Can a Scaffold Enriched with Mesenchymal Stem Cells Be a Good Treatment for Spinal Cord Injury?
Abstract
:1. Spinal Cord Injury
2. Biomaterials and Scaffold
2.1. Collagen
2.2. Fibrin
2.3. Chitosan
2.4. Poly(lactic-co-glycolic) Acid (PLGA)
3. Mesenchymal Stem Cells
4. Combined Approaches of MSCs and Scaffolds
4.1. Combination of MSCs with Collagen in SCI Models
4.2. Combination of MSCs with Fibrin in SCI Models
4.3. Combination of MSCs with Chitosan in SCI Models
4.4. Combination of MSCs with PLGA in SCI Models
5. Clinical Studies with Scaffold Enriched with MSCs for Treatment of Spinal Cord Injury
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anjum, A.; Yazid, M.D.; Daud, M.F.; Idris, J.; Ng, A.M.H.; Naicker, A.S.; Ismail, O.H.R.; Kumar, R.K.A.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Müller-Jensen, L.; Ploner, C.J.; Kroneberg, D.; Schmidt, W.U. Clinical Presentation and Causes of Non-traumatic Spinal Cord Injury: An Observational Study in Emergency Patients. Front. Neurol. 2021, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Marshall, R. Nature of the Non-traumatic Spinal Cord Injury Literature: A Systematic Review. Top. Spinal Cord Inj. Rehabil. 2017, 23, 353–367. [Google Scholar] [CrossRef]
- Grumbles, R.M.; Thomas, C.K. Motoneuron Death after Human Spinal Cord Injury. J. Neurotrauma 2017, 34, 581–590. [Google Scholar] [CrossRef]
- Bains, M.; Hall, E.D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Harry, G.J.; Kraft, A.D. Neuroinflammation and microglia: Considerations and approaches for neurotoxicity assessment. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1265–1277. [Google Scholar] [CrossRef]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wu, C.; Wu, Y.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review). Mol. Med. Rep. 2021, 23, 417. [Google Scholar] [CrossRef]
- Stein, D.M.; Sheth, K.N. Management of Acute Spinal Cord Injury. Contin. Lifelong Learn. Neurol. 2015, 21, 159–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, N.; Balain, B. Spinal cord injury—The role of surgical treatment for neurological improvement. J. Clin. Orthop. Trauma 2017, 8, 99–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshigiri, T.; Sasaki, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Oka, S.; Morita, T.; Hirota, R.; Yoshimoto, M.; Yamashita, T.; et al. Intravenous Infusion of Mesenchymal Stem Cells Alters Motor Cortex Gene Expression in a Rat Model of Acute Spinal Cord Injury. J. Neurotrauma 2019, 36, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Youn Oh, J. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Honmou, O.; Harada, K.; Nakamura, K.; Houkin, K.; Hamada, H.; Kocsis, J.D. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 2006, 129, 2734–2745. [Google Scholar] [CrossRef]
- Sasaki, Y.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Nagahama, H.; Suzuki, J.; Tateyama, D.; Oka, S.; Namioka, T.; Namioka, A.; et al. Synergic Effects of Rehabilitation and Intravenous Infusion of Mesenchymal Stem Cells after Stroke in Rats. Phys. Ther. 2016, 96, 1791–1798. [Google Scholar] [CrossRef] [Green Version]
- Steward, O.; Sharp, K.G.; Yee, K.M.; Hatch, M.N.; Bonner, J.F. Characterization of Ectopic Colonies That Form in Widespread Areas of the Nervous System with Neural Stem Cell Transplants into the Site of a Severe Spinal Cord Injury. J. Neurosci. 2014, 34, 14013–14021. [Google Scholar] [CrossRef] [Green Version]
- Bramanti, P.; Mazzon, E. The combined strategy of mesenchymal stem cells and tissue-engineered scaffolds for spinal cord injury regeneration. Exp. Ther. Med. 2017, 14, 3355–3368. [Google Scholar] [CrossRef] [Green Version]
- Chudickova, M.; Vackova, I.; Urdzikova, L.M.; Jancova, P.; Kekulova, K.; Rehorova, M.; Turnovcova, K.; Jendelova, P.; Kubinova, S. The Effect of Wharton Jelly-Derived Mesenchymal Stromal Cells and Their Conditioned Media in the Treatment of a Rat Spinal Cord Injury. Int. J. Mol. Sci. 2019, 20, 4516. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.; Marconi, G.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, D.; Sousa, R.; Salgado, A. Hydrogels as delivery systems for spinal cord injury regeneration. Mater. Today Bio 2021, 9, 100093. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2011, 1, 10–25. [Google Scholar] [CrossRef]
- Jiao, J.; Peng, C.; Li, C.; Qi, Z.; Zhan, J.; Pan, S. Dual bio-active factors with adhesion function modified electrospun fibrous scaffold for skin wound and infections therapeutics. Sci. Rep. 2021, 11, 457. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, D.; Wang, S.; Song, D.; Yoon, M.-H. Water-insoluble, nanocrystalline, and hydrogel fibrillar scaffolds for biomedical applications. Polym. J. 2018, 50, 637–647. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Breen, A.; O’Brien, T.; Pandit, A. Fibrin as a Delivery System for Therapeutic Drugs and Biomolecules. Tissue Eng. Part B Rev. 2009, 15, 201–214. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Qi, Y.-Y.; Wang, L.-L.; Yin, Z.; Yin, G.-L.; Zou, X.-H.; Ouyang, H.-W. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials 2008, 29, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, M.A.; Nogueira, G.M.; Weska, R.F.; Beppu, M.M. Preparation and Characterization of Insoluble Silk Fibroin/Chitosan Blend Films. Polymers 2010, 2, 719–727. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Chen, J.; Chen, J. Preparation of heparan sulfate-like polysaccharide and application in stem cell chondrogenic differentiation. Carbohydr. Res. 2015, 401, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Xu, L.; Li, J.; Han, Y.; Li, X.; Mao, Y.; Shang, H.; Fan, Z.; Wang, H. Facilitation of facial nerve regeneration using chitosan-β-glycerophosphate-nerve growth factor hydrogel. Acta Oto-Laryngol. 2016, 136, 585–591. [Google Scholar] [CrossRef]
- Itai, S.; Suzuki, K.; Kurashina, Y.; Kimura, H.; Amemiya, T.; Sato, K.; Nakamura, M.; Onoe, H. Cell-encapsulated chitosan-collagen hydrogel hybrid nerve guidance conduit for peripheral nerve regeneration. Biomed. Microdevices 2020, 22, 81. [Google Scholar] [CrossRef]
- Wang, C.; Cao, X.; Zhang, Y. A novel bioactive osteogenesis scaffold delivers ascorbic acid, β-glycerophosphate, and dexamethasone in vivo to promote bone regeneration. Oncotarget 2017, 8, 31612–31625. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [Green Version]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound Tissue Can Utilize a Polymeric Template to Synthesize a Functional Extension of Skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Karamichos, D.; Lakshman, N.; Petroll, W.M. Regulation of Corneal Fibroblast Morphology and Collagen Reorganization by Extracellular Matrix Mechanical Properties. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5030–5037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spill, F.; Andasari, V.; Mak, M.; Kamm, R.D.; Zaman, M.H. Effects of 3D geometries on cellular gradient sensing and polarization. Phys. Biol. 2016, 13, 036008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickström, S.A.; Niessen, C.M. Cell adhesion and mechanics as drivers of tissue organization and differentiation: Local cues for large scale organization. Curr. Opin. Cell Biol. 2018, 54, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Bao, M.; Bruekers, S.M.C.; Huck, W.T.S. Collagen Gels with Different Fibrillar Microarchitectures Elicit Different Cellular Responses. ACS Appl. Mater. Interfaces 2017, 9, 19630–19637. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Houle, J.D.; Xu, J.; Chan, B.P.; Chew, S.Y. Nanofibrous Collagen Nerve Conduits for Spinal Cord Repair. Tissue Eng. Part A 2012, 18, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [Green Version]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Healthc. Mater. 2017, 7, 1701035. [Google Scholar] [CrossRef] [PubMed]
- Luderer, F.; Löbler, M.; Rohm, H.W.; Gocke, C.; Kunna, K.; Köck, K.; Kroemer, H.K.; Weitschies, W.; Schmitz, K.-P.; Sternberg, K. Biodegradable Sirolimus-loaded Poly(lactide) Nanoparticles as Drug Delivery System for the Prevention of In-Stent Restenosis in Coronary Stent Application. J. Biomater. Appl. 2010, 25, 851–875. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 2017, 93, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.-L.; Ji, L.-M.; Zhang, L.-N. Mannotriose induced differentiation of mesenchymal stem cells into neuron-like cells. J. Integr. Neurosci. 2021, 20, 125–130. [Google Scholar] [CrossRef]
- Syková, E.; Homola, A.; Mazanec, R.; Lachmann, H.; Konrádová, L.; Kobylka, P.; Pádr, R.; Neuwirth, J.; Komrska, V.; Vávra, V.; et al. Autologous Bone Marrow Transplantation in Patients with Subacute and Chronic Spinal Cord Injury. Cell Transplant. 2006, 15, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-S.; Fujita, N.; Kawahara, N.; Yui, S.; Nam, E.; Nishimura, R. A Comparison of Neurosphere Differentiation Potential of Canine Bone Marrow-Derived Mesenchymal Stem Cells and Adipose-Derived Mesenchymal Stem Cells. J. Vet. Med. Sci. 2013, 75, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-J.; Liu, H.-Y.; Chang, Y.-T.; Cheng, Y.-H.; Mersmann, H.J.; Kuo, W.-H.; Ding, S.-T. Isolation and Differentiation of Adipose-Derived Stem Cells from Porcine Subcutaneous Adipose Tissues. J. Vis. Exp. 2016, 109, e53886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prpar Mihevc, S.; Kokondoska Grgich, V.; Kopitar, A.N.; Mohorič, L.; Majdič, G. Neural differentiation of canine mesenchymal stem cells/multipotent mesenchymal stromal cells. BMC Vet. Res. 2020, 16, 282. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.-T.; Liang, A.; Liu, S. Concise Reviews: Characteristics and Potential Applications of Human Dental Tissue-Derived Mesenchymal Stem Cells. Stem Cells 2014, 33, 627–638. [Google Scholar] [CrossRef]
- Li, D.; Zou, X.-Y.; El-Ayachi, I.; Romero, L.O.; Yu, Z.; Iglesias-Linares, A.; Cordero-Morales, J.F.; Huang, G.T.-J. Human Dental Pulp Stem Cells and Gingival Mesenchymal Stem Cells Display Action Potential Capacity In Vitro after Neuronogenic Differentiation. Stem Cell Rev. Rep. 2018, 15, 67–81. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Wang, X.; Key, B.; Lee, B.H.; Li, H.; Ye, Q. Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int. 2018, 2018, 1731289. [Google Scholar] [CrossRef] [Green Version]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, A.; Mazzon, E. Dental Mesenchymal Stem Cell Secretome: An Intriguing Approach for Neuroprotection and Neuroregeneration. Int. J. Mol. Sci. 2021, 23, 456. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krabbe, C.; Zimmer, J.; Meyer, M. Neural transdifferentiation of mesenchymal stem cells—A critical review. APMIS 2005, 113, 831–844. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; McNamara, L.M. Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 597661. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mei, X.; Lv, S.; Zhang, Z.; Xu, J.; Sun, D.; Xu, J.; He, X.; Chi, G.; Li, Y. Rat vibrissa dermal papilla cells promote healing of spinal cord injury following transplantation. Exp. Ther. Med. 2018, 15, 3929–3939. [Google Scholar] [CrossRef]
- Peng, Z.; Gao, W.; Yue, B.; Jiang, J.; Gu, Y.; Dai, J.; Chen, L.; Shi, Q. Promotion of neurological recovery in rat spinal cord injury by mesenchymal stem cells loaded on nerve-guided collagen scaffold through increasing alternatively activated macrophage polarization. J. Tissue Eng. Regen. Med. 2016, 12, e1725–e1736. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Xiao, Z.; Yin, W.; Zhao, Y.; Tan, J.; Chen, B.; Jiang, X.; Dai, J. Different functional bio-scaffolds share similar neurological mechanism to promote locomotor recovery of canines with complete spinal cord injury. Biomaterials 2019, 214, 119230. [Google Scholar] [CrossRef]
- Deng, W.-S.; Ma, K.; Liang, B.; Liu, X.-Y.; Xu, H.-Y.; Zhang, J.; Shi, H.-Y.; Sun, H.-T.; Chen, X.Y.; Zhang, S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020, 15, 1686–1700. [Google Scholar] [CrossRef]
- Deng, W.-S.; Liu, X.-Y.; Ma, K.; Liang, B.; Liu, Y.-F.; Wang, R.-J.; Chen, X.-Y.; Zhang, S. Recovery of motor function in rats with complete spinal cord injury following implantation of collagen/silk fibroin scaffold combined with human umbilical cord-mesenchymal stem cells. Rev. Assoc. Med. Bras. 2021, 67, 1342–1348. [Google Scholar] [CrossRef]
- Deng, W.-S.; Yang, K.; Liang, B.; Liu, Y.-F.; Chen, X.-Y.; Zhang, S. Collagen/heparin sulfate scaffold combined with mesenchymal stem cells treatment for canines with spinal cord injury: A pilot feasibility study. J. Orthop. Surg. 2021, 29, 23094990211012293. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, Y.; Xiao, Z.; Chen, B.; Ma, D.; Shen, H.; Gu, R.; Dai, J. Comparison of Regenerative Effects of Transplanting Three-Dimensional Longitudinal Scaffold Loaded-Human Mesenchymal Stem Cells and Human Neural Stem Cells on Spinal Cord Completely Transected Rats. ACS Biomater. Sci. Eng. 2020, 6, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xiao, Z.; Zhao, Y.; Wang, B.; Li, X.; Li, J.; Dai, J. Collagen scaffold combined with human umbilical cord-derived mesenchymal stem cells promote functional recovery after scar resection in rats with chronic spinal cord injury. J. Tissue Eng. Regen. Med. 2018, 12, e1154–e1163. [Google Scholar] [CrossRef]
- Han, S.; Xiao, Z.; Li, X.; Zhao, H.; Wang, B.; Qiu, Z.; Mei, X.; Xu, B.; Fan, C.; Chen, B.; et al. Human placenta-derived mesenchymal stem cells loaded on linear ordered collagen scaffold improves functional recovery after completely transected spinal cord injury in canine. Sci. China Life Sci. 2018, 61, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sabino, L.; Maria, C.; Luca, L.; Valerio, V.; Edda, F.; Giacomo, R.; Gloria, I.; Juan, G.; Antonio, C. Engraftment, neuroglial transdifferentiation and behavioral recovery after complete spinal cord transection in rats. Surg. Neurol. Int. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Chandrababu, K.; Sreelatha, H.V.; Sudhadevi, T.; Anil, A.; Arumugam, S.; Krishnan, L.K. In vivo neural tissue engineering using adipose-derived mesenchymal stem cells and fibrin matrix. J. Spinal Cord Med. 2021, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.; Kostennikov, A.; Zakirova, E.Y.; Galieva, L.R.; Garanina, E.E.; Rogozin, A.A.; Kiassov, A.P.; Rizvanov, A. Adipose-Derived Mesenchymal Stem Cell Application Combined With Fibrin Matrix Promotes Structural and Functional Recovery Following Spinal Cord Injury in Rats. Front. Pharmacol. 2018, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- García, E.; Rodríguez-Barrera, R.; Buzoianu-Anguiano, V.; Flores-Romero, A.; Malagón-Axotla, E.; Guerrero-Godinez, M.; De la Cruz-Castillo, E.; Castillo-Carvajal, L.; Rivas-Gonzalez, M.; Santiago-Tovar, P.; et al. Use of a combination strategy to improve neuroprotection and neuroregeneration in a rat model of acute spinal cord injury. Neural Regen. Res. 2019, 14, 1060–1068. [Google Scholar] [CrossRef]
- Ibarra, A.; Mendieta-Arbesú, E.; Suarez-Meade, P.; Vences, E.E.G.; Martiñón, S.; Rodriguez-Barrera, R.; Lomelí, J.; Flores-Romero, A.; Silva-García, R.; Buzoianu-Anguiano, V.; et al. Motor Recovery after Chronic Spinal Cord Transection in Rats: A Proof-of-Concept Study Evaluating a Combined Strategy. CNS Neurol. Disord. Drug Targets 2019, 18, 52–62. [Google Scholar] [CrossRef]
- Rodríguez-Barrera, R.; Flores-Romero, A.; Buzoianu-Anguiano, V.; Garcia, E.; Soria-Zavala, K.; Incontri-Abraham, D.; Garibay-López, M.; Whaley, J.J.J.-V.; Ibarra, A. Use of a Combination Strategy to Improve Morphological and Functional Recovery in Rats With Chronic Spinal Cord Injury. Front. Neurol. 2020, 11, 189. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.; Shulman, I.; Ogurcov, S.; Kostennikov, A.; Zakirova, E.; Akhmetzyanova, E.; Rogozhin, A.; Masgutova, G.; James, V.; Masgutov, R.; et al. Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models. Biomolecules 2019, 9, 811. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; He, F.; Cao, Z.; Sun, Z.; Chen, Y.; Zhao, H.; Yu, X.; Wang, X.; Yang, Y.; Rosei, F.; et al. Mesenchymal Stem Cell-Laden Hydrogel Microfibers for Promoting Nerve Fiber Regeneration in Long-Distance Spinal Cord Transection Injury. ACS Biomater. Sci. Eng. 2020, 6, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Spejo, A.B.; Chiarotto, G.B.; Ferreira, A.D.F.; Gomes, D.A.; Ferreira, R.S., Jr.; Barraviera, B.; Oliveira, A.L.R. Neuroprotection and immunomodulation following intraspinal axotomy of motoneurons by treatment with adult mesenchymal stem cells. J. Neuroinflamm. 2018, 15, 230. [Google Scholar] [CrossRef] [PubMed]
- Basak, A.T.; Cakici, N.; Bozkurt, G.; Purali, N.; Denkbas, E.B.; Korkusuz, P.; Cetinkaya, D.U. Chitosan channels with stuffed mesenchyme originated stem/progenitor cells for renovate axonal regeneration in complete spinal cord transection. Turk. Neurosurg. 2021, 31, 189–198. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, T.; Chen, Y.; Gao, F.; Guan, F.; Yao, M.-H. A chitosan-based thermosensitive scaffold loaded with bone marrow-derived mesenchymal stem cells promotes motor function recovery in spinal cord injured mice. Biomed. Mater. 2020, 15, 035020. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.-C.; Li, M.; Jiang, W.-T.; Ma, X.; Li, J. Protective effect of brain-derived neurotrophic factor and neurotrophin-3 overexpression by adipose-derived stem cells combined with silk fibroin/chitosan scaffold in spinal cord injury. Neurol. Res. 2020, 42, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Moradi, L.; Katebi, M.; Ai, J.; Azami, M.; Moradveisi, B.; Ostad, S.N. Delivery of injectable thermo-sensitive hydrogel releasing nerve growth factor for spinal cord regeneration in rat animal model. J. Tissue Viability 2020, 29, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, Y.; Ding, J.; Dai, Y.; Le, L.; Wang, L.; Ding, E.; Yang, J. Thermosensitive quaternized chitosan hydrogel scaffolds promote neural differentiation in bone marrow mesenchymal stem cells and functional recovery in a rat spinal cord injury model. Cell Tissue Res. 2021, 385, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Boido, M.; Ghibaudi, M.; Gentile, P.; Favaro, E.; Fusaro, R.; Tonda-Turo, C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci. Rep. 2019, 9, 6402. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Xu, Y.; Wu, J.; Shao, H.; Gao, J.; Feng, X.; Gu, J. A sandwich structured drug delivery composite membrane for improved recovery after spinal cord injury under longtime controlled release. Colloids Surf. B Biointerfaces 2020, 199, 111529. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Zhang, L.-Q.; Zhou, Y.-N.; Chen, X.-M.; Xu, W.-H. Histological and functional outcomes in a rat model of hemisected spinal cord with sustained VEGF/NT-3 release from tissue-engineered grafts. Artif. Cells Nanomed. Biotechnol. 2020, 48, 362–376. [Google Scholar] [CrossRef]
- Ropper, A.E.; Thakor, D.K.; Han, I.; Yu, D.; Zeng, X.; Anderson, J.E.; Aljuboori, Z.; Kim, S.-W.; Wang, H.; Sidman, R.L.; et al. Defining recovery neurobiology of injured spinal cord by synthetic matrix-assisted hMSC implantation. Proc. Natl. Acad. Sci. USA 2017, 114, E820–E829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.-Z.; Zhang, G.-W.; Xu, J.-G.; Chen, S.; Wang, H.; Cao, L.-L.; Liang, B.; Lian, X.-F. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol. Sin. 2017, 38, 623–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, I.-B.; Thakor, D.K.; Ropper, A.E.; Yu, D.; Wang, L.; Kabatas, S.; Zeng, X.; Kim, S.-W.; Zafonte, R.D.; Teng, Y.D. Physical impacts of PLGA scaffolding on hMSCs: Recovery neurobiology insight for implant design to treat spinal cord injury. Exp. Neurol. 2019, 320, 112980. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical Study of NeuroRegen Scaffold Combined with Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, F.; Zhao, Y.; Han, G.; Yin, N.; Li, X.; Chen, B.; Han, S.; Jiang, X.; Yun, C.; et al. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transplant. 2018, 27, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Tang, J.; Zhao, Y.; Zhang, J.; Xiao, Z.; Chen, B.; Han, G.; Yin, N.; Jiang, X.; Zhao, C.; et al. Long-term clinical observation of patients with acute and chronic complete spinal cord injury after transplantation of NeuroRegen scaffold. Sci. China Life Sci. 2021, 65, 909–926. [Google Scholar] [CrossRef]
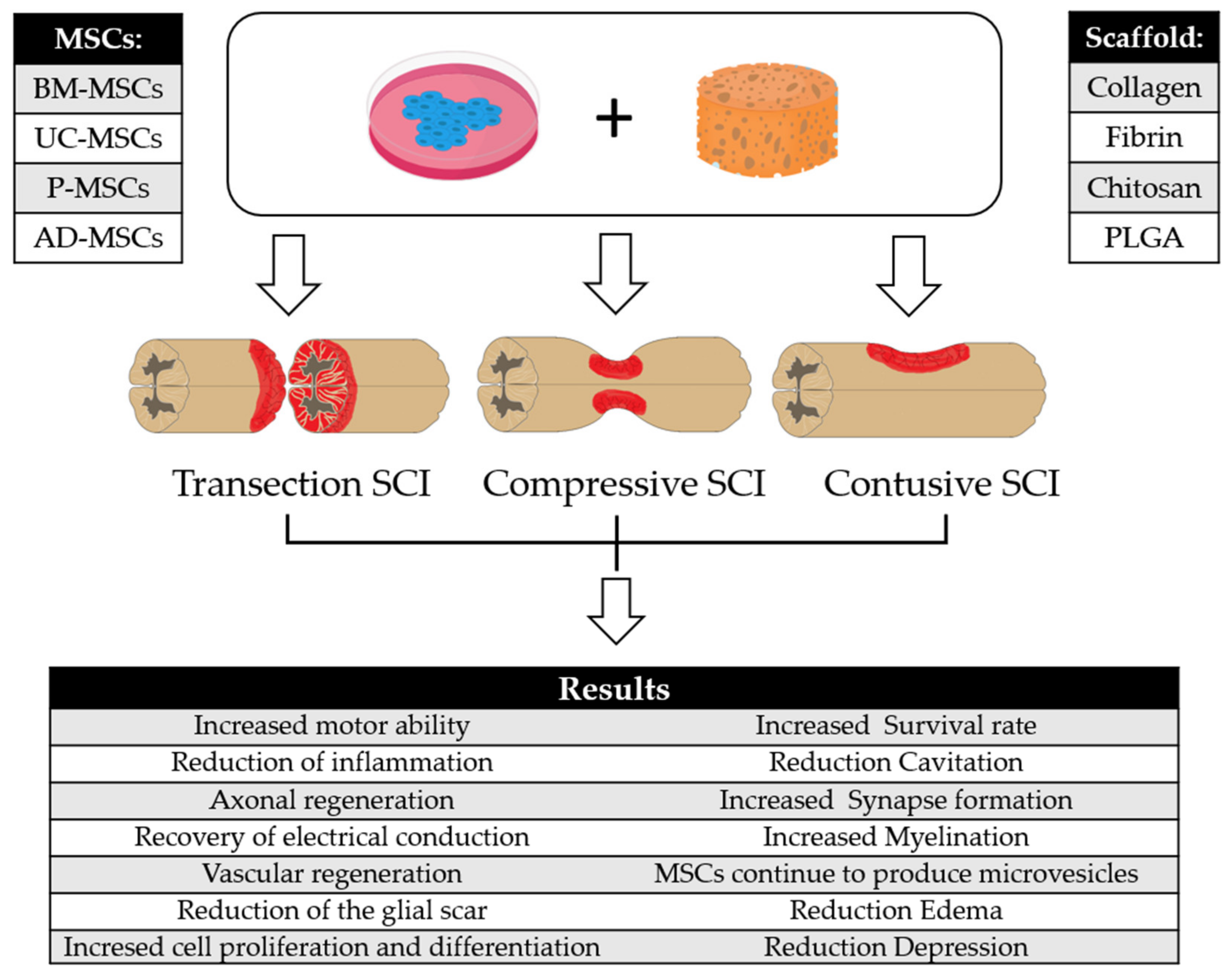
| MSCs | Scaffold | Model | Results | Reference |
|---|---|---|---|---|
| Allogenic BM-MSCs 1 × 106 cells total | 7 µL of rat tail collagen I injection | Rats: completely transected spinal cord Analysis was conducted after 21 days | ↑Axonal regeneration ↑Vascular regeneration ↓Inflammation | [74] |
| Allogenic BM-MSCs 1 × 106 cells total | Porous collagen scaffold with the size of 2 × 2 × 3 mm | Lateral hemisection SCI rat model | ↑Survival rate ↑Motor recovery ↓Glial scar formation | [75] |
| hBM-MSCs 1 × 106 cells total | Collagen scaffold with the dimensions of 0.6–1.5 mm long and 5 mm diameter | Completely transected spinal cord in beagles | ↑Neurogenesis ↑Locomotor recovery | [76] |
| hUC-MSCs 1 × 106 cells total in rats 1 × 107 cells total in beagles | Collagen scaffold 4 mm diameter in rats, 5 mm diameter and 3 mm long in beagle | Completely transected spinal cord in rats and beagles | ↓Lesion area ↑ regeneration of nerve fibers ↑Neurological function | [77] |
| hUC-MSCs 1 × 106 cells total | Silk fibroin/collagen with mass ratio of 3:7 scaffolds with the dimension of 2 mm | Rats: complete spinal cord transection Analysis was conducted after 8 weeks | ↑Axonal regeneration ↑Myelination ↑Locomotion recovery | [78] |
| hUC-MSCs incubated for 1 week in neural differentiation medium 1 × 107 cells total | Collagen/heparin sulphate scaffold with mass ratio of 20:1 | 12 Beagle dogs with spinal cord transections | ↑Locomotion recovery ↑MEP ↑Neurological recovery ↑Nerve fibers ↑IL-10 and TGF-β1 ↓IL-1β and TNF-α | [79] |
| hUC-MSCs 2 × 106 cells total | Longitudinal collagen sponge scaffolds 2 mm in thickness and 3 mm in diameter | Rats: complete spinal cord transection Analysis was conducted after 8 weeks | ↑Motor function ↑NF, GFAP, GAP-43, and class III β-tubulin ↓CSPG ↓CD 68 | [80] |
| hUC-MSCs 1 × 106 cells total | Collagen scaffold 4 mm diameter hydrated scaffold bundle | Rats with chronic spinal cord injury Surgical resection of the glial scar | ↑Persistent motor recovery ↑Electrical conduction ↑Myelin sheath ↓Glial scar formation | [81] |
| hP-MSCs 1 × 107 cells total | Linear-ordered collagen scaffold 5 mm long | Beagle dogs with T8 completely transected spinal cord | ↑Motor recovery ↓Chondroitin sulfate proteoglycans ↑Axonal regeneration ↑Remyelination ↑Synapse formation | [82] |
| MSCs | Scaffold | Model | Results | Reference |
|---|---|---|---|---|
| Sheep BM-MSCs 6 × 106 cells/mL | Fibrin glue injection | Rats with complete spinal cord transection MSCs with fibrin were applied immediately upon transection. Rats were sacrificed after 70 days of treatment. | ↑Locomotor recovery Xenogeneic MSCs showed the expression of early “neuro-like” and “glia-like” differentiation patterns. | [83] |
| AD-MSCs differentiated in vitro toward NPCs and OPCs ∼104 cells suspended in 25 microliters of medium/fibrin | Fibrin glue injection 25 microliters of medium/fibrin | Contusive SCI model in rats Analyses were conducted 28 days after the damage and insertion of the scaffold. | ↑Locomotor recovery only in control with just fibrin ↓Loss of neurons ↓Astrogliosis ↓Cavitation ↓Macrophage infiltration | [84] |
| Allogeneic AD-MSCs 1 × 10 6 cells totals | Fibrin glue injection 18 μL of Baxter | Rats: contusion at Th8 The scaffold was applied two weeks after the SCI and analyzed after 74 days. | ↑Locomotor recovery ↑Tissue retention ↑Cavity volume in the subacute phase ↑H/M wave amplitude ratio ↑neurogenesis ↓Astroglial activation | [85] |
| Allogeneic BM-MSCs 2.5 × 106 cells in 5 µL | Fibrin glue injection 10 μL of Baxter | Contusive SCI model in rats with injection of DPY and INDP The scaffold was transplanted 72 h after the damage, and the necrotic tissue was removed. | ↑Mechanical withdrawal and locomotor recovery ↑Axonal fibers ↑Motor and sensory recovery in animals treated with DPY + INDP + FG + MSCs | [86] |
| Allogeneic BM-MSCs 2.5 × 106 cells in 5 µL | Fibrin glue injection 10 μL of Baxter | Complete spinal cord transection rats with injection of DPY and INDP The scaffold was implanted 60 days after the damage with surgical removal and inhibition of the glial scar. | ↑Locomotor recovery ↑Neuron fibers and the recovery of electric activity ↑BDNF, NT3, GAP-43, and NGF | [87] |
| Allogeneic BM-MSCs 2.5 × 106 cells in 5 µL | Fibrin glue injection 10 μL of Baxter | Contusive rat SCI model with injection of DPY and INDP The scaffold was implanted 60 days after the damage with surgical removal and inhibition of the glial scar. | ↑Motor recovery ↓GAP-43 and BDNF ↓Neuroregeneration | [88] |
| Allogeneic BM-MSCs, AD-MSCs, and DP-MSCs 1 × 106 cells per rat 8 × 106 cells per pig | Fibrin Matrix FM Tissucol (18 μL) for rats FM Tissucol (150 μL) for pigs | Rat: the spinal cord contusion model The scaffolds were applied 2 weeks after injury. Pigs: compression was carried out in addition to contusion. The scaffolds were applied 6 weeks after injury. | In rats: ↑motor activity ↑neural tissue integrity ↑conduction along spinal cord ↓cavitation In pigs: ↑ neural tissue integrity ↓cavitation Partial restoration of the somatosensory spinal pathways No effect of AD-MSCs on microglia No significant improvement in motor activity scores in pigs | [89] |
| Allogenic BM-MSCs 1 × 106 cells | Fibrin hydrogel with an AFG 4 mm in length | Rats with complete spinal cord transection | ↑Regeneration of NF- or GAP-43-positive nerve fibers in the caudal, rostral, and middle sites of the injury area ↑Electrophysiological expression and limb motor functions, host neuron immigration, and neural differentiation of donor MSCs | [90] |
| Allogeneic BM-MSCs 1 × 106 cells | Fibrin sealant | Rats with unilateral cut of the ventral funiculus | MSC therapy is neuroprotective and, when combined with FS, shifts the immune response to a pro-inflammatory profile. ↑Neuronal survival ↓Astrogliosis ↓Synaptic preservation | [91] |
| MSCs | Scaffold | Model | Results | Reference |
|---|---|---|---|---|
| Allogeneic BM-MSCs The BM-MSCs were stuffed into the chitosan channels at a density of 0.5 × 106/10 mL. | Tubular forming of chitosan The tubes were 10 mm in length, 4.1 mm in outer diameter, and the wall thickness was 0.21 mm. | Rats with spinal cord transection | No significant changes in BBB score ↑Axons per unit area ↑Myelin sheath repair | [92] |
| Allogeneic BM-MSCs 1 × 106 per mouse | Thermosensitive composite hydrogel based on chitosan, hydroxyethyl cellulose, collagen, and β-phosphoglycerate | Contusion SCI mice model Analysis was conducted after 28 days. | ↑Locomotor recovery ↓Depression ↓Edema ↑Survival of neurons ↑Neurogenesis ↓Apoptosis ↑Neurotrophic factors | [93] |
| AD-MSCs overexpressing brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) 200,000-cell suspension | Silk fibroin/chitosan scaffold with the dimensions of 2 mm × 2 mm | Rats with spinal cord transection Rats were sacrificed after 12 weeks of treatment. | ↑Locomotor recovery ↓Scar tissues ↑Neuron-like cells ↓Inflammatory cells ↑GAP-43↓GFAP↓CASP-3 | [94] |
| AD-MSCs transfected with lentiviral mediated nerve growth factor 1 × 105 cells | Injectable thermosensitive hydrogel chitosan/β-glycerophosphate/hydroxyethyl cellulose Pores ranging from 100–150 μm | The scaffold was applied in rats one week after the contusive SCI induction, and the evaluations were performed after two months. | ↑Locomotor recovery ↑Cell proliferation ↓Cavitation ↑Spinal cord ECM | [95] |
| Allogeneic BM-MSCs transfected with an adenovirus containing the glial-cell-derived neurotrophic factor gene 2 × 105 cells/10 μL | Thermosensitive quaternary ammonium chloride chitosan/ß-glycerophosphate hydrogel The multiporous three-dimensional structure of the hydrogel scaffolds had an average pore size of 118.56 ± 11.92 μm. | Contusive SCI model in rats Rats were sacrificed after 2, 4, and 6 weeks of treatment. | ↑NeuN, NF-200, and GFAP ↓CSPG ↑Locomotor ↑Neurogenesis ↓Cavitation Increased interconnected and orderly arranged axons in the lesion site 6 weeks after transplantation | [96] |
| BM-MSCs 150,000 cells | 7 µL of chitosan-based hydrogel with β-Glycerol phosphate disodium MSCs were mixed with the hydrogel solution prior to gelation. | Mice with complete spinal cord transection | MSCs continued to produce microvesicles, even with the scaffold ↓ROS level reduction | [97] |
| BM-MSCs 2 × 106 cells | PLA/NGF-PLGA/CS composite membrane The size of the composite membranes used was 5 mm × 8 mm. | Contusion SCI Rats | ↑Neurogenesis ↑Locomotor recovery | [98] |
| MSCs | Scaffold | Model | Results | Reference |
|---|---|---|---|---|
| Allogenic BM-MSCs 30 μL of cell suspension 1 × 106 cells/mL | Acellular spinal cord scaffold + PLGA nanoparticles with VEGF and NT-3 Dimensions of the scaffolds: 3 mm long and 2 mm in diameter | Rats with unilateral hemisection T9 to T11 | ↑Motor recovery ↑Axonal regeneration ↓Macrophage infiltration | [99] |
| hBM-MSCs ∼5 × 105 | PLGA scaffolds tailored to be unique, porous, soft, and smooth dimensions of 1 × 2 × 4 mm | Rats with hemisection T9 to T10 | ↑Motor recovery ↑Axonal regeneration ↑Angiogenesis ↓Neural inflammation ↓Loss of tissue | [100] |
| Allogenic BM-MSCs 10 μL of cell suspension 1 × 107 cells/mL | PLGA scaffold with 50 microchannels in rod shape of 5 cm in length and 3 mm in diameter | Rats with complete transection of the thoracic spinal cord | ↑Nerve regeneration ↑Motor-evoked potential ↑Somatosensory evoked potential ↑Motor recovery ↓ Cystic area Combination with Schwann cell increased these values and promoted the differentiation of MSCs into neuron-like cells | [101] |
| hBM-MSCs ~6 × 104 cells were seeded in the soft scaffold | Soft PLGA scaffold with pore sizes of 350–500 μm. Size of W × H × L: 1 mm × 2 mm × 4 mm | Rats with unilateral hemisection of the midline at the T9-T10 level | ↑Functional recovery ↑Interneuron protection ↓ Loss of tissue ↓Loss of white matter ↓Neural inflammation | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blando, S.; Anchesi, I.; Mazzon, E.; Gugliandolo, A. Can a Scaffold Enriched with Mesenchymal Stem Cells Be a Good Treatment for Spinal Cord Injury? Int. J. Mol. Sci. 2022, 23, 7545. https://doi.org/10.3390/ijms23147545
Blando S, Anchesi I, Mazzon E, Gugliandolo A. Can a Scaffold Enriched with Mesenchymal Stem Cells Be a Good Treatment for Spinal Cord Injury? International Journal of Molecular Sciences. 2022; 23(14):7545. https://doi.org/10.3390/ijms23147545
Chicago/Turabian StyleBlando, Santino, Ivan Anchesi, Emanuela Mazzon, and Agnese Gugliandolo. 2022. "Can a Scaffold Enriched with Mesenchymal Stem Cells Be a Good Treatment for Spinal Cord Injury?" International Journal of Molecular Sciences 23, no. 14: 7545. https://doi.org/10.3390/ijms23147545






