K.; Sarrafzadegan, N.; Amerizadeh, A. Comorbidities with Familial Hypercholesterolemia (FH): A Systematic Review. Curr. Probl. Cardiol. 2022, 101109. [Google Scholar] [CrossRef] [PubMed]
- Murai, T. Cholesterol lowering: Role in cancer prevention and treatment. Biol. Chem. 2015, 396, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Codini, M.; Garcia-Gil, M.; Albi, E. Cholesterol and sphingolipid enriched lipid rafts as therapeutic targets in cancer. Int. J. Mol. Sci. 2021, 22, 726. [Google Scholar] [CrossRef]
- Codini, M.; Cataldi, S.; Lazzarini, A.; Tasegian, A.; Ceccarini, M.R.; Floridi, A.; Lazzarini, R.; Ambesi-Impiombato, F.S.; Curcio, F.; Beccari, T.; et al. Why high cholesterol levels help hematological malignancies: Role of nuclear lipid microdomains. Lipids Health Dis. 2016, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.; Umetani, M. Obesity and Cancer: 27-Hydroxycholesterol, the Missing Link. Int. J. Mol. Sci. 2020, 21, 4822. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.I.; Câtoi, C. Comparative Oncology; Publishing House of the Romanian Academy: Bucharest, Romania, 2007. [Google Scholar]
- Goldstein, J.L.; Brown, M.S. Regulation of low-density lipoprotein receptors: Implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation 2021, 76, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, L.; Bernardini, I.; Pacifico, N.; Peverini, M.; Damaskopoulou, E.; Cataldi, S.; Albi, E. Severe hypocholesterolaemia is often neglected in haematological malignancies. Eur. J. Cancer 2010, 46, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.G.; Angelin, B.; Assmann, G.; Binder, C.J.; Björkhem, I.; Cedazo-Minguez, A.; Cohen, J.; von Eckardstein, A.; Farinaro, E.; Müller-Wieland, D.; et al. Can LDL cholesterol be too low? Possible risks of extremely low levels. J. Intern. Med. 2017, 281, 534–553. [Google Scholar] [CrossRef]
- Pugliese, L.; Bernardini, I.; Viola-Magni, M.P.; Albi, E. Low levels of serum cholesterol/phospholipids are associated with the antiphospholipid antibodies in monoclonal gammopathy. Int. J. Immunopathol. Pharmacol. 2006, 19, 331–337. [Google Scholar] [CrossRef]
- Pugliese, L.; Bernardini, I.; Pacifico, E.; Viola-Magni, M.; Albi, E. Antiphospholipid antibodies in patients with cancer. Int. J. Immunopathol. Pharmacol. 2006, 19, 879–888. [Google Scholar] [CrossRef]
- Wang, W.; Bai, L.; Li, W.; Cui, J. The Lipid Metabolic Landscape of Cancers and New Therapeutic Perspectives. Front. Oncol. 2020, 10, 605154. [Google Scholar] [CrossRef]
- Han, K.T.; Kim, S. Do cholesterol levels and continuity of statin use affect colorectal cancer incidence in older adults under 75 years of age? PLoS ONE 2021, 16, e0250716. [Google Scholar] [CrossRef]
- Lee, Y.R.; Oh, S.S.; Jang, S.I.; Park, E.C. Statin adherence and risk of all-cause, cancer, and cardiovascular mortality among dyslipidemia patients: A time-dependent analysis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2207–2214. [Google Scholar] [CrossRef]
- Lee, J.W.; You, N.Y.; Kim, Y.; Kim, Y.; Kim, J.; Kang, H.T. Statin use and site-specific risk of colorectal cancer in individuals with hypercholesterolemia from the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS). Nutr. Metab. Cardiovasc. Dis. 2019, 29, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Tong, J., III; Mao, Y.; Yang, Z.; Xu, Q.; Zheng, Z.; Zhang, H.; Wang, J.; Zhang, S.; Rong, W.; Zheng, L., III. Baseline Serum Cholesterol Levels Predict the Response of Patients with Advanced Non-Small Cell Lung Cancer to Immune Checkpoint Inhibitor-Based Treatment. Cancer Manag. Res. 2021, 13, 4041–4053. [Google Scholar] [CrossRef] [PubMed]
- Pandrangi, S.L.; Chittineedi, P.; Chikati, R.; Mosquera, J.A.N.; Llaguno, S.N.S.; Mohiddin, G.J.; Lanka, S.; Chalumuri, S.S.; Maddu, N. Role of Lipoproteins in the Pathophysiology of Breast Cancer. Membranes 2022, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Diakowska, D.; Grabowski, K.; Nienartowicz, M.; Zarębski, P.; Fudalej, K.; Markocka-Mączka, K. Circulating Oxidized Low-Density Lipoproteins and Antibodies against Oxidized Low-Density Lipoproteins as Potential Biomarkers of Colorectal Cancer. Gastroenterol. Res. Pract. 2015, 2015, 146819. [Google Scholar] [CrossRef]
- Bersanelli, M.; Cortellini, A.; Buti, S. The interplay between cholesterol (and other metabolic conditions) and immune-checkpoint immunotherapy: Shifting the concept from the “inflamed tumor” to the “inflamed patinets”. Hum. Vaccin. Immunother. 2021, 17, 1930–1934. [Google Scholar] [CrossRef]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C. Potentiating the antitumor response of CD8+ T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef]
- Perrone, F.; Minari, R.; Bersanelli, M.; Bordi, P.; Tiseo, M.; Favari, E.; Sabato, R.; Buti, S. The Prognostic Role of High Blood Cholesterol in Advanced Cancer Patients Treated with Immune Checkpoint Inhibitors. J. Immunother. 2020, 43, 196–203. [Google Scholar] [CrossRef]
- Mbikay, M.; Chrétien, M. The Biological Relevance of PCSK9, When Less is Better. Biochem. Cell Biol. 2022. [Google Scholar] [CrossRef]
- Seidah, N.G. The PCSK9 discovery, an inactive protease with varied functions in hypercholesterolemia, viral infections, and cancer. J. Lipid Res. 2021, 62, 100130. [Google Scholar] [CrossRef]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Steiner, H.; Kwan, W.; Shaffer, T.G.; Walker, S.; Miller, S.; Sagar, A.; Lock, J. Risk and protective factors for juvenile eating disorders. Eur. Child. Adolesc. Psychiatry 2003, 12, 38–46. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Delvecchio, E.; Di Riso, D.; Salcuni, S.; Lis, A.; George, C. Anorexia and attachment: Dysregulated defense and pathological mourning. Front. Psychol. 2014, 5, 1218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lingiardi, V.; McWilliams, N. PDM-2. Manuale Diagnostico Psicodinamico; Cortina: Milano, Italy, 2018. [Google Scholar]
- Esposito, R.; Cieri, F.; Di Giannantonio, M.; Tartaro, A. The role of body image and self-perception in anorexia nervosa: The neuroimaging perspective. J. Neuropsychol. 2018, 12, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Smeets, M.A.; Kosslyn, S.M. Hemispheric differences in body image in anorexia nervosa. Int. J. Eat. Dis. 2001, 29, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, S.; Quattrocchi, C.C. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci. Biobehav. Rev. 2012, 36, 1839–1847. [Google Scholar] [CrossRef]
- Kitada, R.; Johnsrude, I.S.; Kochiyama, T.; Lederman, S.J. Functional specialization andconvergence in the occipito-temporal cortex supporting haptic and visual identification of human faces and body parts: An fMRI study. J. Cogn. Neurosci. 2009, 21, 2027–2045. [Google Scholar] [CrossRef]
- Van Koningsbruggen, M.G.; Peelen, M.V.; Downing, P.E. A causal role for the extrastriate body area in detecting people in real-world scenes. J. Neurosci. 2013, 33, 7003–7010. [Google Scholar] [CrossRef]
- Hummel, D.; Rudolf, A.K.; Brandi, M.L.; Untch, K.H.; Grabhorn, R.; Mohr, H.M. Neural adaptation to thin and fat bodies in the fusiform body area and middle occipital gyrus: An fMRI adaptation study. Hum. Brain Mapp. 2012, 34, 3233–3246. [Google Scholar] [CrossRef]
- Seeger, G.; Braus, D.F.; Ruf, M.; Goldberger, U.; Schmidt, M.H. Body image distortion reveals amygdala activation in patients with anorexia nervosa—A functional magnetic resonance imaging study. Neurosci. Lett. 2002, 326, 25–28. [Google Scholar] [CrossRef]
- Harrison, A.; Tchanturia, K.; Treasure, J. Attentional bias, emotion recognition, and emotion regulation in anorexia: State or trait? Biol. Psychiatry 2010, 68, 755–761. [Google Scholar] [CrossRef]
- Mohr, H.M.; Zimmermann, J.; Röder, C.; Lenz, C.; Overbeck, G.; Grabhorn, R. Separating two components of body image in anorexia nervosa using fMRI. Psychol. Med. 2010, 40, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, A.; Hambrook, D.; Tchanturia, K.; Treasure, J.; Schmidt, U. Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosom. Med. 2010, 2, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, S.; Wehmeier, P.M.; Remschmidt, H. Body image assessment using body size estimation in recent studies on anorexia nervosa. A brief review. Eur. Child Adolesc. Psychiatry 2001, 10, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.; Sowden, P.T. Your hand or mine? The extrastriate body area. NeuroImage 2008, 42, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Tasegian, A.; Curcio, F.; Dalla Ragione, L.; Rossetti, F.; Cataldi, S.; Codini, M.; Ambesi-Impiombato, F.S.; Beccari, T.; Albi, E. Hypovitaminosis D3, Leukopenia, and Human Serotonin Transporter Polymorphism in Anorexia Nervosa and Bulimia Nervosa. Mediators Inflamm. 2016, 2016, 8046479. [Google Scholar] [CrossRef]
- Zák, A.; Vecka, M.; Tvrzická, E.; Novák, F.; Papezová, H.; Hrubý, M.; Lubanda, H.; Stanková, B. Lipid metabolism in anorexia nervosa. Cas. Lek. Cesk. 2003, 142, 280–284. [Google Scholar]
- Matzkin, V.B.; Geissler, C.; Coniglio, R.; Selles, J.; Bello, M. Cholesterol concentrations in patients with Anorexia Nervosa and in healthy controls. Int. J. Psychiatry Nurs. Res. 2006, 11, 1283–1293. [Google Scholar]
- Hussain, A.A.; Hübel, C.; Hindborg, M.; Lindkvist, E.; Kastrup, A.M.; Yilmaz, Z.; Støving, R.K.; Bulik, C.M.; Sjögren, J.M. Increased lipid and lipoprotein concentrations in anorexia nervosa: A systematic review and meta-analysis. Int. J. Eat. Disord. 2019, 52, 611–629. [Google Scholar] [CrossRef]
- Hole, K.; Heiberg, P.L.; Gjestad, C.; Mehus, L.L.; Rø, Ø.; Molden, E. Elevated 4β-hydroxycholesterol/cholesterol ratio in anorexia nervosa patients. Pharmacol. Res. Perspect. 2018, 6, e00430. [Google Scholar] [CrossRef]
- Feillet, F.; Feillet-Coudray, C.; Bard, J.M.; Parra, H.J.; Favre, E.; Kabuth, B.; Fruchart, J.C.; Vidailhet, M. Plasma cholesterol and endogenous cholesterol synthesis during refeeding in anorexia nervosa. Clin. Chim. Acta 2000, 294, 45–56. [Google Scholar] [CrossRef]
- Schmalbach, I.; Herhaus, B.; Pässler, S.; Runst, S.; Berth, H.; Wolff-Stephan, S.; Petrowski, K. Cortisol reactivity in patients with anorexia nervosa after stress induction. Transl. Psychiatry 2020, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J. Cholesterol metabolism in anorexia nervosa and hypercholesterolemia. J. Clin. Endocrinol. Metab. 1974, 38, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, D.; Tallonneau, I.; Vergès, B. Hypercholesterolaemia in anorexia nervosa: Frequency and changes during refeeding. Diabetes Metab. 2009, 35, 57–63. [Google Scholar] [CrossRef]
- Favaro, A.; Caregaro, L.; Di Pascoli, L.; Brambilla, F.; Santonastaso, P. Total serum cholesterol and suicidality in anorexia nervosa. Psychosom. Med. 2004, 66, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Jáuregui-Garrido, B.; Bolaños-Ríos, P.; Santiago-Fernández, M.J.; Jaúregui-Lobera, I. Lipid profile and cardiovascular risk in anorexia nervosa; the effect of nutritional treatment. Nutr. Hosp. 2012, 27, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, S.; Sukkar, S.G.; Rosa, G.M.; Zappi, A.; Bezante, G.P.; Balbi, M.; Brunelli, C. Anorexia nervosa and heart disease: A systematic review. Eat. Weight Disord. 2019, 24, 199–207. [Google Scholar] [CrossRef]
- Tomášová, P.; Procházková, P.; Roubalová, R.; Dvořák, J.; Tlaskalová-Hogenová, H.; Čermáková, M.; Pelantová, H.; Šedivá, B.; Vecka, M.; Papežová, H.; et al. NMR- and MS-Based Untargeted Metabolomic Study of Stool and Serum Samples from Patients with Anorexia Nervosa. J. Proteome Res. 2022, 21, 778–787. [Google Scholar] [CrossRef]
- Albi, E.; Peloso, I.; Magni, M.V. Nuclear membrane sphingomyelin-cholesterol changes in rat liver after hepatectomy. Biochem. Biophys. Res. Commun. 1999, 262, 692–695. [Google Scholar] [CrossRef]
- Albi, E.; Magni, M.V. The presence and the role of chromatin cholesterol in rat liver regeneration. J. Hepatol. 2002, 36, 395–400. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, K.; Wang, X.; Jia, Z.; Yang, Y.; Duan, Y.; Huang, L.; Wu, Z.X.; Zhang, J.Y.; Ding, X. Cholesterol promotes EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1 signaling-mediated ERRα re-expression. Mol. Cancer 2022, 21, 77. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wu, D.; Ren, X.; Wu, W.; Zuo, R.; Zeng, Q.; Wang, B.; He, X.; Yun, J.; et al. ERRalpha is an aggressive factor in lung adenocarcinoma indicating poor prognostic outcomes. Cancer Manag. Res. 2019, 11, 8111–8123. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gizzi, G.; Cataldi, S.; Mazzeschi, C.; Delvecchio, E.; Ceccarini, M.R.; Codini, M.; Albi, E. Hypercholesterolemia in Cancer and in Anorexia Nervosa: A Hypothesis for a Crosstalk. Int. J. Mol. Sci. 2022, 23, 7466. https://doi.org/10.3390/ijms23137466
Gizzi G, Cataldi S, Mazzeschi C, Delvecchio E, Ceccarini MR, Codini M, Albi E. Hypercholesterolemia in Cancer and in Anorexia Nervosa: A Hypothesis for a Crosstalk. International Journal of Molecular Sciences. 2022; 23(13):7466. https://doi.org/10.3390/ijms23137466
Chicago/Turabian StyleGizzi, Giulia, Samuela Cataldi, Claudia Mazzeschi, Elisa Delvecchio, Maria Rachele Ceccarini, Michela Codini, and Elisabetta Albi. 2022. "Hypercholesterolemia in Cancer and in Anorexia Nervosa: A Hypothesis for a Crosstalk" International Journal of Molecular Sciences 23, no. 13: 7466. https://doi.org/10.3390/ijms23137466
APA StyleGizzi, G., Cataldi, S., Mazzeschi, C., Delvecchio, E., Ceccarini, M. R., Codini, M., & Albi, E. (2022). Hypercholesterolemia in Cancer and in Anorexia Nervosa: A Hypothesis for a Crosstalk. International Journal of Molecular Sciences, 23(13), 7466. https://doi.org/10.3390/ijms23137466







