A Cruciform Petal-like (ZIF-8) with Bactericidal Activity against Foodborne Gram-Positive Bacteria for Antibacterial Food Packaging
Abstract
:1. Introduction
2. Results and Discussion
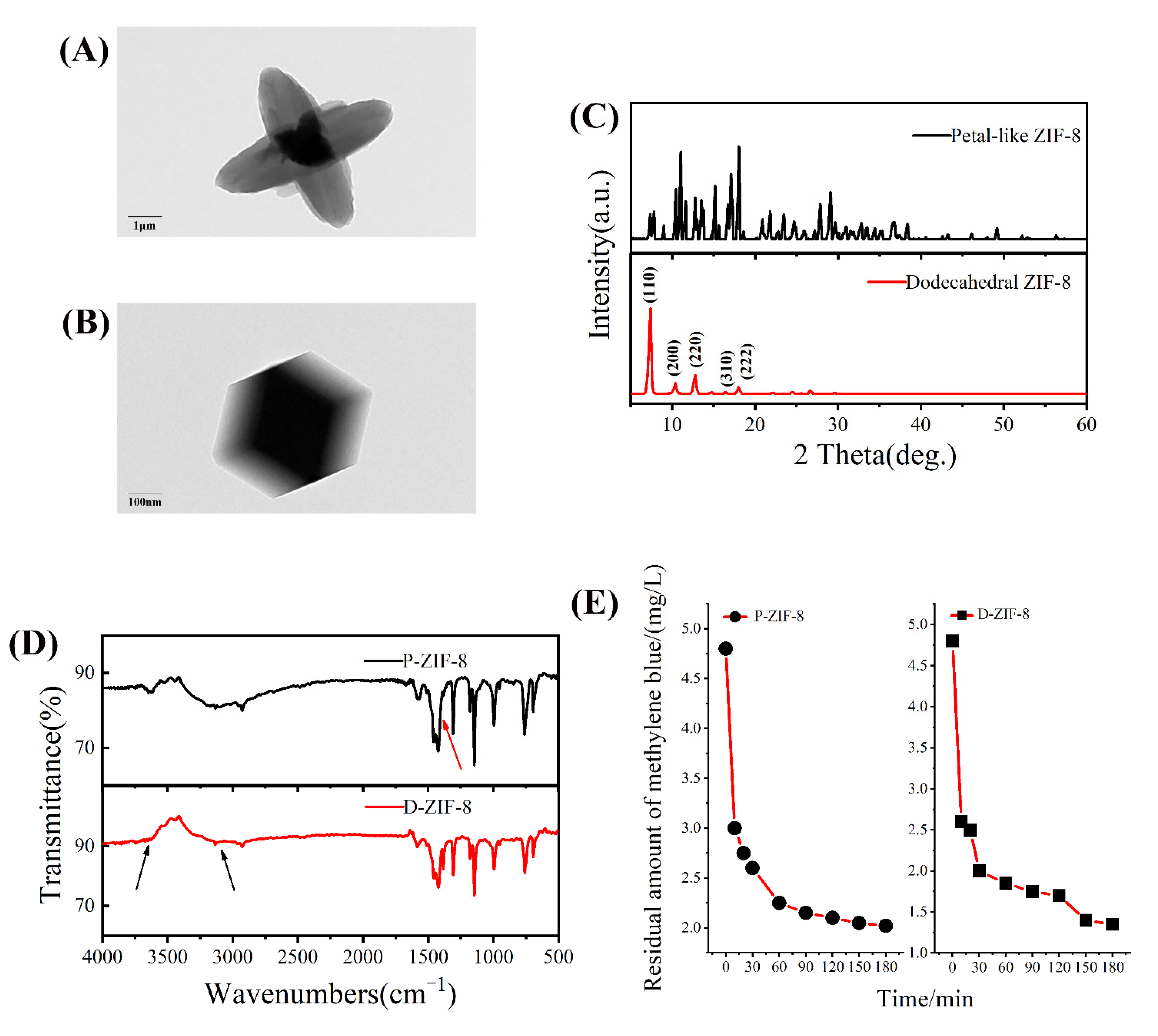
2.1. Characterization of ZIF-8
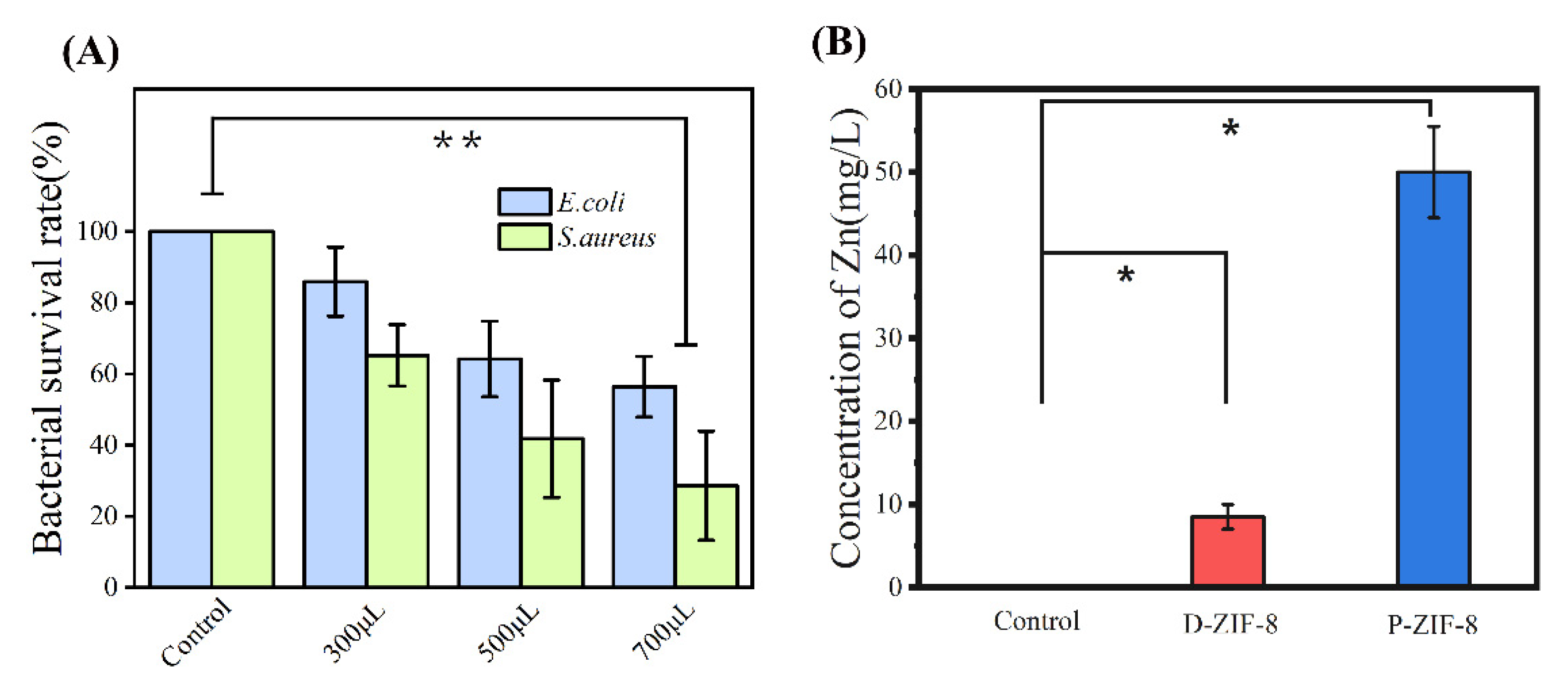
2.2. Antibacterial Activity against Foodborne Bacteria of ZIF-8
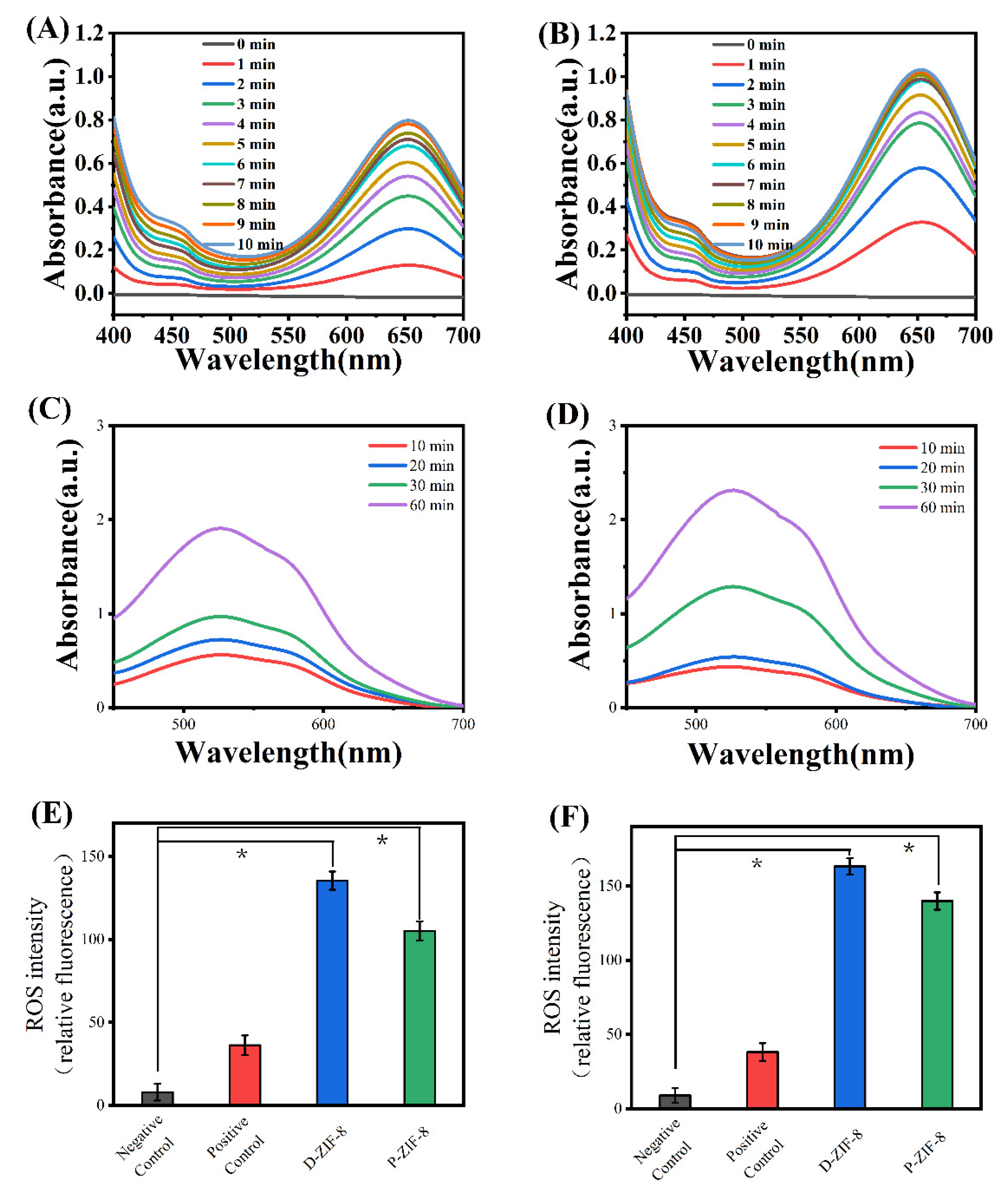
2.3. Antibacterial Mechanism of ROS Generation by ZIF-8
2.4. Antibacterial Mechanism of ZIF-8 Involves Active Zn Compounds
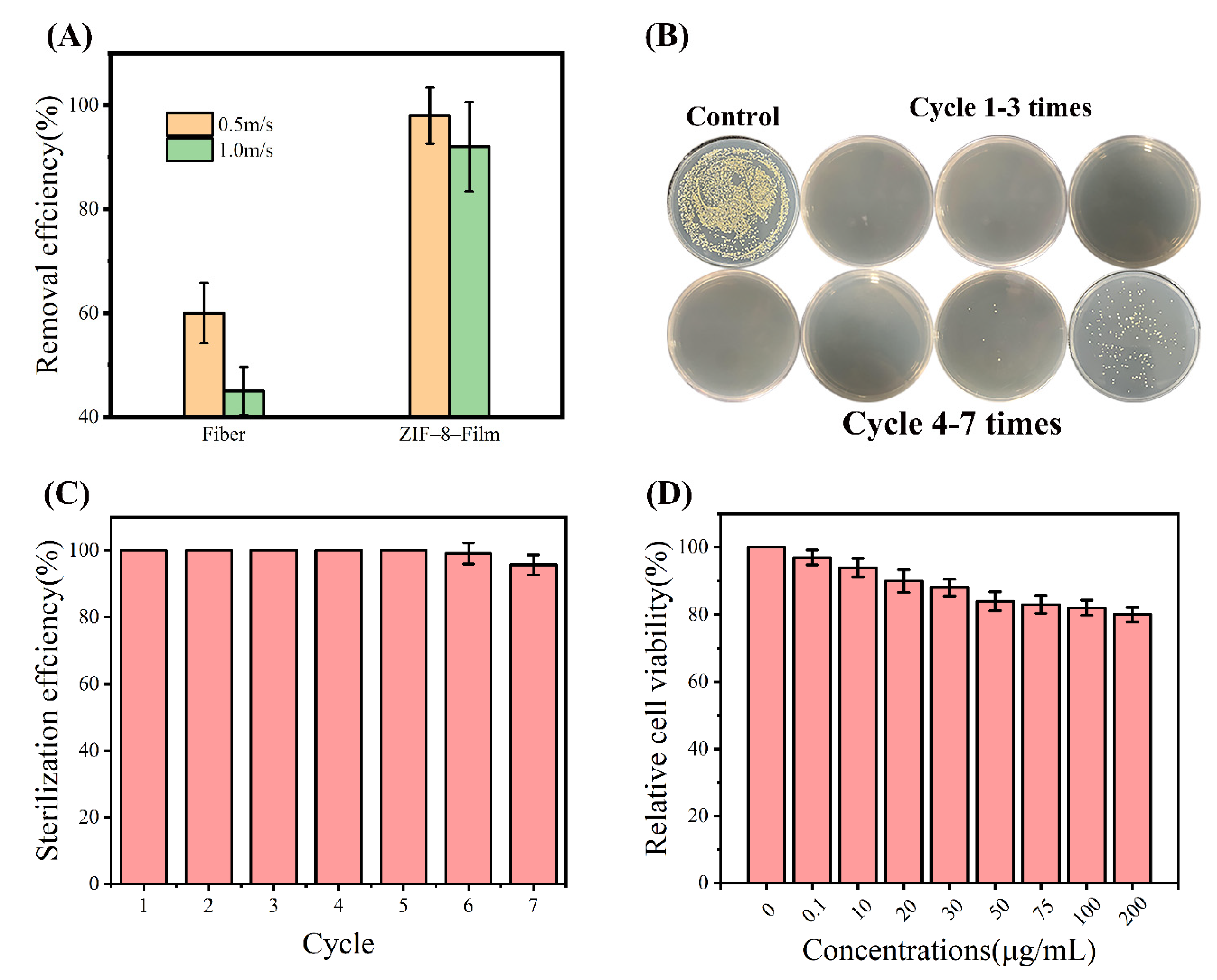
2.5. Synthesis and Filtering Ability of ZIF-8-Film
2.6. Antibacterial Activity of the ZIF-8-Film
3. Materials and Methods
3.1. Strains and Reagents
3.2. Synthesis of ZIF-8
3.3. Materials Characterization
3.4. Antibacterial Assays
3.4.1. Cultivation of Bacteria
3.4.2. Instant Antibacterial Effect
3.4.3. Measuring the Minimum Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
3.5. Photocatalysis Experiment
3.6. Determination of H2O2 Produced by ZIF-8
3.7. Superoxide Anion Detection
3.8. Determination of Hydroxyl Radical
3.9. Intracellular ROS Detection
3.10. Antibacterial Assays of Zine Ion
3.11. Antibacterial Assays of MOF Extract
3.12. Preparation of ZIF-8-Film
3.13. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.N.M.A. Food safety and public health issues in Bangladesh: A regulatory concern. Eur. Food Feed Law Rev. 2013, 8, 31–40. [Google Scholar]
- Fukuda, K. Food safety in a globalized world. Bull. World Health Organ. 2015, 93, 212. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Park, G.; Han, S. Biocompatible, antibacterial, polymeric hydrogels active against multidrug-resistant Staphylococcus aureus strains for food packaging applications. Food Control 2021, 123, 107695. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Broussolle, V.; Colin, P.; Nguyen-The, C.; Prieto, M. The adaptive response of bacterial food-borne pathogens in the environment, host and food: Implications for food safety. Int. J. Food Microbiol. 2015, 213, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, M.; Tauxe, R.; Hedberg, C. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol. Infect. 2009, 137, 307–315. [Google Scholar]
- Zhou, C.; Koshani, R.; OBrieen, B.; Ronholm, J.; Cao, X.; Wang, Y. Bio-inspired mechano-bactericidal nanostructures: A promising strategy for eliminating surface foodborne bacteria. Curr. Opin. Food Sci. 2021, 39, 110–119. [Google Scholar] [CrossRef]
- Omerović, N.; Djisalov, M.; Živojević, K.; Mladenović, M.; Vunduk, J.; Milenković, I.; Knežević, N.Ž.; Gadjanski, I.; Vidić, J. Antimicrobial nanoparticles and biodegradable polymer composites for active food packaging applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2428–2454. [Google Scholar]
- Han, J. A review of food packaging technologies and innovations. Innov. Food Packag. 2014, 3–12. [Google Scholar] [CrossRef]
- Sothornvit, R. Nanostructured materials for food packaging systems: New functional properties. Curr. Opin. Food Sci. 2019, 25, 82–87. [Google Scholar] [CrossRef]
- Wang, Y.; Malkmes, M.J.; Jiang, C.; Wang, P.; Zhu, L.; Zhang, H.; Zhang, Y.; Huang, H.; Jiang, L. Antibacterial mechanism and transcriptome analysis of ultra-small gold nanoclusters as an alternative of harmful antibiotics against Gram-negative bacteria. J. Hazard. Mater. 2021, 416, 126236. [Google Scholar] [CrossRef]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T.F. Antibacterial applications of metal–organic frameworks and their composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Zhu, Y.; Wan, X.; Zhang, X.; Zhang, J. Colloidal semiconductor nanocrystals for biological photodynamic therapy applications: Recent progress and perspectives. Prog. Nat. Sci. 2020, 30, 443–455. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Kandler, O.J.B.R. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Shen, B.; Song, Z.; Jiang, L. Permeabilized TreS-Expressing Bacillus subtilis Cells Decorated with Glucose Isomerase and a Shell of ZIF-8 as a Reusable Biocatalyst for the Coproduction of Trehalose and Fructose. J. Agric. Food Chem. 2020, 68, 4464–4472. [Google Scholar] [CrossRef]
- Liu, T.; Malkmes, M.J.; Zhu, L.; Huang, H.; Jiang, L. Metal-organic frameworks coupling simultaneous saccharication and fermentation for enhanced butyric acid production from rice straw under visible light by Clostridium tyrobutyricum CtΔack: cat1. Bioresour. Technol. 2021, 332, 125117. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Feng, X.; Li, J.; Hao, Y.; Zhang, J.; Wang, H.; Yin, A.; Zhou, J.; Ma, X. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Taheri, M.; Ashok, D.; Sen, T.; Enge, T.G.; Verma, N.K.; Tricoli, A.; Lowe, A.; Nisbet, D.R.; Tsuzuki, T. Stability of ZIF-8 nanopowders in bacterial culture media and its implication for antibacterial properties. Chem. Eng. J. 2021, 413, 127511. [Google Scholar] [CrossRef]
- Pei, Z.; Fei, P.; Zhang, A.; Guo, J.; Hao, J.; Jia, J.; Dong, H.; Shen, Q.; Wei, L.; Jia, H.; et al. Thermal oxygen sensitization modification and its visible light catalytic antibacterial performance for ZIF-8. J. Alloys Compd. 2022, 904, 164055. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, H.; Zhang, K.; Ma, G.; Zhu, L.; Zhou, L.; Yan, B. Oxygen-defective MnO2/ZIF-8 nanorods with enhanced antibacterial activity under solar light. Chem. Eng. J. 2022, 428, 131349. [Google Scholar] [CrossRef]
- Kalati, M.; Kamran, A. Optimizing the metal ion release and antibacterial activity of ZnO@ ZIF-8 by modulating its synthesis method. New J. Chem. 2021, 45, 22924–22931. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhao, M.; Lin, Y. Stability of ZIF-8 in water under ambient conditions. Microporous Mesoporous Mater. 2019, 279, 201–210. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, M.; Yang, Y.; Lin, Y. Hydrolysis and condensation of ZIF-8 in water. Microporous Mesoporous Mater. 2019, 288, 109568. [Google Scholar] [CrossRef]
- Moh, P.Y.; Brenda, M.; Anderson, M.W.; Attfield, M.P. Crystallisation of solvothermally synthesised ZIF-8 investigated at the bulk, single crystal and surface level. Cryst. Eng. Comm. 2013, 15, 9672–9678. [Google Scholar] [CrossRef]
- Torad, N.L.; Hu, M.; Kamachi, Y.; Takai, K.; Imura, M.; Naito, M.; Yamauchi, Y. Facile synthesis of nanoporous carbons with controlled particle sizes by direct carbonization of monodispersed ZIF-8 crystals. Chem. Commun. 2013, 49, 2521–2523. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Cui, J.; Feng, Y.; Lin, T.; Tan, Z.; Zhong, C.; Jia, S. Mesoporous metal–organic framework with well-defined cruciate flower-like morphology for enzyme immobilization. ACS Appl. Mater. Interfaces 2017, 9, 10587–10594. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef]
- Chin, M.; Cisneros, C.; Araiza, S.M.; Vargas, K.M.; Ishihara, K.M.; Tian, F. Rhodamine B degradation by nanosized zeolitic imidazolate framework-8 (ZIF-8). RSC Adv. 2018, 8, 26987–26997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, H.-P.; Wang, C.-C.; Zhang, Y.-W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Das, B.; Kumar, A.; Tripathy, S.; Roy, S.; Sahu, S.K. One-pot synthesis of multifunctional nanoscale metal-organic frameworks as an effective antibacterial agent against multidrug-resistant Staphylococcus aureus. Nanotechnology 2017, 28, 095102. [Google Scholar] [CrossRef] [PubMed]
- Singbumrung, K.; Motina, K.; Pisitsak, P.; Chitichotpanya, P.; Wongkasemjit, S.; Inprasit, T. Polymers, Preparation of Cu-BTC/PVA fibers with antibacterial applications. Fiber Polym. 2018, 19, 1373–1378. [Google Scholar] [CrossRef]
- Hoop, M.; Walde, C.F.; Riccò, R.; Mushtaq, F.; Terzopoulou, A.; Chen, X.-Z.; de Mello, A.J.; Doonan, C.J.; Falcaro, P.; Nelson, B.J. Biocompatibility characteristics of the metal organic framework ZIF-8 for therapeutical applications. Appl. Mater. Today 2018, 11, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, Y.; Sun, M.; Hanif, A.; Wu, H.; Gu, Q.; Ok, Y.S.; Tsang, D.C.; Li, J.; Yu, J. Thermally treated zeolitic imidazolate framework-8 (ZIF-8) for visible light photocatalytic degradation of gaseous formaldehyde. Chem. Sci. 2020, 11, 6670–6681. [Google Scholar] [CrossRef]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Comm. 2010, 46, 8017–8019. [Google Scholar] [CrossRef]
- Javed, H.; Metz, J.; Eraslan, T.C.; Mathieu, J.; Wang, B.; Wu, G.; Tsai, A.-L.; Wong, M.S.; Alvarez, P. Discerning the relevance of superoxide in PFOA degradation. Environ. Sci. Technol. Lett. 2020, 7, 653–658. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. Compounds, A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloys Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Tu, Y.; Lei, C.; Deng, F.; Chen, Y.; Wang, Y.; Zhang, Z. Core–shell ZIF-8@ polydopamine nanoparticles obtained by mitigating the polydopamine coating induced self-etching of MOFs: Prototypical metal ion reservoirs for sticking to and killing bacteria. New J. Chem. 2021, 45, 8701–8713. [Google Scholar] [CrossRef]
- Schulte, M.; Mattay, D.; Kriegel, S.; Hellwig, P.; Friedrich, T. Inhibition of Escherichia coli respiratory complex I by Zn2+. Biochemistry 2014, 53, 6332–6339. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Sustainable microbial cell nanofactory for zinc oxide nanoparticles production by zinc-tolerant probiotic Lactobacillus plantarum strain TA4. Microb. Cell Factories. 2020, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.; Wu, Y.; Li, Y. Hollow Zn/Co ZIF particles derived from core–shell ZIF-67@ ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem. Int. Ed. Engl. 2015, 127, 11039–11043. [Google Scholar] [CrossRef]
- Säbel, C.E.; Neureuther, J.M.; Siemann, S. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 2010, 397, 218–226. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Liao, Y.; Yu, H.; Liang, Z.; Zhang, B.; Lan, X.; Luo, R.; Wang, Y. Superhydrophilic versus normal polydopamine coating: A superior and robust platform for synergistic antibacterial and antithrombotic properties. Chem. Eng. J. 2020, 402, 126196. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Z.; Zhang, X.; Diao, D. Superhydrophobic, photo-sterilize, and reusable mask based on graphene nanosheet-embedded carbon (GNEC) film. Nano Res. 2021, 14, 1110–1115. [Google Scholar] [CrossRef]
- Seymour, I.; Appleton, H. Foodborne viruses and fresh produce. J. Appl. Microbiol. 2001, 91, 759. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Cao, X.; Liu, Q.; Wang, H.; Kong, B. Preparation and functional properties of poly (vinyl alcohol)/ethyl cellulose/tea polyphenol electrospun nanofibrous films for active packaging material. Food Control. 2021, 130, 108331. [Google Scholar] [CrossRef]
- Shevtsova, T.; Caxallaro, G.; Lazzara, G.; Milioto, S.; Donchak, V.; Harhay, K.; Korolko, S.; Budkowski, A.; Stetsyshyn, Y. Temperature-responsive hybrid nanomaterials based on modified halloysite nanotubes uploaded with silver nanoparticles. Colloid Surf. A 2022, 641, 128525. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinell, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef]
- Shen, B.; Wang, B.; Zhu, L.; Jiang, L.J.N. Properties of cobalt-and nickel-doped ZIF-8 framework materials and their application in heavy-metal removal from wastewater. Nanomaterials 2020, 10, 1636. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, B.; Wang, Y.; Wang, X.; Amal, F.E.; Zhu, L.; Jiang, L. A Cruciform Petal-like (ZIF-8) with Bactericidal Activity against Foodborne Gram-Positive Bacteria for Antibacterial Food Packaging. Int. J. Mol. Sci. 2022, 23, 7510. https://doi.org/10.3390/ijms23147510
Shen B, Wang Y, Wang X, Amal FE, Zhu L, Jiang L. A Cruciform Petal-like (ZIF-8) with Bactericidal Activity against Foodborne Gram-Positive Bacteria for Antibacterial Food Packaging. International Journal of Molecular Sciences. 2022; 23(14):7510. https://doi.org/10.3390/ijms23147510
Chicago/Turabian StyleShen, Bowen, Yuxian Wang, Xinlong Wang, Fatima Ezzahra Amal, Liying Zhu, and Ling Jiang. 2022. "A Cruciform Petal-like (ZIF-8) with Bactericidal Activity against Foodborne Gram-Positive Bacteria for Antibacterial Food Packaging" International Journal of Molecular Sciences 23, no. 14: 7510. https://doi.org/10.3390/ijms23147510
APA StyleShen, B., Wang, Y., Wang, X., Amal, F. E., Zhu, L., & Jiang, L. (2022). A Cruciform Petal-like (ZIF-8) with Bactericidal Activity against Foodborne Gram-Positive Bacteria for Antibacterial Food Packaging. International Journal of Molecular Sciences, 23(14), 7510. https://doi.org/10.3390/ijms23147510





