Nematic-to-Isotropic Phase Transition in Poly(L-Lactide) with Addition of Cyclodextrin during Abiotic Degradation Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Degradation Study
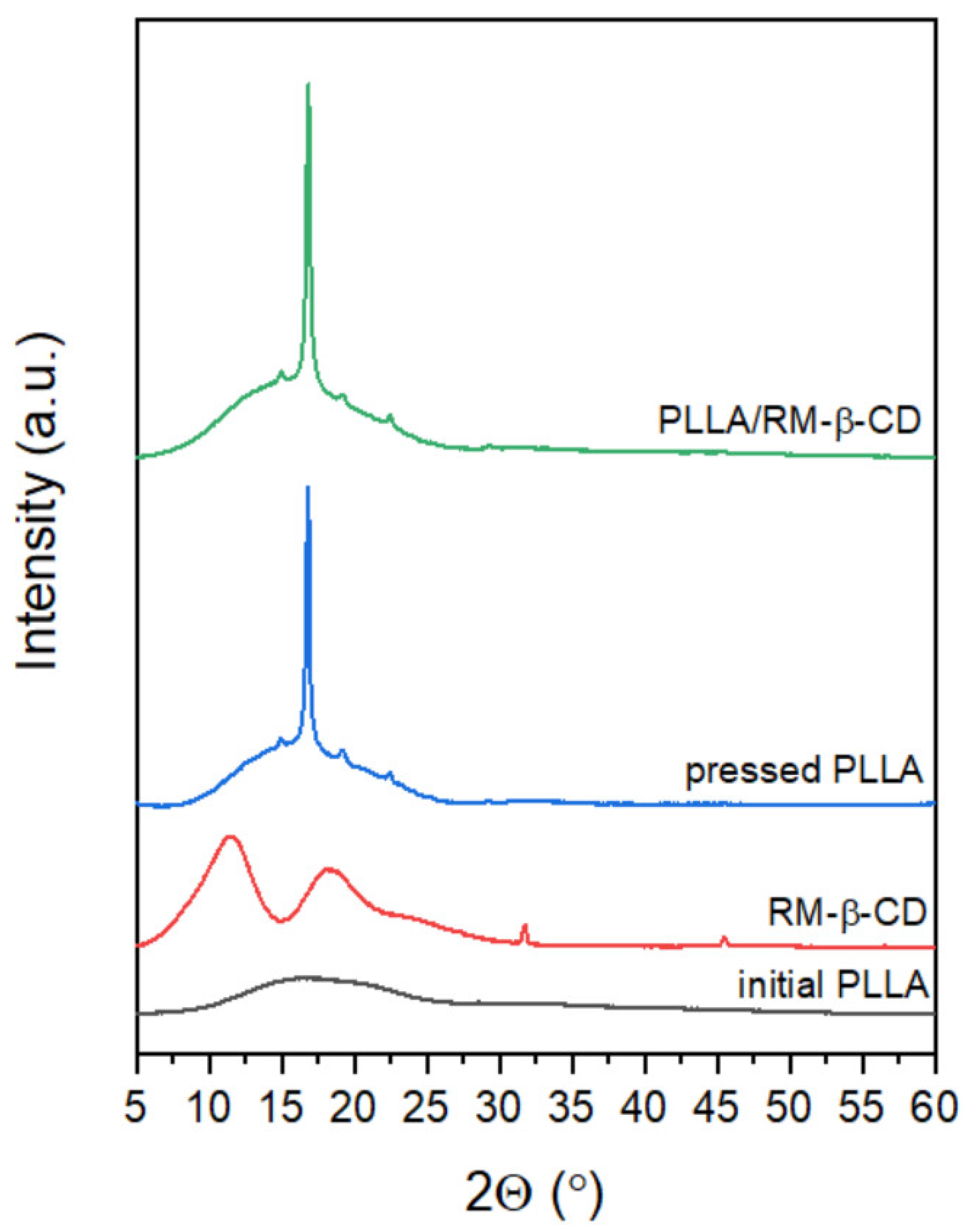
2.2. Liquid Crystal Properties
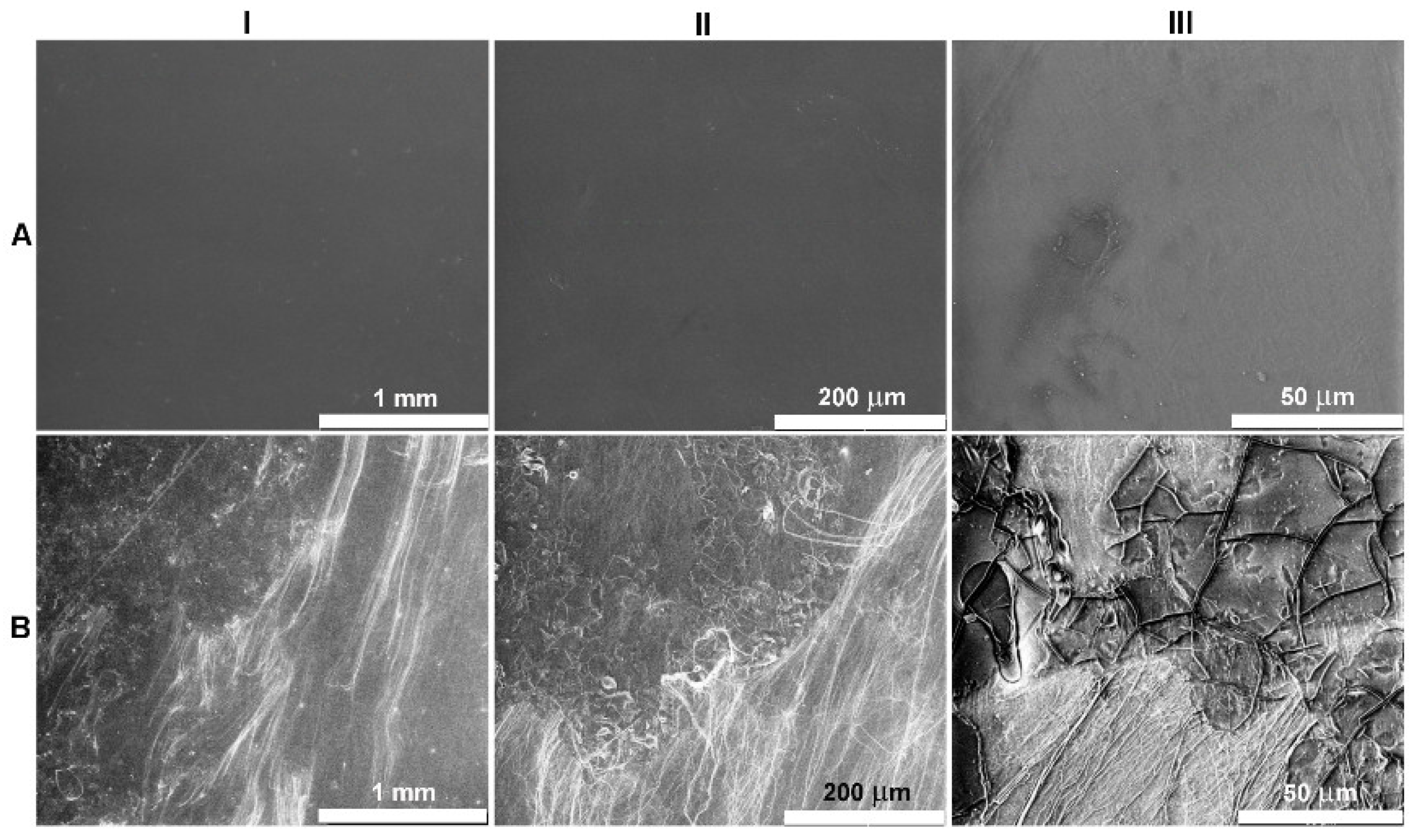
2.3. Surface Characteristics
3. Materials and Methods
3.1. Materials
3.2. PLLA Films Preparation
3.3. Abiotic Degradation Study
3.4. Characterization
3.4.1. Polarized Optical Microscopy (POM)
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. Atomic Force Microscopy (AFM)
3.4.4. Differential Scanning Calorimetry (DSC)
3.4.5. X-ray Diffraction and Residual Stress Analysis
3.4.6. Gel Permeation Chromatography (GPC)
3.4.7. Contact Angle Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Kumar, S. Microbial enzyme in food biotechnology. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Elsevier: Oxford, UK, 2019; Chapter 2. [Google Scholar] [CrossRef]
- Dipak, R.; Pravin, G.; Kripal, S.; Dipak, S. FTIR, 1H NMR spectral, powder X-ray diffraction and DSC studies of “β-cyclodextrin-para-chlorobenzonitrile” inclusion complex. Res. J. Chem. Sci. 2012, 2, 60–63. [Google Scholar]
- Maazaoui, R.; Abderrahim, R. Applications of cyclodextrins: Formation of inclusion complexes and their characterization. Int. J. Adv. Res. 2015, 3, 1030. [Google Scholar]
- Zhou, Y.; Song, Y.; Zhen, W.; Wang, W. The Effects of structure of inclusion complex between β-cyclodextrin and poly(L-lactic acid) on its performance. Macromol. Res. 2015, 23, 1103–1111. [Google Scholar] [CrossRef]
- He, Y.; Inoue, Y. α-Cyclodextrin-enhanced crystallization of poly(3-hydroxybutyrate). Biomacromolecules 2003, 4, 1865–1867. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Inoue, Y. Effect of α-cyclodextrin on the crystallization of poly(3-hydroxybutyrate). J. Polym. Sci. B Polym. Phys. 2004, 42, 3461–3469. [Google Scholar] [CrossRef]
- Zhang, L.; Zhenand, W.; Zhou, Y. Structure and properties of poly(lactic acid)/poly(lactic acid)-α-cyclodextrin inclusion compound composites. J. Polym. Eng. 2016, 37, 897–909. [Google Scholar] [CrossRef]
- Almenar, E.; Auras, R.; Harte, B.; Rubino, M. Beta-cyclodextrins as nucleating agents for poly(lactic acid). (Board of Trustees Operating) WO2009032199A1. U.S. Patent No. 12/201,452, 5 March 2009. [Google Scholar]
- Byun, Y.; Rodriguez, K.; Han, J.H.; Kim, J.T. Improved thermal stability of polylactic acid (PLA) composite film via PLA-β-cyclodextrin-inclusion complex systems. Int. J. Biol. Macromol. 2015, 8, 1591–1598. [Google Scholar] [CrossRef]
- Kowalewska, A.; Nowacka, M. Supramolecular interactions in hybrid polylactide blends—The structures, mechanisms and properties. Molecules 2020, 25, 3351. [Google Scholar] [CrossRef]
- Fenyvesi, F.; Nguyen, T.L.P.; Haimhoffer, Á.; Rusznyák, Á.; Vasvári, G.; Bácskay, I.; Vecsernyés, M.; Ignat, S.-R.; Dinescu, S.; Costache, M.; et al. Cyclodextrin complexation improves the solubility and Caco-2 permeability of chrysin. Materials 2020, 13, 3618. [Google Scholar] [CrossRef]
- Szente, L. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Suzuki, T.; Ei, A.; Takada, Y.; Uehara, H.; Yamanobeand, T.; Takahashi, K. Modification of physical properties of poly(L-lactic acid) by addition of methyl-β-cyclodextrin. Beilstein J. Org. Chem. 2014, 10, 2997–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champagne, P.L.; Kumar, R.; Ling, C.C. Supramolecular liquid crystals based on cyclodextrins. In Cyclodextrin Applications in Medicine, Food, Environment and Liquid Crystals; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Chapter 7. [Google Scholar] [CrossRef]
- Fleischmann, E.K.; Zentel, R. Liquid-crystalline ordering as a concept in materials science: From semiconductors to stimuli-responsive devices. Angew. Chem. Int. Ed. 2013, 52, 8810–8827. [Google Scholar] [CrossRef] [PubMed]
- Liquid Crystals—IUPAC. Available online: https://iupac.org/materialschemistryedu/computing/liquid-crystals/ (accessed on 29 June 2021).
- Urbanski, M.; Reyes, C.G.; Noh, J.H.; Sharma, A.; Geng, Y.; Subba, V.; Jampani, R.; Lagerwall, J.P.F. Liquid crystals in micron-scale droplets, shells and fibers. J. Phys. Condens. Matter 2017, 29, 133003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puskás, I. Highly ordered architectures built from cyclodextrin complexes. Cyclodext. News 2017, 31, 1–22. [Google Scholar]
- Szente, L.; Puskás, I.; Csabai, K.; Fenyvesi, É. Supramolecular proteoglycan aggregate mimics: Cyclodextrin-assisted biodegradable polymer assemblies for electrostatic-driven drug delivery. Chem. Asian J. 2014, 9, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Mathiowitz, E.; Jacob, J.S.; Jong, Y.S.; Chickering, D.E., III; Edwards, E.E. Liquid crystalline polymers. (Brown University Research Foundation) WO2001068745A2. U.S. Patent No. WO 01/68745 A2, 20 September 2001. [Google Scholar]
- Hsiao, B.S.; Shaw, T.; Samulski, E.T. Pressure-induced phases in a thermotropic polyester. Macromolecules 1988, 21, 543–545. [Google Scholar] [CrossRef]
- Baptista, C.; Azagury, A.; Baker, C.; Mathiowitz, E. The characterization and quantification of the induced mesophases of poly-L-lactic acid. Polymer 2021, 226, 123822. [Google Scholar] [CrossRef]
- Baptista, C.; Azagury, A.; Shin, H.; Baker, C.; Ly, E.; Lee, R.; Mathiowitz, E. The effect of temperature and pressure on polycaprolactone morphology. Polymer 2020, 191, 122227. [Google Scholar] [CrossRef]
- Baker, C.; Azagury, A.; Mathiowitz, E. Effect of pressure on poly-L-lactic acid morphology. Polymer 2016, 99, 250–262. [Google Scholar] [CrossRef]
- Janeczek, H.; Duale, K.; Sikorska, W.; Godzierz, M.; Kordyka, A.; Marcinkowski, A.; Hercog, A.; Musioł, M.; Kowalczuk, M.; Christova, D.; et al. Poly(L-lactide) liquid crystals with tailor-made properties toward a specific nematic mesophase texture. ACS Sustain. Chem. Eng. 2022, 10, 3323–3334. [Google Scholar] [CrossRef]
- Stoclet, G.; Seguela, R.; Vanmansart, C.; Rochas, C.; Lefebvre, J.-M. WAXS study of the structural reorganization of semi-crystalline polylactide under tensile drawing. Polymer 2012, 53, 519–528. [Google Scholar] [CrossRef]
- Qiu, J.; Murata, T.; Wu, X.; Kudo, M.; Sakai, E. Plastic deformation mechanism of crystalline polymer materials during the rolling process. J. Mater. Sci. 2013, 48, 1920–1931. [Google Scholar] [CrossRef]
- Stoclet, G.; Seguela, R.; Lefebvre, J.M.; Elkoun, S.; Vanmansart, C. Strain-induced molecular ordering in polylactide upon uniaxial stretching. Macromolecules 2010, 43, 1488–1498. [Google Scholar] [CrossRef]
- Park, J.B.; Uhlmann, D.R. Recovery of deformed polymers. III. Thermal properties. J. Appl. Phys. 1973, 44, 201–206. [Google Scholar] [CrossRef]
- Hasan, O.; Boyce, M. Energy storage during inelastic deformation of glassy polymers. Polymer 1993, 34, 5085–5092. [Google Scholar] [CrossRef]
- Rydz, J.; Adamus, G.; Wolna-Stypka, K.; Marcinkowski, A.; Misiurska-Marczak, M.; Kowalczuk, M.M. Degradation of polylactide in paraffin and selected proticmedia. Polym. Degrad. Stab. 2013, 98, 316–324. [Google Scholar] [CrossRef]
- Gonzalez Ausejo, J.; Rydz, J.; Musioł, M.; Sikorska, W.; Sobota, M.; Włodarczyk, K.; Adamus, G.; Janeczek, H.; Kwiecień, I.; Hercog, A.; et al. A comparative study of three-dimensional printing directions: The degradation and toxicological profile of a PLA/PHA blend. Polym. Degrad. Stab. 2018, 152, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Rydz, J.; Wolna-Stypka, K.; Musioł, M.; Szeluga, U.; Janeczek, H.; Kowalczuk, M. Further evidence of polylactide degradation in paraffin and in selected proticmedia. A thermal analysis of eroded polylactide films. Polym. Degrad. Stab. 2013, 98, 1450–1457. [Google Scholar] [CrossRef]
- Yllmaz, V.T.; Karadag, A.; Jgbudak, H. Thermal decomposition of β-cyclodextrin inclusion complexes of ferrocene and their derivatives. Thermochim. Acta 1995, 261, 107–118. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, T.; Wan, J.; Liu, C.; Liu, W.; Wang, R. Predict the glass transition temperature and plasticization of β-cyclodextrin/water binary system by molecular dynamics simulation. Carbohydr. Res. 2015, 401, 89–95. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, T.; Xu, P.; Zhao, X.; Li, X.; Dong, W.; Ma, P.; Chen, M. Crystallization modification of poly(lactide) by using nucleating agents and stereocomplexation. e-Polymers 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Montserrat, S.; Colomer, R. The effect of the molecular weight on the glass transition temperature in amorphous poly(ethylene terephthalate). Polym. Bull. 1984, 12, 173–180. [Google Scholar] [CrossRef]
- Musioł, M.; Rydz, J.; Janeczek, H.; Kordyka, A.; Andrzejewski, J.; Sterzyński, T.; Jurczyk, S.; Cristea, M.; Musioł, K.; Kampik, M.; et al. (Bio)degradable biochar composites—Studies on degradation and electrostatic properties. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2022, 275, 115515. [Google Scholar] [CrossRef]
- Pokorny, R.; Seda, L.; Grof, Z.; Hajova, H.; Kosek, J. Diffusion in semi-crystalline polymers. Comput. Aided Chem. Eng. 2009, 26, 961–966. [Google Scholar] [CrossRef]
- Chan, C.L.C.; Bay, M.M.; Jacucci, G.; Vadrucci, R.; Williams, C.A.; van de Kerkhof, G.T.; Parker, R.M.; Vynck, K.; Frka-Petesic, B.; Vignolini, S. Visual appearance of chiral nematic cellulose-based photonic films: Angular and polarization independent color response with a twist. Adv. Mater. 2019, 31, 1905151. [Google Scholar] [CrossRef] [PubMed]
- Aldred, M.P.; Hudson, R.; Kitney, S.P.; Vlachos, P.; Liedtke, A.; Woon, K.L.; O’Neill, M.; Kelly, S.M. Electroluminescent segmented liquid crystalline trimers. Liq. Cryst. 2008, 35, 413–427. [Google Scholar] [CrossRef]
- Kumar, T.A.; Le, K.V.; Aya, S.; Kang, S.; Araoka, F.; Ishikawa, K.; Dhara, S.; Takezoe, H. Anchoring transition in a nematic liquid crystal doped with chiral agents. Phase Transit. 2012, 85, 888–899. [Google Scholar] [CrossRef]
- Demus, D.; Richter, L. Textures of Liquid Crystals; Verlag Chemie: Leipzig, Germany, 1978. [Google Scholar]
- Nayak, R.A.; Bhat, S.A.; Shanker, G.; Rao, D.S.S.; Yelamaggad, C.V. Highly frustrated liquid crystal phases in optically active dimers: Synthesis and rich phase transitional behavior. New J. Chem. 2019, 43, 2148–2162. [Google Scholar] [CrossRef]
- García, A.; Leonardi, D.; Vasconi, M.D.; Hinrichsen, L.I.; Lamas, M.C. Characterization of albendazole-randomly methylated-β-cyclodextrin inclusion complex and in vivo evaluation of its antihelmitic activity in a murine model of trichinellosis. PLoS ONE 2014, 9, e113296, 7 pages. [Google Scholar] [CrossRef] [Green Version]
- DeVries, A. X-ray photographic studies of liquid crystals I. A cybotactic nematic phase. Mol. Cryst. Liq. Cryst. 1970, 10, 219–236. [Google Scholar] [CrossRef]
- Chen, L.; Hu, T.H.; Xie, H.L.; Zhang, H.L. A mixed cyclodextrin-biphenyl thermotropic liquid crystal: Synthesis, liquid-crystalline properties, and supramolecular organization. J. Polym. Sci. A Polym. Chem. 2010, 48, 2838–2845. [Google Scholar] [CrossRef]
- Reheman, A.; Hu, S.; Cao, L.; Xie, D.; Yan, G.; Wang, J. Liquid-crystalline behaviour and electrorheological effect of phthalocyanine-based ionic liquid crystals. Liq. Cryst. 2021, 48, 1321–1330. [Google Scholar] [CrossRef]
- Na, B.; Zou, S.; Lv, R.; Luo, M.; Pan, H.; Yin, Q. Unusual cold crystallization behavior in physically aged poly(L-lactide). J. Phys. Chem. B 2011, 115, 10844–10848. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lee, S.C.; Jeong, Y.G. Influences of physical aging on enthalpy relaxation behavior, gas permeability, and dynamic mechanical property of polylactide films with various D-isomer contents. Macromol. Res. 2010, 18, 346–351. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Inoue, Y. Enthalpy relaxation and embrittlement of poly(L-lactide) during physical aging. Macromolecules 2007, 40, 9664–9671. [Google Scholar] [CrossRef]
- Iain, J.; Cowie, M.G.; Arrighi, V. Physical aging. PART III: Position annihilation lifetime spectroscopy. In Polymer Physics: From Suspensions to Nanocomposites and Beyond; Utracki, L.A., Jamieson, A.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Chapter 9. [Google Scholar] [CrossRef]
- Graca, M.; Wieczorek, S.A.; Holyst, R. Growth of polystyrene domains in isotropic, nematic and smectic phase of 8CB liquid crystal. Macromolecules 2003, 36, 6903–6913. [Google Scholar] [CrossRef]
- Miyata, T.; Masuko, T. Crystallization behavior of poly(L-lactide). Polymer 1998, 39, 5515–5521. [Google Scholar] [CrossRef]
- Kumar, B.R.; Rao, T.S. AFM studies on surface morphology, topography and texture of nanostructured zinc aluminum oxide thin films. Dig. J. Nanomater. Bios. 2012, 7, 1881–1889. [Google Scholar]
- Asylum research—An Oxford Instruments Company, Using AFM to measure surface roughness. 2020. Available online: https://www.azonano.com/article.aspx?ArticleID=5473 (accessed on 21 May 2021).
- Majoinen, J.; Kontturi, E.; Ikkala, O.; Gray, D.G. SEM imaging of chiral nematic films cast from cellulose nanocrystal suspensions. Cellulose 2012, 19, 1599–1605. [Google Scholar] [CrossRef]
- Bartczak, Z.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)—Morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- ISO 15814; Implants for surgery—Copolymers and blends based on polylactide—In vitro degradation testing. International Organization for Standardization: Geneva, Switzerland, 1999.
- Sikorska, W.; Richert, J.; Rydz, J.; Musioł, M.; Adamus, G.; Janeczek, H.; Kowalczuk, M. Degradability studies of poly(L-lactide) after multireprocessingexperiments in extruder. Polym. Degrad. Stab. 2012, 97, 1891–1897. [Google Scholar] [CrossRef]







| Sample | Initial PLLA | Pressed PLLA | Pressed PLLA/RM-β-CD | |||
|---|---|---|---|---|---|---|
| 50 °C | 70 °C | 50 °C | 70 °C | 50 °C | 70 °C | |
| Before degradation | ||||||
| Mw [g·mol−1] | 179,000 | 177,000 | 184,000 | |||
| Mw/Mn | 2.0 | 2.1 | 2.3 | |||
| 3rd day | ||||||
| Mw [g·mol−1] | N/A | 28,000 | N/A | 46,000 | N/A | 45,000 |
| Mw/Mn | N/A | 3.8 | N/A | 3.0 | N/A | 3.5 |
| 7th day | ||||||
| Mw [g·mol−1] | 136,000 | 7,000 | 157,000 | 16,000 | 159,000 | 17,000 |
| Mw/Mn | 4.5 | 1.9 | 2.4 | 3.3 | 4.5 | 3.3 |
| 21st day | ||||||
| Mw [g·mol−1] | 113,000 | N/A | 116,000 | N/A | 119,000 | N/A |
| Mw/Mn | 2.0 | N/A | 2.8 | N/A | 2.0 | N/A |
| 70st day | ||||||
| Mw [g·mol−1] | 20,000 | N/A | 29,000 | N/A | 21,000 | N/A |
| Mw/Mn | 4.0 | N/A | 3.2 | N/A | 3.0 | N/A |
| Sample | Initial PLLA | Pressed PLLA | Pressed PLLA/RM-β-CD | |||
|---|---|---|---|---|---|---|
| 50 °C | 70 °C | 50 °C | 70 °C | 50 °C | 70 °C | |
| Before degradation | ||||||
| Tg [°C] | 60.0 | 58.9 | 62.1 | |||
| Δcp [J/g °C] | 0.59 | 0.57 | 0.48 | |||
| Tcc [°C] | 125.6 | 126.2 | 122.0 | |||
| ΔHcc [J/g] | −8.08 | −4.00 | −9.17 | |||
| Tm [°C] | 152.4 | 148.9 | 150.3 | |||
| ΔHm [J/g] | 8.8 | 7.91 | 13.77 | |||
| 21st day | ||||||
| Tg [°C] | 58.8 | 45.7 | 58.6 | 48.0 | 59.5 | 47.9 |
| Δcp [J/g°C] | 0.60 | 0.59 | 0.24 | 0.55 | 0.60 | 0.58 |
| Tcc [°C] | 108.8 | - | 97.6 | - | 110.5 | 95.0 |
| ΔHcc [J/g] | −30.13 | - | −12.65 | - | −19.25 | −8.28 |
| Tm [°C] | 147.8/152.2 | 135.1 | 154.5 | 134.9/139.6 | 156.6 | 139.0 |
| ΔHm [J/g] | 31.11 | 58.04 | 12.70 | 61.88 | 19.45 | 55.73 |
| 70th day | ||||||
| Tg [°C] | 51.8 | 0.5/23.2 | 54.7 | −4.4/39.0 | 54.6 | −11.8/38.9 |
| Δcp [J/g°C] | 0.51 | 0.05/0.59 | 0.56 | 0.11/0.48 | 0.54 | 0.04/0.50 |
| Tcc [°C] | - | - | 96.2 | - | 111.1 | - |
| ΔHcc [J/g] | - | - | −5.28 | - | −3.42 | - |
| Tm [°C] | 132.8/149.8 | 91.4 | 152.3 | 81.2/99.5 | 153.0 | 82.8/112.3 |
| ΔHm [J/g] | 39.43 | 52.42 | 36.68 | 46.31 | 32.53 | 38.54 |
| Sample | Lattice Parameters [Å] | Lattice Volume [Å3] | Crystallite Size [nm] | Lattice Strain [%] | Residual Stress [MPa] | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| reference polylactide 1 | 10.84 | 6.19 | 28.95 | 1942.5 | |||
| Pressed PLLA24 | 10.72 | 6.21 | 29.16 | 1940.8 | 41 ± 8 | 0.05 ± 0.01 | 4.8 ± 1.0 |
| Pressed PLLA/RM-β-CD | 10.67 | 6.19 | 28.41 | 1875.9 | 30 ± 1 | 0.91 ± 0.44 | −2.8 ± 0.7 |
| Pressed PLLA_1d_50 | 10.73 | 6.19 | 28.56 | 1897.5 | 18 ± 3 | 0.50 ± 0.06 | 6.7 ± 0.9 |
| Pressed PLLA_1d_70 | 10.84 | 6.20 | 29.00 | 1948.8 | 25 ± 6 | 0.46 ± 0.06 | 13.8 ± 1.7 |
| Initial PLLA_70d_50 | 10.64 | 6.13 | 28.94 | 1889.6 | 66 ± 6 | 0.05 ± 0.01 | 5.7 ± 0.6 |
| Sample | Initial PLLA | Pressed PLLA | Pressed PLLA/RM-β-CD |
|---|---|---|---|
| Before degradation | |||
| Rq [nm] | 1.3 ± 0.2 | 3.3 ± 0.3 | 3.9 ± 0.4 |
| Ra [nm] | 1.0 ± 0.2 | 2.6 ± 0.3 | 3.1 ± 0.4 |
| Image Z range * [nm] | 7.7 ± 1.4 | 19.7 ± 3.0 | 28.5 ± 9.4 |
| 1 day | |||
| Rq [nm] | 1.0 ± 0.1 | 1.5 ± 0.3 | 2.4 ± 0.7 |
| Ra [nm] | 0.8 ± 0.1 | 1.2 ± 0.3 | 1.9 ± 0.5 |
| Image Z range * [nm] | 7.2 ± 0.5 | 10.8 ± 2.8 | 17.3 ± 6.4 |
| 70 days | |||
| Rq [nm] | 2.4 ± 0.8 | 2.8 ± 0.1 | 3.4 ± 0.3 |
| Ra [nm] | 1.9 ± 0.7 | 2.1 ± 0.1 | 2.6 ± 0.1 |
| Image Z range * [nm] | 18.1 ± 1.9 | 22.7 ± 4.7 | 28.1 ± 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydz, J.; Duale, K.; Janeczek, H.; Sikorska, W.; Marcinkowski, A.; Musioł, M.; Godzierz, M.; Kordyka, A.; Sobota, M.; Peptu, C.; et al. Nematic-to-Isotropic Phase Transition in Poly(L-Lactide) with Addition of Cyclodextrin during Abiotic Degradation Study. Int. J. Mol. Sci. 2022, 23, 7693. https://doi.org/10.3390/ijms23147693
Rydz J, Duale K, Janeczek H, Sikorska W, Marcinkowski A, Musioł M, Godzierz M, Kordyka A, Sobota M, Peptu C, et al. Nematic-to-Isotropic Phase Transition in Poly(L-Lactide) with Addition of Cyclodextrin during Abiotic Degradation Study. International Journal of Molecular Sciences. 2022; 23(14):7693. https://doi.org/10.3390/ijms23147693
Chicago/Turabian StyleRydz, Joanna, Khadar Duale, Henryk Janeczek, Wanda Sikorska, Andrzej Marcinkowski, Marta Musioł, Marcin Godzierz, Aleksandra Kordyka, Michał Sobota, Cristian Peptu, and et al. 2022. "Nematic-to-Isotropic Phase Transition in Poly(L-Lactide) with Addition of Cyclodextrin during Abiotic Degradation Study" International Journal of Molecular Sciences 23, no. 14: 7693. https://doi.org/10.3390/ijms23147693
APA StyleRydz, J., Duale, K., Janeczek, H., Sikorska, W., Marcinkowski, A., Musioł, M., Godzierz, M., Kordyka, A., Sobota, M., Peptu, C., Koseva, N., & Kowalczuk, M. (2022). Nematic-to-Isotropic Phase Transition in Poly(L-Lactide) with Addition of Cyclodextrin during Abiotic Degradation Study. International Journal of Molecular Sciences, 23(14), 7693. https://doi.org/10.3390/ijms23147693










