Extracellular Vesicles—Oral Therapeutics of the Future
Abstract
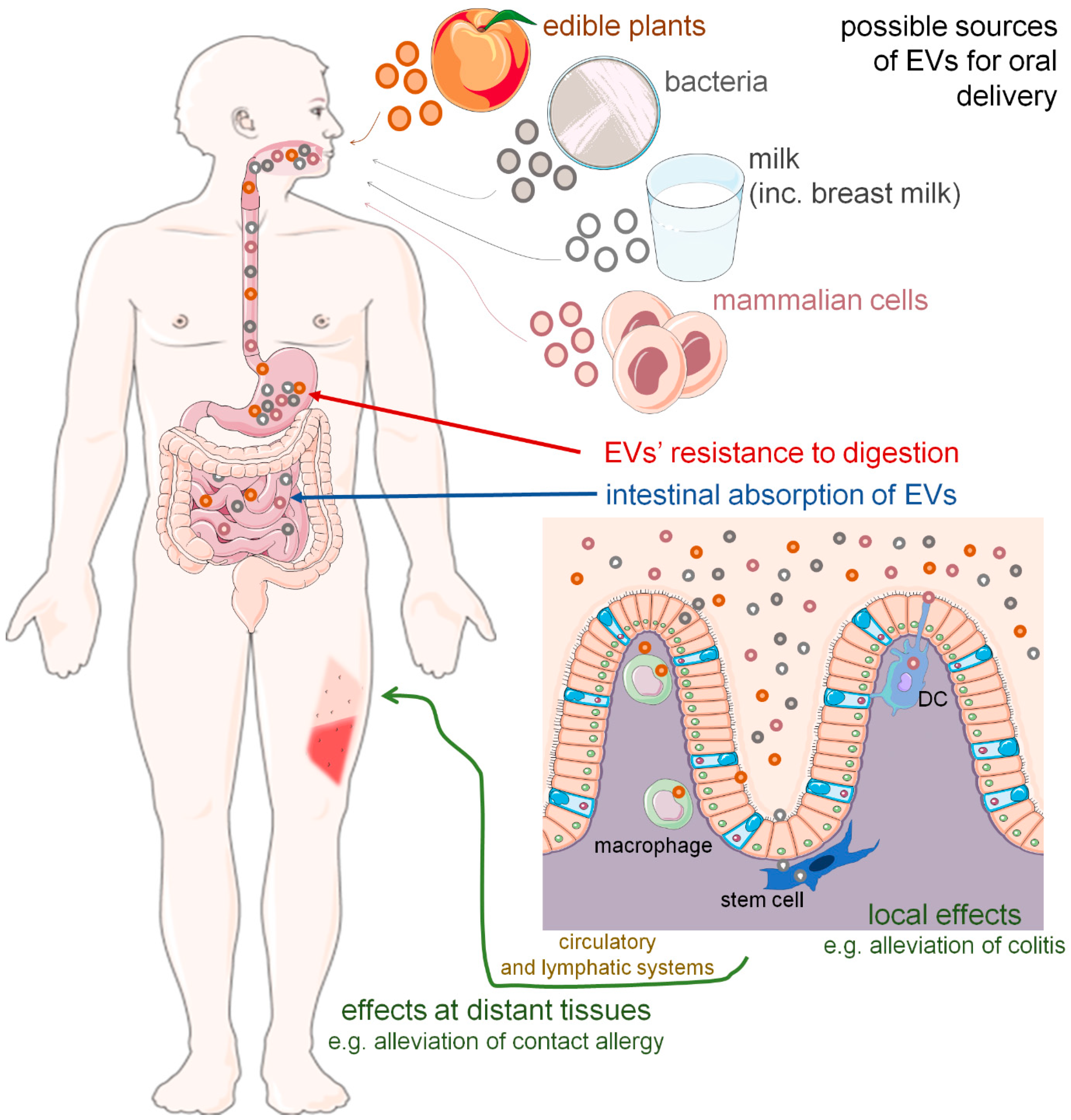
:1. Introduction
2. Plant-Derived EVs in Cross-Kingdom Delivery—Truth or Imagination?
3. Bacterial EVs—Another Source for Consideration?
4. “Milky Way”
5. Mammalian Cell-Derived EVs—A New Perspective
6. Upon Arrival at Destination—EVs in the Intestines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazimek, K.; Bryniarski, K.; Santocki, M.; Ptak, W. Exosomes as Mediators of Intercellular Communication: Clinical Implications. Pol. Arch. Intern. Med. 2015, 125, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular Vesicles: A Bright Star of Nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef]
- Nazimek, K.; Bryniarski, K. Perspectives in Manipulating EVs for Therapeutic Applications: Focus on Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 4623. [Google Scholar] [CrossRef]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Division of Molecular and Cellular Medicine, National Cancer Center Research Institute, Tokyo, Japan Drug Delivery Application of Extracellular Vesicles; Insight into Production, Drug Loading, Targeting, and Pharmacokinetics. AIMS Bioeng. 2017, 4, 73–92. [Google Scholar] [CrossRef]
- Kim, D.H.; Kothandan, V.K.; Kim, H.W.; Kim, K.S.; Kim, J.Y.; Cho, H.J.; Lee, Y.; Lee, D.-E.; Hwang, S.R. Noninvasive Assessment of Exosome Pharmacokinetics In Vivo: A Review. Pharmaceutics 2019, 11, 649. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of Extracellular Vesicles Following Administration into Animals: A Systematic Review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.A.; Goulding, A.M.; Deo, S.K. Direct Detection and Quantification of MicroRNAs. Anal. Biochem. 2009, 387, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.C.; Maragh, S.; Ghiran, I.C.; Jones, J.C.; DeRose, P.C.; Elsheikh, E.; Vreeland, W.N.; Wang, L. Measurement and Standardization Challenges for Extracellular Vesicle Therapeutic Delivery Vectors. Nanomedicine 2020, 15, 2149–2170. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Zhou, Y.; Chen, X. New Insight into Inter-Kingdom Communication: Horizontal Transfer of Mobile Small RNAs. Front. Microbiol. 2017, 8, 768. [Google Scholar] [CrossRef]
- Bordoni, L.; Gabbianelli, R. The Neglected Nutrigenomics of Milk: What Is the Role of Inter-Species Transfer of Small Non-Coding RNA? Food Biosci. 2021, 39, 100796. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Fernández-Alonso, N.; Tomás-Zapico, C.; Visioli, F.; Iglesias-Gutierrez, E.; Dávalos, A. Breast Milk MicroRNAs Harsh Journey towards Potential Effects in Infant Development and Maturation. Lipid Encapsulation Can Help. Pharmacol. Res. 2018, 132, 21–32. [Google Scholar] [CrossRef]
- Yu, L.; Deng, Z.; Liu, L.; Zhang, W.; Wang, C. Plant-Derived Nanovesicles: A Novel Form of Nanomedicine. Front. Bioeng. Biotechnol. 2020, 8, 584391. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2021, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular Vesicles from Plants: Current Knowledge and Open Questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.-I.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola Exosome-like Nanovesicles to Systemically Deliver Nucleic Acid Medicine via Oral Administration. Mol. Ther. Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Guzman, J.R.; Conlin, V.S.; Jobin, C. Diet, Microbiome, and the Intestinal Epithelium: An Essential Triumvirate? BioMed Res. Int. 2013, 2013, 425146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi Ghiasi, M.; Rahimi, E.; Amirkhani, Z.; Salehi, R. Leucine-Rich Repeat-Containing G-Protein Coupled Receptor 5 Gene Overexpression of the Rat Small Intestinal Progenitor Cells in Response to Orally Administered Grape Exosome-like Nanovesicles. Adv. Biomed. Res. 2018, 7, 125. [Google Scholar] [PubMed]
- Barker, N.; Clevers, H. Leucine-Rich Repeat-Containing G-Protein-Coupled Receptors as Markers of Adult Stem Cells. Gastroenterology 2010, 138, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Snow, J.W.; Hale, A.E.; Isaacs, S.K.; Baggish, A.L.; Chan, S.Y. Ineffective Delivery of Diet-Derived MicroRNAs to Recipient Animal Organisms. RNA Biol. 2013, 10, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Zhu, Y.; Sun, B.; Shao, Y.; Jing, A.; Wang, J.; Xiao, Z. Assessing the Survival of Exogenous Plant MicroRNA in Mice. Food Sci. Nutr. 2014, 2, 380–388. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, L.; Chu, Q.; Sun, S.; Wu, Y.; Tong, Z.; Fang, W.; Timko, M.P.; Fan, L. Large-Scale Identification of Extracellular Plant MiRNAs in Mammals Implicates Their Dietary Intake. PLoS ONE 2021, 16, e0257878. [Google Scholar] [CrossRef]
- Otsuka, K.; Yamamoto, Y.; Matsuoka, R.; Ochiya, T. Maintaining Good MiRNAs in the Body Keeps the Doctor Away?: Perspectives on the Relationship between Food-Derived Natural Products and MicroRNAs in Relation to Exosomes/Extracellular Vesicles. Mol. Nutr. Food Res. 2018, 62, 1700080. [Google Scholar] [CrossRef]
- Zempleni, J.; Baier, S.R.; Howard, K.M.; Cui, J. Gene Regulation by Dietary MicroRNAs. Can. J. Physiol. Pharmacol. 2015, 93, 1097–1102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous Plant MIR168a Specifically Targets Mammalian LDLRAP1: Evidence of Cross-Kingdom Regulation by MicroRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Baier, S.R.; Nguyen, C.; Xie, F.; Wood, J.R.; Zempleni, J. MicroRNAs Are Absorbed in Biologically Meaningful Amounts from Nutritionally Relevant Doses of Cow Milk and Affect Gene Expression in Peripheral Blood Mononuclear Cells, HEK-293 Kidney Cell Cultures, and Mouse Livers. J. Nutr. 2014, 144, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Zhang, C.-Y. Diet-Derived MicroRNAs: Unicorn or Silver Bullet? Genes Nutr. 2017, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Mar-Aguilar, F.; Arreola-Triana, A.; Mata-Cardona, D.; Gonzalez-Villasana, V.; Rodríguez-Padilla, C.; Reséndez-Pérez, D. Evidence of Transfer of MiRNAs from the Diet to the Blood Still Inconclusive. PeerJ 2020, 8, e9567. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; Lόpez de las Hazas, M.C.; Margollés, A.; Dávalos, A.; García-Ruiz, A. Eating MicroRNAs: Pharmacological Opportunities for Cross-kingdom Regulation and Implications in Host Gene and Gut Microbiota Modulation. Br. J. Pharmacol. 2021, 178, 2218–2245. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, D.; Rizzetto, L.; Tocci, N.; Rivero, D.; Asquini, E.; Si-Ammour, A.; Bonechi, E.; Ballerini, C.; Viola, R. Plant MicroRNAs as Novel Immunomodulatory Agents. Sci. Rep. 2016, 6, 25761. [Google Scholar] [CrossRef] [Green Version]
- Reiner, A.T.; Somoza, V. Extracellular Vesicles as Vehicles for the Delivery of Food Bioactives. J. Agric. Food Chem. 2019, 67, 2113–2119. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia Muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of Serotonin Signaling/Metabolism by Akkermansia Muciniphila and Its Extracellular Vesicles through the Gut-Brain Axis in Mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Ashrafian, F.; Behrouzi, A.; Shahriary, A.; Ahmadi Badi, S.; Davari, M.; Khatami, S.; Rahimi Jamnani, F.; Fateh, A.; Vaziri, F.; Siadat, S.D. Comparative Study of Effect of Akkermansia Muciniphila and Its Extracellular Vesicles on Toll-like Receptors and Tight Junction. Gastroenterol. Hepatol. Bed Bench 2019, 12, 163–168. [Google Scholar] [PubMed]
- Lee, K.-E.; Kim, J.-K.; Han, S.-K.; Lee, D.Y.; Lee, H.-J.; Yim, S.-V.; Kim, D.-H. The Extracellular Vesicle of Gut Microbial Paenalcaligenes Hominis Is a Risk Factor for Vagus Nerve-Mediated Cognitive Impairment. Microbiome 2020, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-S.; Ban, M.; Choi, E.-J.; Moon, H.-G.; Jeon, J.-S.; Kim, D.-K.; Park, S.-K.; Jeon, S.G.; Roh, T.-Y.; Myung, S.-J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia Muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fábrega, M.-J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badía, J.; Giménez, R.; Gálvez, J.; Baldomà, L. Intestinal Anti-Inflammatory Effects of Outer Membrane Vesicles from Escherichia Coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus Rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef]
- Kaparakis, M.; Turnbull, L.; Carneiro, L.; Firth, S.; Coleman, H.A.; Parkington, H.C.; Le Bourhis, L.; Karrar, A.; Viala, J.; Mak, J.; et al. Bacterial Membrane Vesicles Deliver Peptidoglycan to NOD1 in Epithelial Cells. Cell. Microbiol. 2010, 12, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Khan, A.; Ghosh, T.; Mondal, S.; Mallick, A.I. Gut Microbe-Derived Outer Membrane Vesicles: A Potential Platform to Control Cecal Load of Campylobacter jejuni. ACS Infect. Dis. 2021, 7, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Schild, S.; Nelson, E.J.; Camilli, A. Immunization with Vibrio cholerae Outer Membrane Vesicles Induces Protective Immunity in Mice. Infect. Immun. 2008, 76, 4554–4563. [Google Scholar] [CrossRef] [Green Version]
- Keenan, J.; Oliaro, J.; Domigan, N.; Potter, H.; Aitken, G.; Allardyce, R.; Roake, J. Immune Response to an 18-Kilodalton Outer Membrane Antigen Identifies Lipoprotein 20 as a Helicobacter pylori Vaccine Candidate. Infect. Immun. 2000, 68, 3337–3343. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; You, L.; Zhang, Z.; Cui, X.; Zhong, H.; Sun, X.; Ji, C.; Chi, X. Biological Properties of Milk-Derived Extracellular Vesicles and Their Physiological Functions in Infant. Front. Cell Dev. Biol. 2021, 9, 693534. [Google Scholar] [CrossRef]
- Feng, X.; Chen, X.; Zheng, X.; Zhu, H.; Qi, Q.; Liu, S.; Zhang, H.; Che, J. Latest Trend of Milk Derived Exosomes: Cargos, Functions, and Applications. Front. Nutr. 2021, 8, 747294. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-Related MicroRNAs Are Abundant in Breast Milk Exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, M.; Wang, T.; Liang, Y.; Zhong, Z.; Wang, X.; Zhou, Q.; Chen, L.; Lang, Q.; He, Z.; et al. Lactation-Related MicroRNA Expression Profiles of Porcine Breast Milk Exosomes. PLoS ONE 2012, 7, e43691. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, X.; Yu, J.; Zen, K.; Zhang, C.-Y.; Li, L. Immune Modulatory Function of Abundant Immune-Related MicroRNAs in Microvesicles from Bovine Colostrum. Protein Cell 2013, 4, 197–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Herwijnen, M.J.C.; Driedonks, T.A.P.; Snoek, B.L.; Kroon, A.M.T.; Kleinjan, M.; Jorritsma, R.; Pieterse, C.M.J.; Nolte-’t Hoen, E.N.M.; Wauben, M.H.M. Abundantly Present MiRNAs in Milk-Derived Extracellular Vesicles Are Conserved Between Mammals. Front. Nutr. 2018, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Chen, T.; Xie, M.; Li, M.; Zeng, B.; Sun, R.; Zhu, Y.; Ye, D.; Wu, J.; Sun, J.; et al. Oral Administration of Bovine and Porcine Milk Exosome Alter MiRNAs Profiles in Piglet Serum. Sci. Rep. 2020, 10, 6983. [Google Scholar] [CrossRef]
- Lukasik, A.; Zielenkiewicz, P. In Silico Identification of Plant MiRNAs in Mammalian Breast Milk Exosomes–A Small Step Forward? PLoS ONE 2014, 9, e99963. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, B.; Buschmann, D.; Paul, V.; Pfaffl, M.W. Postprandial Transfer of Colostral Extracellular Vesicles and Their Protein and MiRNA Cargo in Neonatal Calves. PLoS ONE 2020, 15, e0229606. [Google Scholar] [CrossRef]
- Auerbach, A.; Vyas, G.; Li, A.; Halushka, M.; Witwer, K. Uptake of Dietary Milk MiRNAs by Adult Humans: A Validation Study. F1000Research 2016, 5, 721. [Google Scholar] [CrossRef]
- Sadri, M.; Shu, J.; Kachman, S.D.; Cui, J.; Zempleni, J. Milk Exosomes and MiRNA Cross the Placenta and Promote Embryo Survival in Mice. Reproduction 2020, 160, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Leiferman, A.; Shu, J.; Grove, R.; Cui, J.; Adamec, J.; Zempleni, J. A Diet Defined by Its Content of Bovine Milk Exosomes and Their RNA Cargos Has Moderate Effects on Gene Expression, Amino Acid Profiles and Grip Strength in Skeletal Muscle in C57BL/6 Mice. J. Nutr. Biochem. 2018, 59, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Go, G.; Jeon, J.; Lee, G.; Lee, J.H.; Lee, S.H. Bovine Milk Extracellular Vesicles Induce the Proliferation and Differentiation of Osteoblasts and Promote Osteogenesis in Rats. J. Food Biochem. 2021, 45, e13705. [Google Scholar] [CrossRef] [PubMed]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk Exosomes Are Bioavailable and Distinct MicroRNA Cargos Have Unique Tissue Distribution Patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef] [Green Version]
- Parry, H.A.; Mobley, C.B.; Mumford, P.W.; Romero, M.A.; Haun, C.T.; Zhang, Y.; Roberson, P.A.; Zempleni, J.; Ferrando, A.A.; Vechetti, I.J., Jr.; et al. Bovine Milk Extracellular Vesicles (EVs) Modification Elicits Skeletal Muscle Growth in Rats. Front. Physiol. 2019, 10, 436. [Google Scholar] [CrossRef]
- Zhou, F.; Paz, H.A.; Sadri, M.; Cui, J.; Kachman, S.D.; Fernando, S.C.; Zempleni, J. Dietary Bovine Milk Exosomes Elicit Changes in Bacterial Communities in C57BL/6 Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G618–G624. [Google Scholar] [CrossRef]
- Du, C.; Quan, S.; Nan, X.; Zhao, Y.; Shi, F.; Luo, Q.; Xiong, B. Effects of Oral Milk Extracellular Vesicles on the Gut Microbiome and Serum Metabolome in Mice. Food Funct. 2021, 12, 10938–10949. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, 1901251. [Google Scholar] [CrossRef]
- Yun, B.; Maburutse, B.E.; Kang, M.; Park, M.R.; Park, D.J.; Kim, Y.; Oh, S. Short Communication: Dietary Bovine Milk-Derived Exosomes Improve Bone Health in an Osteoporosis-Induced Mouse Model. J. Dairy Sci. 2020, 103, 7752–7760. [Google Scholar] [CrossRef]
- Reif, S.; Elbaum-Shiff, Y.; Koroukhov, N.; Shilo, I.; Musseri, M.; Golan-Gerstl, R. Cow and Human Milk-Derived Exosomes Ameliorate Colitis in DSS Murine Model. Nutrients 2020, 12, 2589. [Google Scholar] [CrossRef]
- Roefs, M.T.; Sluijter, J.P.G.; Vader, P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol. 2020, 30, 990–1013. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Yin, Y.; Jang, S.C.; Lässer, C.; Wennmalm, S.; Hoffmann, H.J.; Li, L.; Gho, Y.S.; Nilsson, J.A.; Lötvall, J. Endosomal Signalling via Exosome Surface TGFβ-1. J. Extracell. Vesicles 2019, 8, 1650458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieters, B.C.H.; Arntz, O.J.; Bennink, M.B.; Broeren, M.G.A.; van Caam, A.P.M.; Koenders, M.I.; van Lent, P.L.E.M.; van den Berg, W.B.; de Vries, M.; van der Kraan, P.M.; et al. Commercial Cow Milk Contains Physically Stable Extracellular Vesicles Expressing Immunoregulatory TGF-β. PLoS ONE 2015, 10, e0121123. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Weiskirchen, R.; Melnik, B.C. Milk Exosomes Prevent Intestinal Inflammation in a Genetic Mouse Model of Ulcerative Colitis: A Pilot Experiment. Inflamm. Intest. Dis. 2020, 5, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kittana, H.; Shu, J.; Kachman, S.D.; Cui, J.; Ramer-Tait, A.E.; Zempleni, J. Dietary Depletion of Milk Exosomes and Their MicroRNA Cargos Elicits a Depletion of MiR-200a-3p and Elevated Intestinal Inflammation and CXCL9 Expression in Mdr1a−/- Mice. Curr. Dev. Nutr. 2019, 3, nzz122. [Google Scholar] [CrossRef]
- Xie, M.-Y.; Chen, T.; Xi, Q.-Y.; Hou, L.-J.; Luo, J.-Y.; Zeng, B.; Li, M.; Sun, J.-J.; Zhang, Y.-L. Porcine Milk Exosome MiRNAs Protect Intestinal Epithelial Cells against Deoxynivalenol-Induced Damage. Biochem. Pharmacol. 2020, 175, 113898. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, M.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wei, L.-M.; Li, M.; Lin, D.-L.; Jiang, Q.-Y.; et al. Porcine Milk-Derived Exosomes Promote Proliferation of Intestinal Epithelial Cells. Sci. Rep. 2016, 6, 33862. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Y.; Yan, D.; Wang, Y.; Xu, X.; Zhao, Y.; Xiao, T. Protective Effects of Human Milk-Derived Exosomes on Intestinal Stem Cells Damaged by Oxidative Stress. Cell Transplant. 2020, 29, 096368972091269. [Google Scholar] [CrossRef] [Green Version]
- Hock, A.; Miyake, H.; Li, B.; Lee, C.; Ermini, L.; Koike, Y.; Chen, Y.; Määttänen, P.; Zani, A.; Pierro, A. Breast Milk-Derived Exosomes Promote Intestinal Epithelial Cell Growth. J. Pediatr. Surg. 2017, 52, 755–759. [Google Scholar] [CrossRef]
- Arntz, O.J.; Pieters, B.C.H.; Oliveira, M.C.; Broeren, M.G.A.; Bennink, M.B.; de Vries, M.; van Lent, P.L.E.M.; Koenders, M.I.; van den Berg, W.B.; van der Kraan, P.M.; et al. Oral Administration of Bovine Milk Derived Extracellular Vesicles Attenuates Arthritis in Two Mouse Models. Mol. Nutr. Food Res. 2015, 59, 1701–1712. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; López de las Hazas, M.-C.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for MiRNA-Based Therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-Derived Exosomes for Oral Delivery of Paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S.; et al. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Induces Senescence in the Primary Tumor but Accelerates Cancer Metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; El-Magd, M.A.; AlSadrah, S.A. Therapeutic Effect of Camel Milk and Its Exosomes on MCF7 Cells In Vitro and In Vivo. Integr. Cancer Ther. 2018, 17, 1235–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Chen, G.; Dong, Y.; Yang, J.; Liu, Y.; Song, H.; Song, H.; Wang, Y. Liraglutide-Loaded Milk Exosomes Lower Blood Glucose When Given by Sublingual Route. ChemMedChem 2022, 17, e202100758. [Google Scholar] [CrossRef] [PubMed]
- Grossen, P.; Portmann, M.; Koller, E.; Duschmalé, M.; Minz, T.; Sewing, S.; Pandya, N.J.; van Geijtenbeek, S.K.; Ducret, A.; Kusznir, E.-A.; et al. Evaluation of Bovine Milk Extracellular Vesicles for the Delivery of Locked Nucleic Acid Antisense Oligonucleotides. Eur. J. Pharm. Biopharm. 2021, 158, 198–210. [Google Scholar] [CrossRef]
- Carobolante, G.; Mantaj, J.; Ferrari, E.; Vllasaliu, D. Cow Milk and Intestinal Epithelial Cell-Derived Extracellular Vesicles as Systems for Enhancing Oral Drug Delivery. Pharmaceutics 2020, 12, 226. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Du, X.; Li, J.; Lönnerdal, B. Human Milk Exosomes and Their MicroRNAs Survive Digestion in Vitro and Are Taken up by Human Intestinal Cells. Mol. Nutr. Food Res. 2017, 61, 1700082. [Google Scholar] [CrossRef]
- Rani, P.; Vashisht, M.; Golla, N.; Shandilya, S.; Onteru, S.K.; Singh, D. Milk MiRNAs Encapsulated in Exosomes Are Stable to Human Digestion and Permeable to Intestinal Barrier in Vitro. J. Funct. Foods 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Sukreet, S.; Zhang, H.; Adamec, J.; Cui, J.; Zempleni, J. Identification of Glycoproteins on the Surface of Bovine Milk Exosomes and Intestinal Cells That Facilitate Exosome Uptake in Human Colon Carcinoma Caco-2 Cells. FASEB J. 2017, 31, 646.25. [Google Scholar] [CrossRef]
- Kusuma, R.J.; Manca, S.; Friemel, T.; Sukreet, S.; Nguyen, C.; Zempleni, J. Human Vascular Endothelial Cells Transport Foreign Exosomes from Cow’s Milk by Endocytosis. Am. J. Physiol.-Cell Physiol. 2016, 310, C800–C807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reif, S.; Elbaum Shiff, Y.; Golan-Gerstl, R. Milk-Derived Exosomes (MDEs) Have a Different Biological Effect on Normal Fetal Colon Epithelial Cells Compared to Colon Tumor Cells in a MiRNA-Dependent Manner. J. Transl. Med. 2019, 17, 325. [Google Scholar] [CrossRef]
- Ross, M.; Atalla, H.; Karrow, N.; Mallard, B.A. The Bioactivity of Colostrum and Milk Exosomes of High, Average, and Low Immune Responder Cows on Human Intestinal Epithelial Cells. J. Dairy Sci. 2021, 104, 2499–2510. [Google Scholar] [CrossRef]
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine Milk Contains MicroRNA and Messenger RNA that are Stable under Degradative Conditions. J. Dairy Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef] [Green Version]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine Milk-Derived Exosomes for Drug Delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The Potential of Exosomes From Cow Milk for Oral Delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine Milk Exosomes Contain MicroRNA and MRNA and Are Taken up by Human Macrophages. J. Dairy Sci. 2015, 98, 2920–2933. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. HucMSC Exosome-Derived GPX1 is Required for the Recovery of Hepatic Oxidant Injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Aminzadeh, M.A.; Fournier, M.; Akhmerov, A.; Jones-Ungerleider, K.C.; Valle, J.B.; Marbán, E. Casein-Enhanced Uptake and Disease-Modifying Bioactivity of Ingested Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12045. [Google Scholar] [CrossRef]
- Ptak, W.; Nazimek, K.; Askenase, P.W.; Bryniarski, K. From Mysterious Supernatant Entity to MiRNA-150 in Antigen-Specific Exosomes: A History of Hapten-Specific T Suppressor Factor. Arch. Immunol. Ther. Exp. 2015, 63, 345–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryniarski, K.; Ptak, W.; Jayakumar, A.; Püllmann, K.; Caplan, M.J.; Chairoungdua, A.; Lu, J.; Adams, B.D.; Sikora, E.; Nazimek, K.; et al. Antigen-Specific, Antibody-Coated, Exosome-like Nanovesicles Deliver Suppressor T-Cell MicroRNA-150 to Effector T Cells to Inhibit Contact Sensitivity. J. Allergy Clin. Immunol. 2013, 132, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryniarski, K.; Ptak, W.; Martin, E.; Nazimek, K.; Szczepanik, M.; Sanak, M.; Askenase, P.W. Free Extracellular MiRNA Functionally Targets Cells by Transfecting Exosomes from Their Companion Cells. PLoS ONE 2015, 10, e0122991. [Google Scholar] [CrossRef] [Green Version]
- Nazimek, K.; Askenase, P.W.; Bryniarski, K. Antibody Light Chains Dictate the Specificity of Contact Hypersensitivity Effector Cell Suppression Mediated by Exosomes. Int. J. Mol. Sci. 2018, 19, 2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazimek, K.; Bryniarski, K. Increasing the Therapeutic Efficacy of Extracellular Vesicles From the Antigen-Specific Antibody and Light Chain Perspective. Front. Cell Dev. Biol. 2021, 9, 790722. [Google Scholar] [CrossRef]
- Nazimek, K.; Ptak, W.; Nowak, B.; Ptak, M.; Askenase, P.W.; Bryniarski, K. Macrophages Play an Essential Role in Antigen-Specific Immune Suppression Mediated by T CD8+ Cell-Derived Exosomes. Immunology 2015, 146, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Nazimek, K.; Bustos-Morán, E.; Blas-Rus, N.; Nowak, B.; Ptak, W.; Askenase, P.W.; Sánchez-Madrid, F.; Bryniarski, K. Syngeneic Red Blood Cell-Induced Extracellular Vesicles Suppress Delayed-Type Hypersensitivity to Self-Antigens in Mice. Clin. Exp. Allergy 2019, 49, 1487–1499. [Google Scholar] [CrossRef]
- Nazimek, K.; Bryniarski, K.; Ptak, W.; Groot Kormelink, T.; Askenase, P.W. Orally Administered Exosomes Suppress Mouse Delayed-Type Hypersensitivity by Delivering MiRNA-150 to Antigen-Primed Macrophage APC Targeted by Exosome-Surface Anti-Peptide Antibody Light Chains. Int. J. Mol. Sci. 2020, 21, 5540. [Google Scholar] [CrossRef] [PubMed]
- Wąsik, M.; Nazimek, K.; Nowak, B.; Askenase, P.W.; Bryniarski, K. Delayed-Type Hypersensitivity Underlying Casein Allergy is Suppressed by Extracellular Vesicles Carrying MiRNA-150. Nutrients 2019, 11, 907. [Google Scholar] [CrossRef] [Green Version]
- Nazimek, K.; Bustos-Morán, E.; Blas-Rus, N.; Nowak, B.; Totoń-Żurańska, J.; Seweryn, M.T.; Wołkow, P.; Woźnicka, O.; Szatanek, R.; Siedlar, M.; et al. Antibodies Enhance the Suppressive Activity of Extracellular Vesicles in Mouse Delayed-Type Hypersensitivity. Pharmaceuticals 2021, 14, 734. [Google Scholar] [CrossRef]
- Nazimek, K.; Bryniarski, K. Approaches to Inducing Antigen-Specific Immune Tolerance in Allergy and Autoimmunity: Focus on Antigen-Presenting Cells and Extracellular Vesicles. Scand. J. Immunol. 2020, 91, e12881. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional Specialization within the Intestinal Immune System. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Chang, X.; Wang, S.-L.; Zhao, S.-B.; Shi, Y.-H.; Pan, P.; Gu, L.; Yao, J.; Li, Z.-S.; Bai, Y. Extracellular Vesicles with Possible Roles in Gut Intestinal Tract Homeostasis and IBD. Mediat. Inflamm. 2020, 2020, 1945832. [Google Scholar] [CrossRef]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by Food: Impact on Gut Immunity and Immune Cell Function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef]
- Cieza, R.J.; Cao, A.T.; Cong, Y.; Torres, A.G. Immunomodulation for Gastrointestinal Infections. Expert Rev. Anti-Infect. Ther. 2012, 10, 391–400. [Google Scholar] [CrossRef]
- Kayama, H.; Takeda, K. Regulation of Intestinal Homeostasis by Innate and Adaptive Immunity. Int. Immunol. 2012, 24, 673–680. [Google Scholar] [CrossRef]
- Lee, Y.; Kamada, N.; Moon, J.J. Oral Nanomedicine for Modulating Immunity, Intestinal Barrier Functions, and Gut Microbiome. Adv. Drug Deliv. Rev. 2021, 179, 114021. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, Z.; Yao, J.; Jin, Y. Extracellular Vesicles-Mediated Interaction within Intestinal Microenvironment in Inflammatory Bowel Disease. J. Adv. Res. 2022, 37, 221–233. [Google Scholar] [CrossRef]
- Diaz-Garrido, N.; Cordero, C.; Olivo-Martinez, Y.; Badia, J.; Baldomà, L. Cell-to-Cell Communication by Host-Released Extracellular Vesicles in the Gut: Implications in Health and Disease. Int. J. Mol. Sci. 2021, 22, 2213. [Google Scholar] [CrossRef]
- Filip, R. An Update on the Role of Extracellular Vesicles in the Pathogenesis of Necrotizing Enterocolitis and Inflammatory Bowel Diseases. Cells 2021, 10, 3202. [Google Scholar] [CrossRef]
- López de las Hazas, M.-C.; del Pozo-Acebo, L.; Hansen, M.S.; Gil-Zamorano, J.; Mantilla-Escalante, D.C.; Gómez-Coronado, D.; Marín, F.; Garcia-Ruiz, A.; Rasmussen, J.T.; Dávalos, A. Dietary Bovine Milk MiRNAs Transported in Extracellular Vesicles are Partially Stable during GI Digestion, are Bioavailable and Reach Target Tissues but Need a Minimum Dose to Impact on Gene Expression. Eur. J. Nutr. 2022, 61, 1043–1056. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Morishita, M.; Nishikawa, M.; Takakura, Y. Role of Extracellular Vesicle Surface Proteins in the Pharmacokinetics of Extracellular Vesicles. Mol. Pharm. 2018, 15, 1073–1080. [Google Scholar] [CrossRef]
- Nolte, M.A.; Nolte-’t Hoen, E.N.M.; Margadant, C. Integrins Control Vesicular Trafficking; New Tricks for Old Dogs. Trends Biochem. Sci. 2021, 46, 124–137. [Google Scholar] [CrossRef]
- Staubach, S.; Bauer, F.N.; Tertel, T.; Börger, V.; Stambouli, O.; Salzig, D.; Giebel, B. Scaled Preparation of Extracellular Vesicles from Conditioned Media. Adv. Drug Deliv. Rev. 2021, 177, 113940. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Aarts, J.; Boleij, A.; Pieters, B.C.H.; Feitsma, A.L.; van Neerven, R.J.J.; ten Klooster, J.P.; M’Rabet, L.; Arntz, O.J.; Koenders, M.I.; van de Loo, F.A.J. Flood Control: How Milk-Derived Extracellular Vesicles Can Help to Improve the Intestinal Barrier Function and Break the Gut–Joint Axis in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 703277. [Google Scholar] [CrossRef]
| Disease/Condition | Source of EVs | EVs’ Action | References |
|---|---|---|---|
| DSS-induced colitis | grapefruit | relieving symptoms by inhibiting the production of proinflammatory cytokines | [17] |
| broccoli | activation of AMPK in DCs, preventing DC activation and inducing tolerogenic phenotype, reduction in proinflammatory cytokines | [19] | |
| grape | increasing the proliferation of intestinal stem cells | [20] | |
| Akkermansia muciniphila | decreasing the production of proinflammatory cytokines in the colon and infiltration by inflammatory cells | [43] | |
| Escherichia coli Nissle 1917 | ameliorating inflammation in the gut, reducing the increased levels of colonic proinflammatory cytokines, improving intestinal barrier functions | [44] | |
| Lactobacillus rhamnosus GG | reversal of overexpression of NF-κB and NLRP3 signaling pathways involved in inflammation | [45] | |
| cow and human milk | reducing the histopathological changes and shortening of the colon, reducing the production of proinflammatory cytokines | [70] | |
| tamoxifen-induced ulcerative colitis | bovine milk | improvement of colon condition, macroscopic reduction of colon inflammation | [74] |
| deoxynivalenol-induced small intestine damage | porcine milk | restoring the disturbed proliferation of intestinal cells, reversal of apoptosis and intercellular junction disorders | [76] |
| alcohol-induced liver damage | ginger | enhancing the expression of liver detoxifying/antioxidant genes, inhibiting the production of reactive oxygen species | [24] |
| CCl4-induced liver failure | human umbilical cord mesenchymal stem cells | reduction in oxidative stress and apoptosis | [99] |
| diabetes | Akkermansia muciniphila | improving intestinal integrity by regulation of tight junctions through AMPK activation, improving glucose tolerance | [40] |
| bacterial infections | Campylobacter jejuni | immune protection against infection with C. jejuni, reducing the bacterial load in cecum | [47] |
| Vibrio cholerae | strong stimulation of antibody production, long-term protective immune response against V. cholerae | [48] | |
| Helicobacter pylori | reducing bacterial numbers, production of H. pylori-specific antibodies | [49] | |
| osteoporosis | bovine milk | improving bone mineral density, inhibition of bone resorption, restoration of disturbed intestinal microbiota | [69] |
| arthritis | bovine milk | reducing proinflammatory cytokines, improved histology of joints | [80] |
| muscular dystrophy | human cardiac stromal cells | improvement of cardiac ejection fraction, muscle strength and exercise capacity | [100] |
| solid tumors | cow milk | reducing the primary tumor burden | [83] |
| camel milk | induction of cancer cell apoptosis, reduction of angiogenesis and metastasis in tumor tissues | [84] | |
| delayed-type hypersensitivity to food allergens (ovalbumin, casein) | suppressor T cells | suppressing cutaneous symptoms of hypersensitivity reaction | [108,109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieślik, M.; Nazimek, K.; Bryniarski, K. Extracellular Vesicles—Oral Therapeutics of the Future. Int. J. Mol. Sci. 2022, 23, 7554. https://doi.org/10.3390/ijms23147554
Cieślik M, Nazimek K, Bryniarski K. Extracellular Vesicles—Oral Therapeutics of the Future. International Journal of Molecular Sciences. 2022; 23(14):7554. https://doi.org/10.3390/ijms23147554
Chicago/Turabian StyleCieślik, Martyna, Katarzyna Nazimek, and Krzysztof Bryniarski. 2022. "Extracellular Vesicles—Oral Therapeutics of the Future" International Journal of Molecular Sciences 23, no. 14: 7554. https://doi.org/10.3390/ijms23147554






