Underlying Mechanisms of Reductive Amination on Pd-Catalysts: The Unique Role of Hydroxyl Group in Generating Sterically Hindered Amine
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Tests of the Prepared Palladium Catalysts
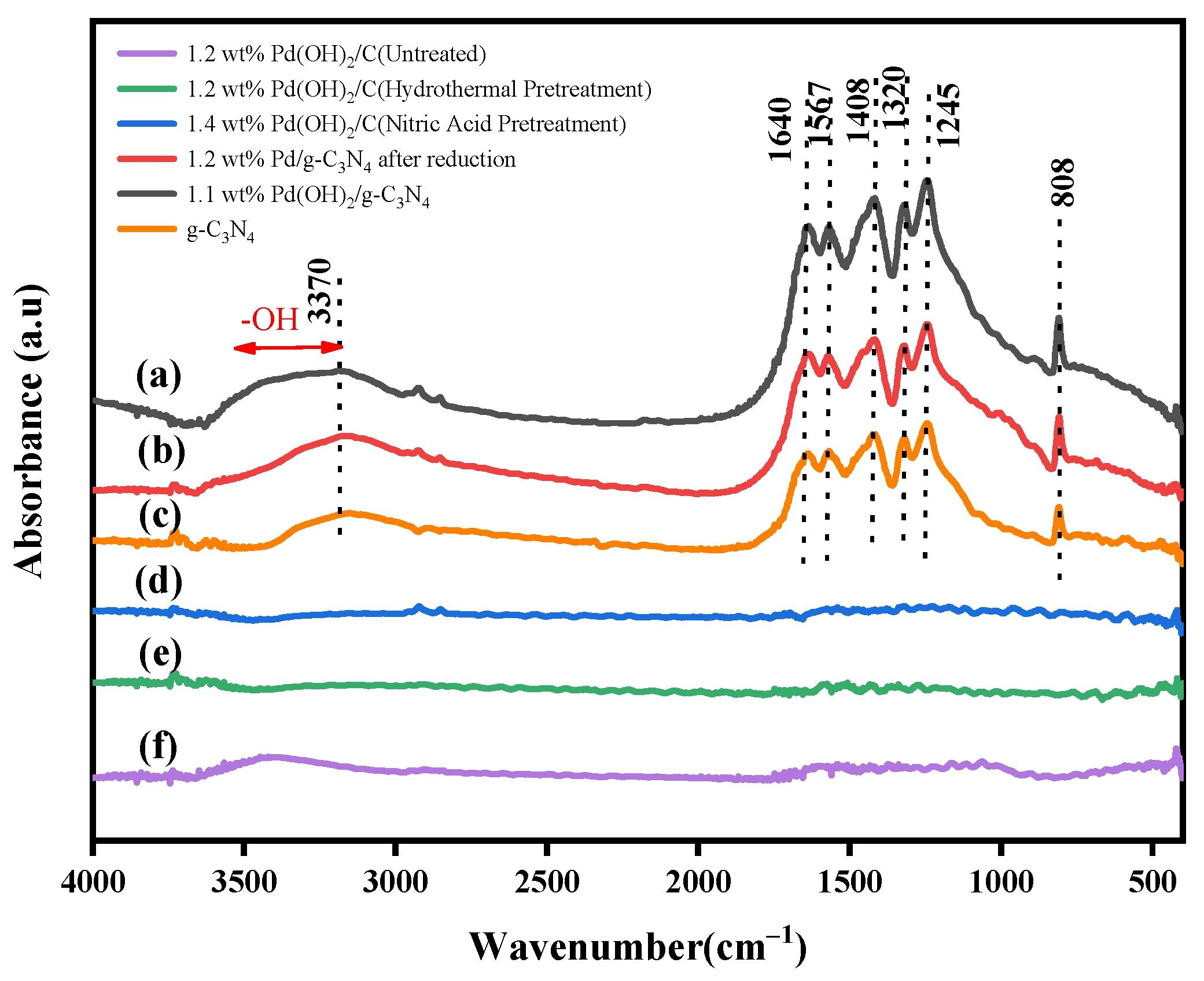
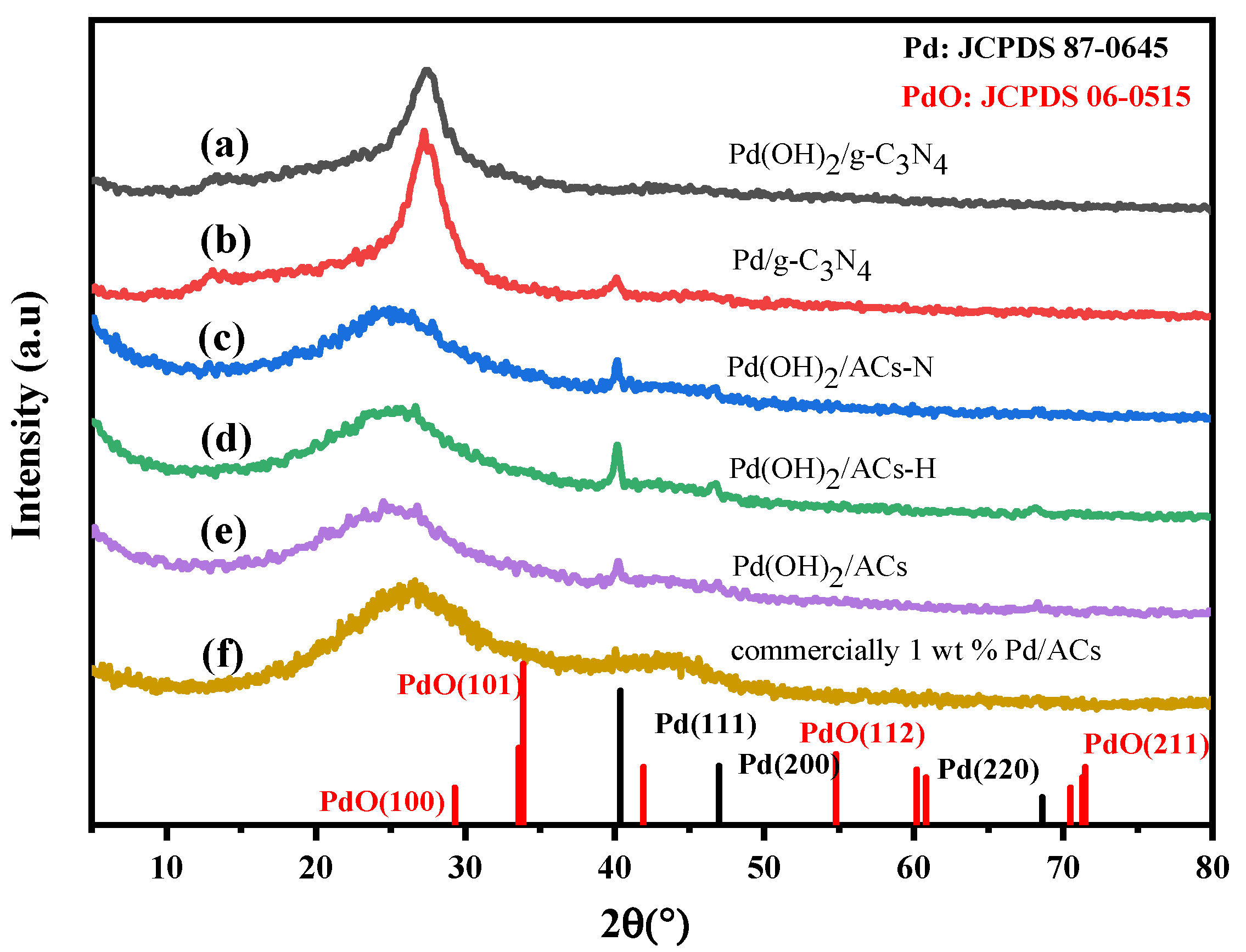
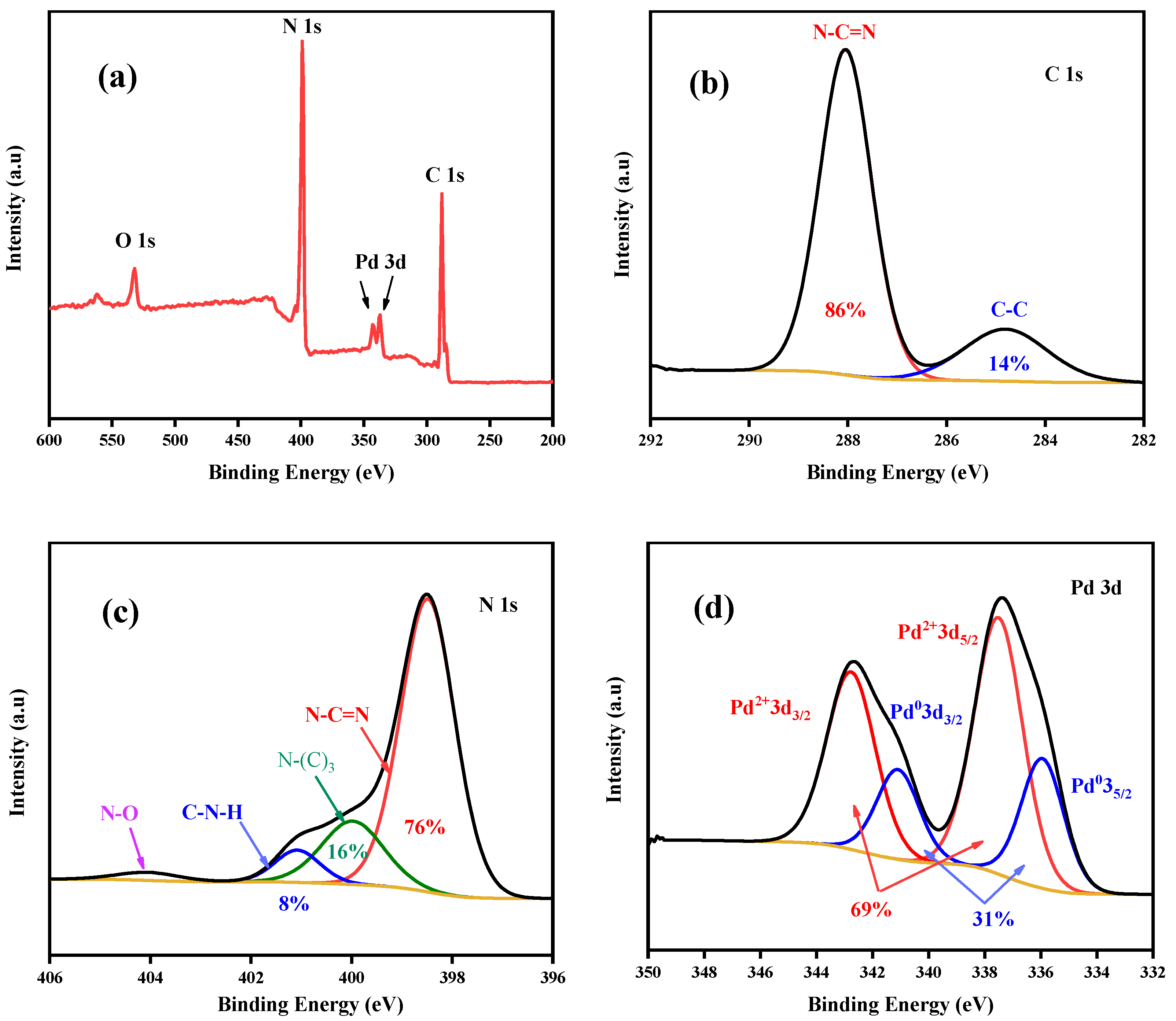
2.2. Characterization of the Prepared Pd(OH)2/g-C3N4 and Other Palladium Catalysts
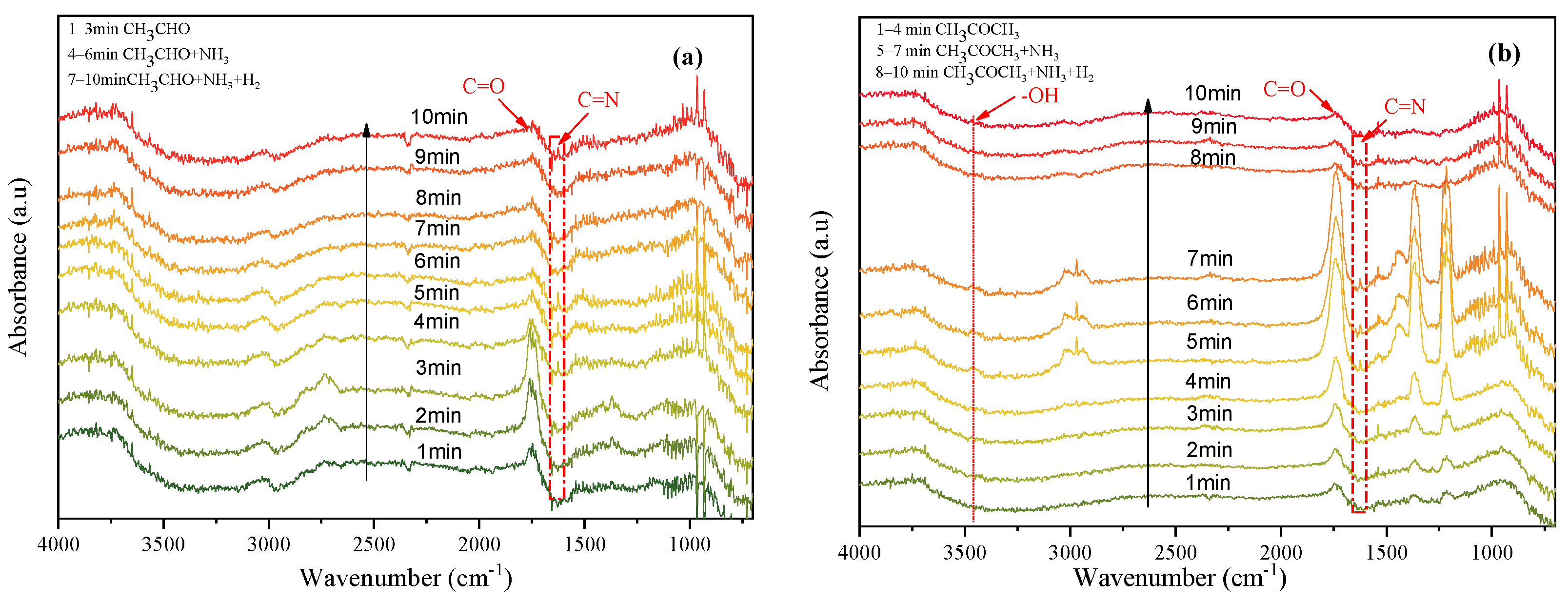
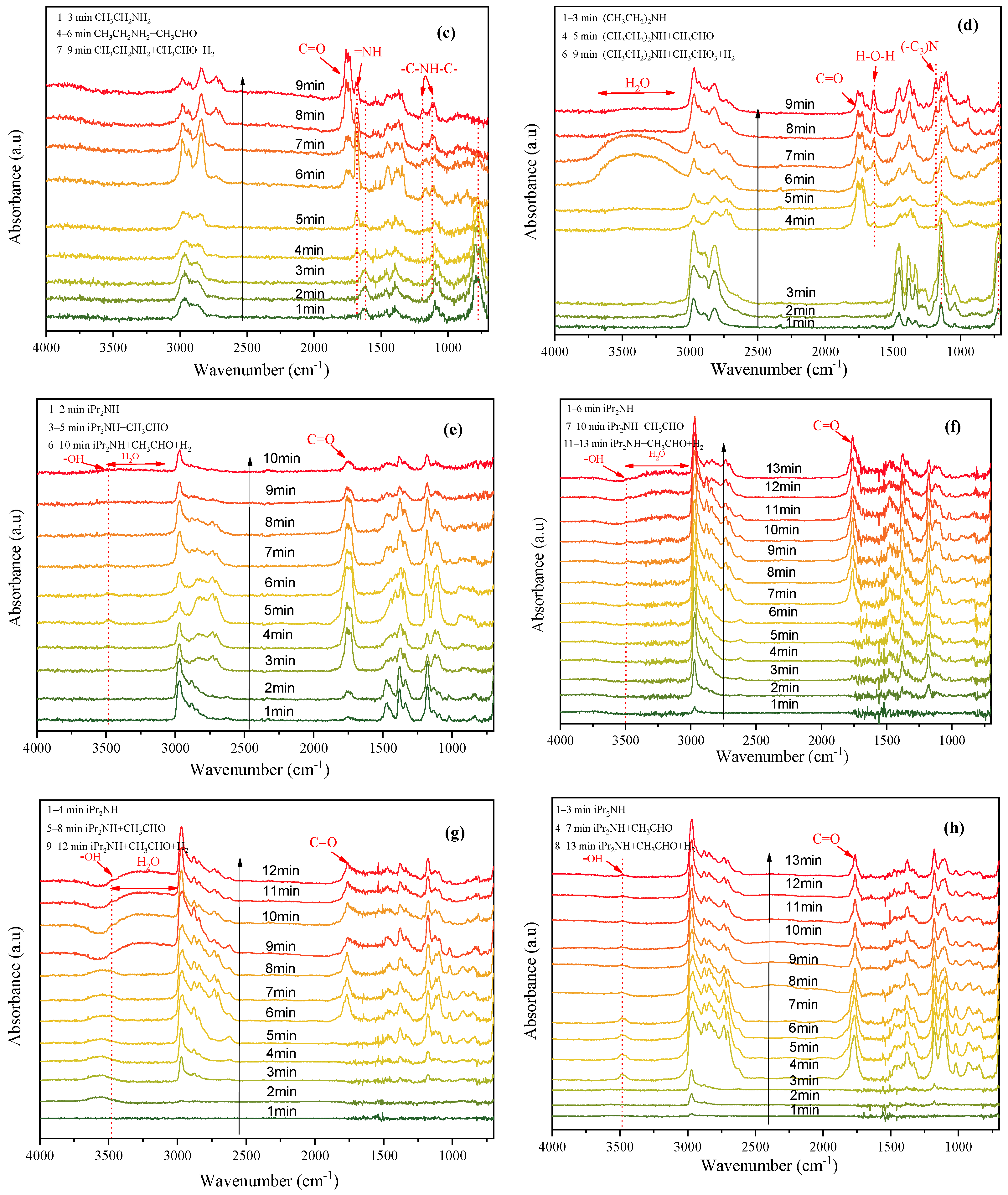
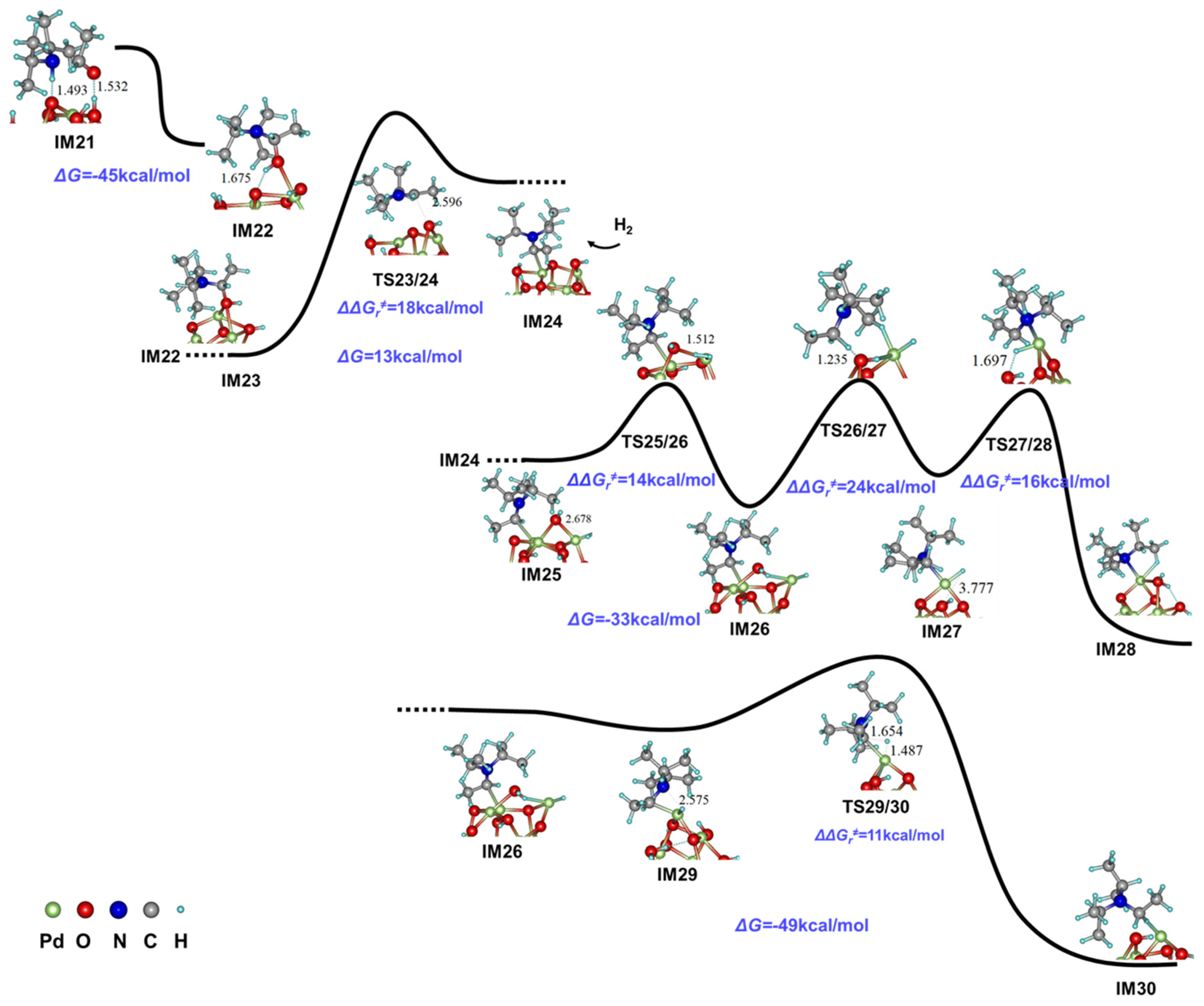
2.3. Mechanism Studies
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Characterization Techniques
3.3. Preparation of the Supports and Palladium Catalysts
3.3.1. Preparation of g-C3N4 Support
3.3.2. Pretreatment of Activated Carbon
3.3.3. Preparation of the Pd/ACs Catalyst
3.3.4. Preparation of the Pd(OH)2/g-C3N4 and Pd(OH)2/ACs Catalysts
3.4. General Procedure for the Preparation of Sterically Hindered Amine and Recycling Experiments
3.5. General Operation Procedure for the In Situ FT-IR Spectra
3.6. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afanasyev, O.I.; Kuchuk, E.; Usanov, D.L.; Chusov, D. Reductive Amination in the Synthesis of Pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Whyman, R. Review of Methods for the Catalytic Hydrogenation of Carboxamides. Chem. Rev. 2014, 114, 5477–5510. [Google Scholar] [CrossRef] [PubMed]
- Shamim, T.; Kumar, V.; Paul, S. Silica-Functionalized CuI: An Efficient and Selective Catalyst for N-Benzylation, Allylation, and Alkylation of Primary and Secondary Amines in Water. Synth. Commun. 2014, 44, 620–632. [Google Scholar] [CrossRef]
- Coleman, G.H. The Reaction of Alkylchloroamines with Grignard Reagents. J. Am. Chem. Soc. 1933, 55, 3001–3005. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. 3d-Metal Catalyzed N- and C-Alkylation Reactions via Borrowing Hydrogen or Hydrogen Autotransfer. Chem. Rev. 2019, 119, 2524–2549. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Chandrashekhar, V.G.; Natte, K.; Kamer, P.C.J.; Beller, M.; Jagadeesh, R.V. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines. Chem. Soc. Rev. 2020, 49, 6273–6328. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen. Chem. Rev. 2020, 120, 9583–9674. [Google Scholar] [CrossRef]
- Dong, C.; Wu, Y.; Wang, H.; Peng, J.; Li, Y.; Samart, C.; Ding, M. Facile and Efficient Synthesis of Primary Amines via Reductive Amination over a Ni/Al2O3 Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 7318–7327. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.-M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Lator, A.; Gaillard, Q.G.; Mérel, D.S.; Lohier, J.-F.; Gaillard, S.; Poater, A.; Renaud, J.-L. Room-Temperature Chemoselective Reductive Alkylation of Amines Catalyzed by a Well-Defined Iron(II) Complex Using Hydrogen. J. Org. Chem. 2019, 84, 6813–6829. [Google Scholar] [CrossRef] [PubMed]
- Kapnang, H.; Charles, G.; Sondengam, B.L.; Hemo, J.H. Bis (alcoxymethyl) amines: Preparation et reduction; N-dimethylation selective d′amines primaires. Tetrahedron Lett. 1977, 18, 3469–3472. [Google Scholar] [CrossRef]
- Tadanier, J.; Hallas, R.; Martin, J.R.; Stanaszek, R.S. Observations relevant to the mechanism of the reductive aminations of ketones with sodium cyanoborohydride and ammonium acetate. Tetrahedron 1981, 37, 1309–1316. [Google Scholar] [CrossRef]
- Gomez, S.; Peters, J.A.; Maschmeyer, T. The Reductive Amination of Aldehydes and Ketones and the Hydrogenation of Nitriles: Mechanistic Aspects and Selectivity Control. Adv. Synth. Catal. 2002, 344, 1037–1057. [Google Scholar] [CrossRef]
- Yagafarov, N.Z.; Kolesnikov, P.N.; Usanov, D.L.; Novikov, V.V.; Nelyubina, Y.V.; Chusov, D. The synthesis of sterically hindered amines by a direct reductive amination of ketones. Chem. Commun. 2016, 52, 1397–1400. [Google Scholar] [CrossRef]
- Hünig, S.; Kiessel, M. Spezifische Protonenacceptoren als Hilfsbasen bei Alkylierungs- und Dehydrohalogenierungsreaktionen. Chem. Ber. 1958, 91, 380–392. [Google Scholar] [CrossRef]
- Step, E.N.; Turro, N.J.; Gande, M.E.; Klemchuk, P.P. Mechanism of Polymer Stabilization by Hindered-Amine Light Stabilizers (HALS). Model Investigations of the Interaction of Peroxy Radicals with HALS Amines and Amino Ethers. Macromolecules 1994, 27, 2529–2539. [Google Scholar] [CrossRef]
- Chelucci, G. Metal-complexes of optically active amino- and imino-based pyridine ligands in asymmetric catalysis. Coord. Chem. Rev. 2013, 257, 1887–1932. [Google Scholar] [CrossRef]
- Stephan, D.W. Frustrated Lewis pairs: From concept to catalysis. Acc. Chem. Res. 2015, 48, 306–316. [Google Scholar] [CrossRef]
- Luo, D.; He, Y.; Yu, X.; Wang, F.; Zhao, J.; Zheng, W.; Jiao, H.; Yang, Y.; Li, Y.; Wen, X. Intrinsic mechanism of active metal dependent primary amine selectivity in the reductive amination of carbonyl compounds. J. Catal. 2021, 395, 293–301. [Google Scholar] [CrossRef]
- Podyacheva, E.; Afanasyev, O.I.; Tsygankov, A.A.; Makarova, M.; Chusov, D. Hitchhiker′s Guide to Reductive Amination. Synthesis-Stuttgart 2019, 51, 2667–2677. [Google Scholar]
- Karageorge, G.N.; Macor, J.E. Synthesis of novel serotonergics and other N-alkylamines using simple reductive amination using catalytic hydrogenation with Pd/C. Tetrahedron Lett. 2011, 52, 5117–5119. [Google Scholar] [CrossRef]
- Guo, M.; Jayakumar, S.; Luo, M.; Kong, X.; Li, C.; Li, H.; Chen, J.; Yang, Q. The promotion effect of pi-pi interactions in Pd NPs catalysed selective hydrogenation. Nat. Commun. 2022, 13, 1770. [Google Scholar] [CrossRef]
- Neri, G.; Musolino, M.G.; Milone, C.; Pietropaolo, D.; Galvagno, S. Particle size effect in the catalytic hydrogenation of 2,4-dinitrotoluene over Pd/C catalysts. Appl. Catal. A 2001, 208, 307–316. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, H.; Liu, R.; Hao, J.; Yu, Y.; Zhao, F. Influence of metal particle size on the hydrogenation of maleic anhydride over Pd/C catalysts in scCO2. Catal. Today 2009, 148, 368–372. [Google Scholar] [CrossRef]
- Suo, Y.; Hsing, I.M. Size-controlled synthesis and impedance-based mechanistic understanding of Pd/C nanoparticles for formic acid oxidation. Electrochim. Acta 2009, 55, 210–217. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Zhao, H.; Zheng, X.; Wu, L.; Pan, H.; Zhu, J.; Chen, Y.; Lu, J. Size-dependent catalytic activity over carbon-supported palladium nanoparticles in dehydrogenation of formic acid. J. Catal. 2017, 352, 371–381. [Google Scholar] [CrossRef]
- Chinthaginjala, J.K.; Villa, A.; Su, D.S.; Mojet, B.L.; Lefferts, L. Nitrite reduction over Pd supported CNFs: Metal particle size effect on selectivity. Catal. Today 2012, 183, 119–123. [Google Scholar] [CrossRef]
- Sun, W.; Wu, S.; Lu, Y.; Wang, Y.; Cao, Q.; Fang, W. Effective Control of Particle Size and Electron Density of Pd/C and Sn-Pd/C Nanocatalysts for Vanillin Production via Base-Free Oxidation. ACS Catal. 2020, 10, 7699–7709. [Google Scholar] [CrossRef]
- Edwards, J.K.; Thomas, A.; Solsona, B.E.; Landon, P.; Carley, A.F.; Hutchings, G.J. Comparison of supports for the direct synthesis of hydrogen peroxide from H2 and O2 using Au–Pd catalysts. Catal. Today 2007, 122, 397–402. [Google Scholar] [CrossRef]
- Xu, X.; Luo, J.; Li, L.; Zhang, D.; Wang, Y.; Li, G. Unprecedented catalytic performance in amine syntheses via Pd/g-C3N4 catalyst-assisted transfer hydrogenation. Green Chem. 2018, 20, 2038–2046. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, J.; Liu, G.; Zhou, Y.; Han, K.; Ye, H. G-C3N4-coated activated carbon-supported Pd catalysts for 4-CBA hydrogenation: Effect of nitrogen species. Catal. Sci. Technol. 2015, 5, 3926–3930. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Li, H.; Su, D.; Antonietti, M. Highly Selective Hydrogenation of Phenol and Derivatives over a Pd@Carbon Nitride Catalyst in Aqueous Media. J. Am. Chem. Soc. 2011, 133, 2362–2365. [Google Scholar] [CrossRef] [PubMed]
- Heinen, A.W.; Peters, J.A.; Bekkum, H. The Reductive Amination of Benzaldehyde Over Pd/C Catalysts: Mechanism and Effect of Carbon Modifications on the Selectivity. Eur. J. Org. Chem. 2000, 2000, 2501–2506. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, J.; Varadan, V.K. Functionalization of carbon nanotubes by potassium permanganate assisted with phase transfer catalyst. Smart Mater. Struct. 2002, 11, 962–965. [Google Scholar] [CrossRef]
- Carmo, M.; Linardi, M.; Rocha Poco, J.G. H2O2 treated carbon black as electrocatalyst support for polymer electrolyte membrane fuel cell applications. Int. J. Hydrogen Energy 2008, 33, 6289–6297. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.; Li, X.; Lu, C.; Liu, H. Effect of Nitric Acid Pretreatment on the Properties of Activated Carbon and Supported Palladium Catalysts. Ind. Eng. Chem. Res. 2005, 44, 5478–5482. [Google Scholar] [CrossRef]
- Xu, T.-Y.; Zhang, Q.-F.; Yang, H.-F.; Li, X.-N.; Wang, J.-G. Role of Phenolic Groups in the Stabilization of Palladium Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 9783–9789. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.Z.; Yan, N. A remarkable solvent effect on reductive amination of ketones. Mol. Catal. 2018, 454, 87–93. [Google Scholar] [CrossRef]
- Liu, J.; Fitzgerald, A.E.; Mani, N.S. Reductive Amination by Continuous-Flow Hydrogenation: Direct and Scalable Synthesis of a Benzylpiperazine. Synthesis-Stuttgart 2012, 44, 2469–2473. [Google Scholar] [CrossRef]
- Moro Ouma, C.N.; Modisha, P.; Bessarabov, D. Insight into the adsorption of a liquid organic hydrogen carrier, perhydro-i-dibenzyltoluene (i = m, o, p), on Pt, Pd and PtPd planar surfaces. RSC Adv. 2018, 8, 31895–31904. [Google Scholar] [CrossRef] [PubMed]
- Santarossa, G.; Iannuzzi, M.; Vargas, A.; Baiker, A. Adsorption of Naphthalene and Quinoline on Pt, Pd and Rh: A DFT Study. ChemPhysChem 2008, 9, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, F.; Thomazeau, C.; Raybaud, P.; Toulhoat, H. Adsorption of Unsaturated Hydrocarbons on Pd(111) and Pt(111): A DFT Study. J. Phys. Chem. B 2003, 107, 12287–12295. [Google Scholar] [CrossRef]
- Han, M.; Wang, H.; Zhao, S.; Hu, L.; Huang, H.; Liu, Y. One-step synthesis of CoO/g-C3N4 composites by thermal decomposition for overall water splitting without sacrificial reagents. Inorg. Chem. Front. 2017, 4, 1691–1696. [Google Scholar] [CrossRef]
- Qi, F.; Li, Y.; Wang, Y.; Wang, Y.; Liu, S.; Zhao, X. Ag-Doped g-C3N4 film electrode: Fabrication, characterization and photoelectrocatalysis property. RSC Adv. 2016, 6, 81378–81385. [Google Scholar] [CrossRef]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 52, 5155–5164. [Google Scholar] [CrossRef]
- Baranova, E.A.; Le Page, Y.; Ilin, D.; Bock, C.; MacDougall, B.; Mercier, P.H.J. Size and composition for 1–5nm Ø PtRu alloy nano-particles from Cu Kα X-ray patterns. J. Alloys Compd. 2009, 471, 387–394. [Google Scholar] [CrossRef][Green Version]
- Bakker, J.J.W.; Neut, A.G.v.d.; Kreutzer, M.T.; Moulijn, J.A.; Kapteijn, F. Catalyst performance changes induced by palladium phase transformation in the hydrogenation of benzonitrile. J. Catal. 2010, 274, 176–191. [Google Scholar] [CrossRef]
- Amorim, C.; Keane, M.A. Palladium supported on structured and nonstructured carbon: A consideration of Pd particle size and the nature of reactive hydrogen. J. Colloid Interface Sci. 2008, 322, 196–208. [Google Scholar] [CrossRef]
- Albers, P.W.; Möbus, K.; Wieland, S.D.; Parker, S.F. The fine structure of Pearlman’s catalyst. Phys. Chem. Chem. Phys. 2015, 17, 5274–5278. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, A.; Haider, A.; Nguyen, C.V.; Lee, K.R.; Choung, S.; Han, J.W.; Baek, S.-H.; Shin, C.-H.; Jung, K.-D. Structural effect of Nitrogen/Carbon on the stability of anchored Ru catalysts for CO2 hydrogenation to formate. Chem. Eng. J. 2022, 433, 133571. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Yuranov, I.; Suvorova, E.I.; Buffat, P.A.; Kiwi-Minsker, L. Highly dispersed gold on activated carbon fibers for low-temperature CO oxidation. J. Catal. 2004, 224, 8–17. [Google Scholar] [CrossRef]
- Das, T.K.; Banerjee, S.; Vishwanadh, B.; Joshi, R.; Sudarsan, V. On the nature of interaction between Pd nanoparticles and C3N4 support. Solid State Sci. 2018, 83, 70–75. [Google Scholar] [CrossRef]
- García, G.; Silva-Chong, J.; Rodríguez, J.L.; Pastor, E. Spectroscopic elucidation of reaction pathways of acetaldehyde on platinum and palladium in acidic media. J. Solid State Electrochem. 2014, 18, 1205–1213. [Google Scholar] [CrossRef]
- Knöpke, L.R.; Nemati, N.; Köckritz, A.; Brückner, A.; Bentrup, U. Reaction Monitoring of Heterogeneously Catalyzed Hydrogenation of Imines by Coupled ATR-FTIR, UV/Vis, and Raman Spectroscopy. ChemCatChem 2010, 2, 273–280. [Google Scholar] [CrossRef]
- Tammer, M.G. Sokrates: Infrared and Raman characteristic group frequencies: Tables and charts. Colloid Polym. Sci. 2004, 283, 235. [Google Scholar] [CrossRef]
- Aguirre, A.; Collins, S.E. Insight into the mechanism of acetonitrile hydrogenation in liquid phase on Pt/Al2O3 by ATR-FTIR. Catal. Today 2019, 336, 22–32. [Google Scholar] [CrossRef]
- Litvak, I.; Anker, Y.; Cohen, H. On-line in situ determination of deuterium content in water via FTIR spectroscopy. RSC Adv. 2018, 8, 28472–28479. [Google Scholar] [CrossRef]
- Raskó, J.; Kiss, J. Adsorption and surface reactions of acetonitrile on Al2O3-supported noble metal catalysts. Appl. Catal. A 2006, 298, 115–126. [Google Scholar] [CrossRef]
- Wu, X.; Ge, Q.; Zhu, X. Vapor phase hydrodeoxygenation of phenolic compounds on group 10 metal-based catalysts: Reaction mechanism and product selectivity control. Catal. Today 2021, 365, 143–161. [Google Scholar] [CrossRef]
- Ge, X.; Luo, C.; Qian, C.; Yu, Z.; Chen, X. RANEY® nickel-catalyzed reductive N-methylation of amines with paraformaldehyde: Theoretical and experimental study. RSC Adv. 2014, 4, 43195–43203. [Google Scholar] [CrossRef]
- Sanyal, U.; Yuk, S.F.; Koh, K.; Lee, M.S.; Stoerzinger, K.; Zhang, D.; Meyer, L.C.; Lopez-Ruiz, J.A.; Karkamkar, A.; Holladay, J.D.; et al. Hydrogen Bonding Enhances the Electrochemical Hydrogenation of Benzaldehyde in the Aqueous Phase. Angew. Chem. Int. Ed. 2021, 60, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Brigas, A.F.; Clegg, W.; Dillon, C.J.; Fonseca, C.F.C.; Johnstone, R.A.W. Metal-assisted reactions. Part 29.1 Structure and hydrogenolysis of C–N bonds in derivatives of aromatic amines. Bond length and electronegativity changes from X-ray crystallographic data. J. Chem. Soc. Perkin Trans. 2 2001, 8, 1315–1324. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB-An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All spd-Block Elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Using Hessian updating to increase the efficiency of a Hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005, 1, 61–69. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Kilpatrick, N.J.; Garrett, B.C. Reaction-path interpolation models for variational transition-state theory. J. Chem. Phys. 1983, 78, 2438–2442. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical-reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Lu, T. Gau_xtb: A Gaussian Interface for xtb Code. Available online: http://sobereva.com/soft/gau_xtb (accessed on 17 May 2021).




| Entry | Catalyst | Temp./°C | Time/h | Conv./% | Sel./% | Yield b/% |
|---|---|---|---|---|---|---|
| 1 | Raney Ni | 100 | 7 | 70 | Trace | Trace |
| 2 | Raney Co | 30 | 4 | 55 | Trace | Trace |
| 3 | Raney Cu | 100 | 7 | 46 | Trace | Trace |
| 4 | 5.0 wt% Ru/ACs g | 30 | 5 | Trace | Trace | Trace |
| 5 | 5.0 wt% Rh/ACs | 30 | 5 | Trace | Trace | Trace |
| 6 | 5.0 wt% Pt/ACs | 30 | 5 | 51 | 61 | 31 |
| 7 | 5.0 wt% Pd/ACs | 30 | 4 | 94 | 85 | 80 |
| 8 | 1.0 wt% Pd/ACs | 30 | 4 | 90 | 76 | 68 |
| 9 | Pd(AcO)2+Xphos | 60 | 7 | 28 | 46 | 13 |
| 10 | 10.0 wt% Pd(OH)2/ACs | 30 | 4 | 97 | 95 | 92 |
| 11 | 1.2 c,1.4 d,1.2 e wt% Pd(OH)2/ACs | 30 | 4 | 65/62/33 | 86 c/89 d/36 e | 56/55/12 |
| 12 | 1.1 wt% Pd(OH)2/g-C3N4 | 30 | 4 | 60/77 | 97/95 f | 58/73(60) |
| 13 | 1.2 wt% Pd/g-C3N4 | 30 | 4 | 33 | 30 | 10 |
| 14 | 1.2 wt% Pd/ACs | 30 | 4 | 80 | 66 | 53 |
| 15 | 1.3 wt% PdO/ACs | 30 | 4 | 22 | 36 | 8 |
| 16 | 1.1 wt% PdO/g-C3N4 | 30 | 4 | 30 | trace | trace |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Sub 1 | Sub 2 | Product | Temp (℃) | t (h) | Conv. (%) | Sel. (%) | Yield. a (%) |
| 1 | 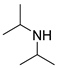 |  |  | 90 | 6 | 90 | >99 | >89(83) |
| 2 | 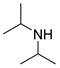 |  |  | 30 | 6 | 99 | 87 | 86(81) |
| 3 | 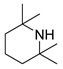 |  | 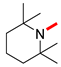 | 90 | 6 | >99 | >99 | >99(98) |
| 4 | 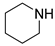 | 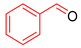 |  | 90 | 6 | >99 | >99 | >99(93) |
| 5 | 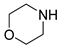 |  |  | 90 | 6 | >99 | >99 | >99(94) |
| 6 | 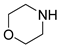 |  |  | 90 | 6 | 85 | 85 | 72(62) |
| 7 | 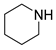 |  |  | 30 | 6 | >99 | 70 | 70(63) |
| 8 | 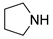 | 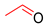 |  | 30 | 6 | 90 | 40 | 36(28) |
| 9 |  |  |  | 90 | 6 | >99 | >99 | >99(98) |
| 10 |  |  | 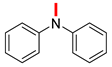 | 90 | 6 | 85 | 12 | 10(8) |
| 11 | 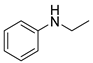 |  | 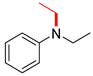 | 30 | 6 | 40 | 38 | 15(12) |
| 12 | 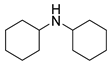 |  |  | 90 | 6 | >99 | >99 | >99(92) |
| 13 | 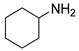 |  |  | 90 | 6 | 70 | 75 | 52(43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Z.; Ge, X.; Zhou, S. Underlying Mechanisms of Reductive Amination on Pd-Catalysts: The Unique Role of Hydroxyl Group in Generating Sterically Hindered Amine. Int. J. Mol. Sci. 2022, 23, 7621. https://doi.org/10.3390/ijms23147621
Hong Z, Ge X, Zhou S. Underlying Mechanisms of Reductive Amination on Pd-Catalysts: The Unique Role of Hydroxyl Group in Generating Sterically Hindered Amine. International Journal of Molecular Sciences. 2022; 23(14):7621. https://doi.org/10.3390/ijms23147621
Chicago/Turabian StyleHong, Zeng, Xin Ge, and Shaodong Zhou. 2022. "Underlying Mechanisms of Reductive Amination on Pd-Catalysts: The Unique Role of Hydroxyl Group in Generating Sterically Hindered Amine" International Journal of Molecular Sciences 23, no. 14: 7621. https://doi.org/10.3390/ijms23147621
APA StyleHong, Z., Ge, X., & Zhou, S. (2022). Underlying Mechanisms of Reductive Amination on Pd-Catalysts: The Unique Role of Hydroxyl Group in Generating Sterically Hindered Amine. International Journal of Molecular Sciences, 23(14), 7621. https://doi.org/10.3390/ijms23147621







