Gypenoside A from Gynostemma pentaphyllum Attenuates Airway Inflammation and Th2 Cell Activities in a Murine Asthma Model
Abstract
:1. Introduction
2. Results
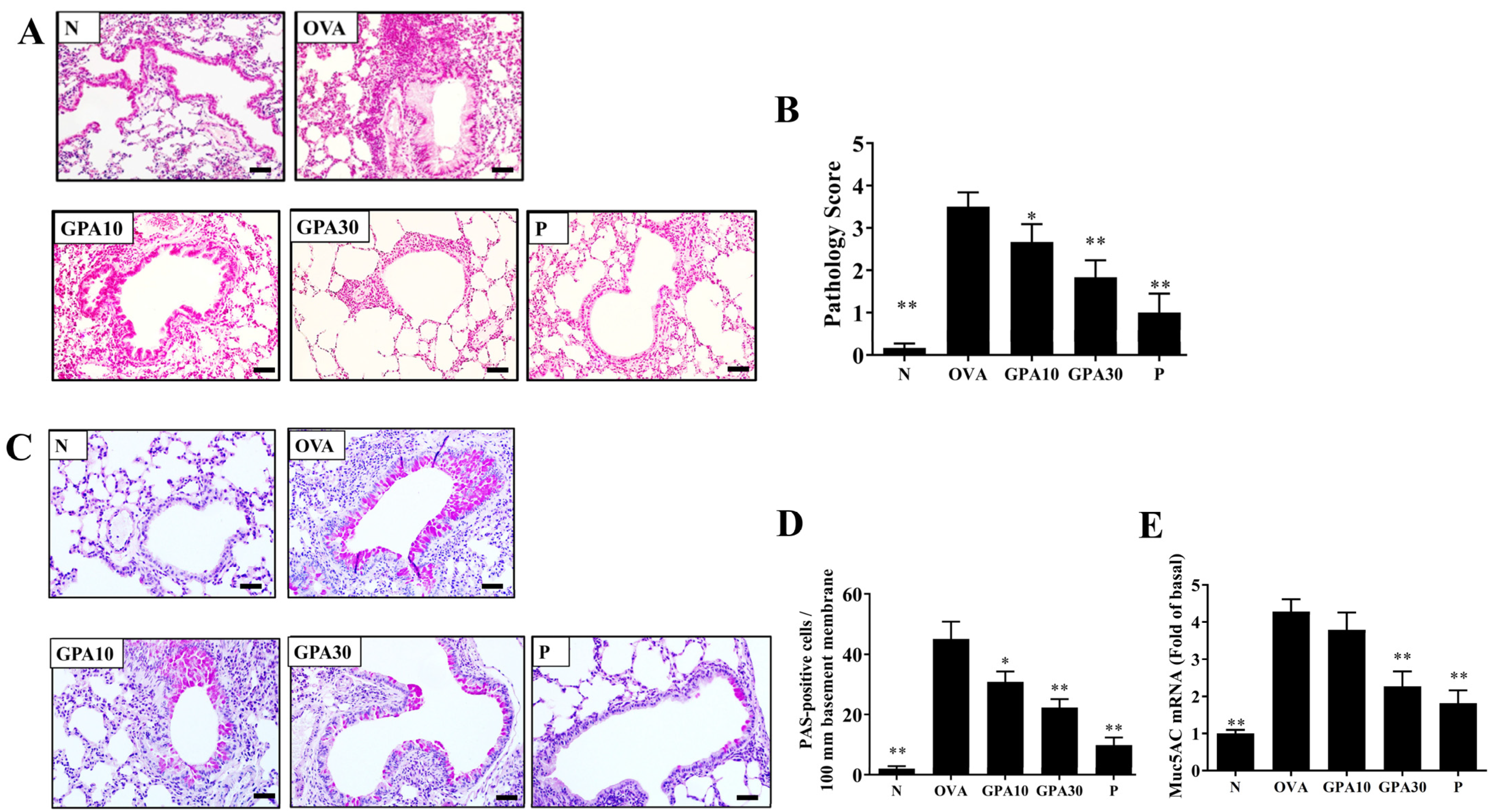
2.1. Gypenoside A Attenuated Eosinophil Infiltration and Goblet Cell Hyperplasia
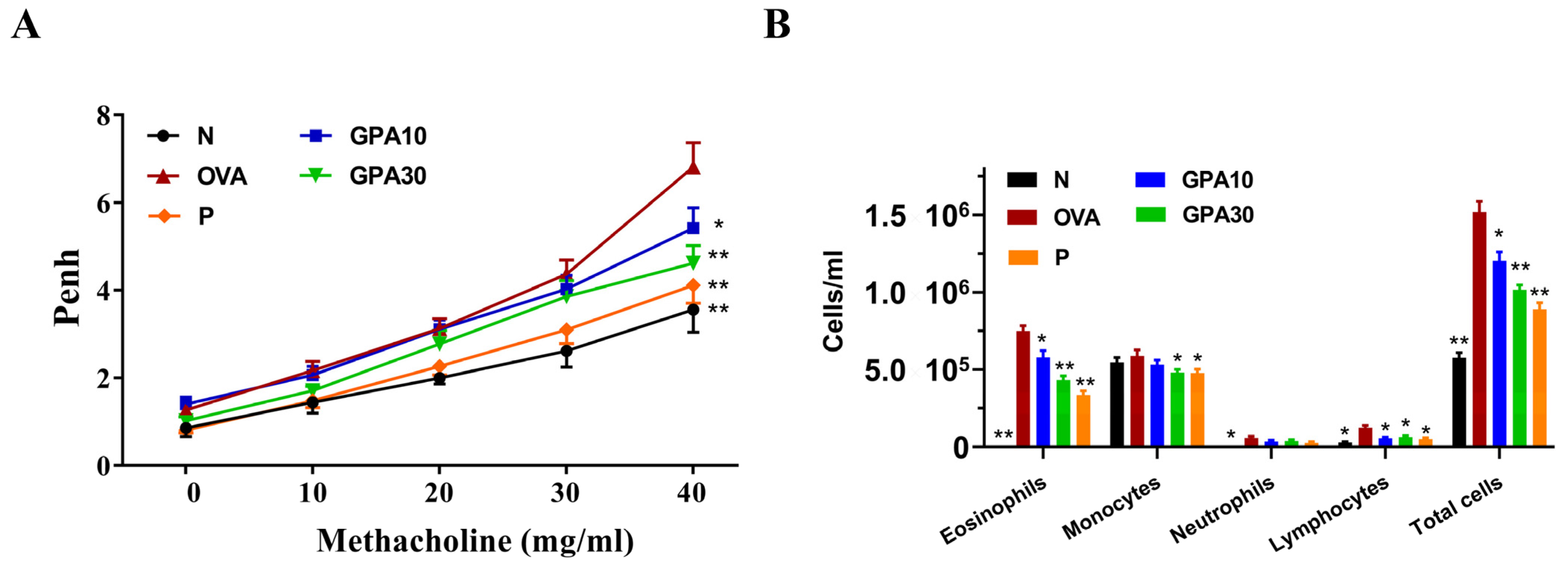
2.2. Gypenoside A Mitigated AHR and Eosinophil Infiltration in Bronchoalveolar Lavage Fluid
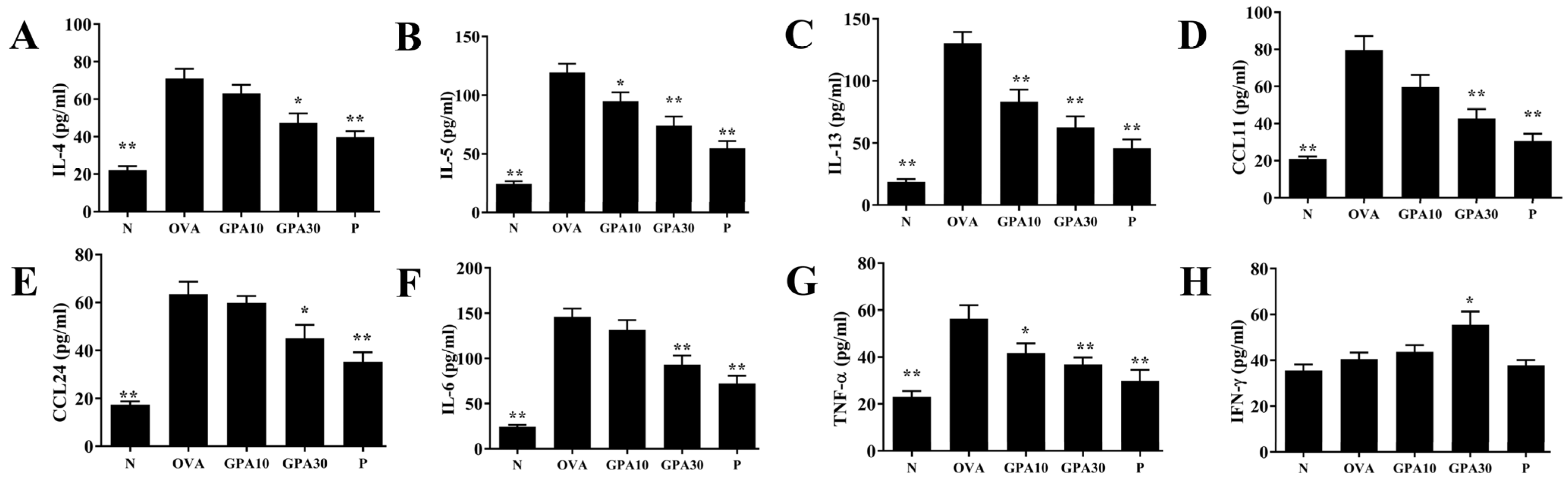
2.3. Gypenoside A Regulates Chemokine and Cytokine Secretion in BALF
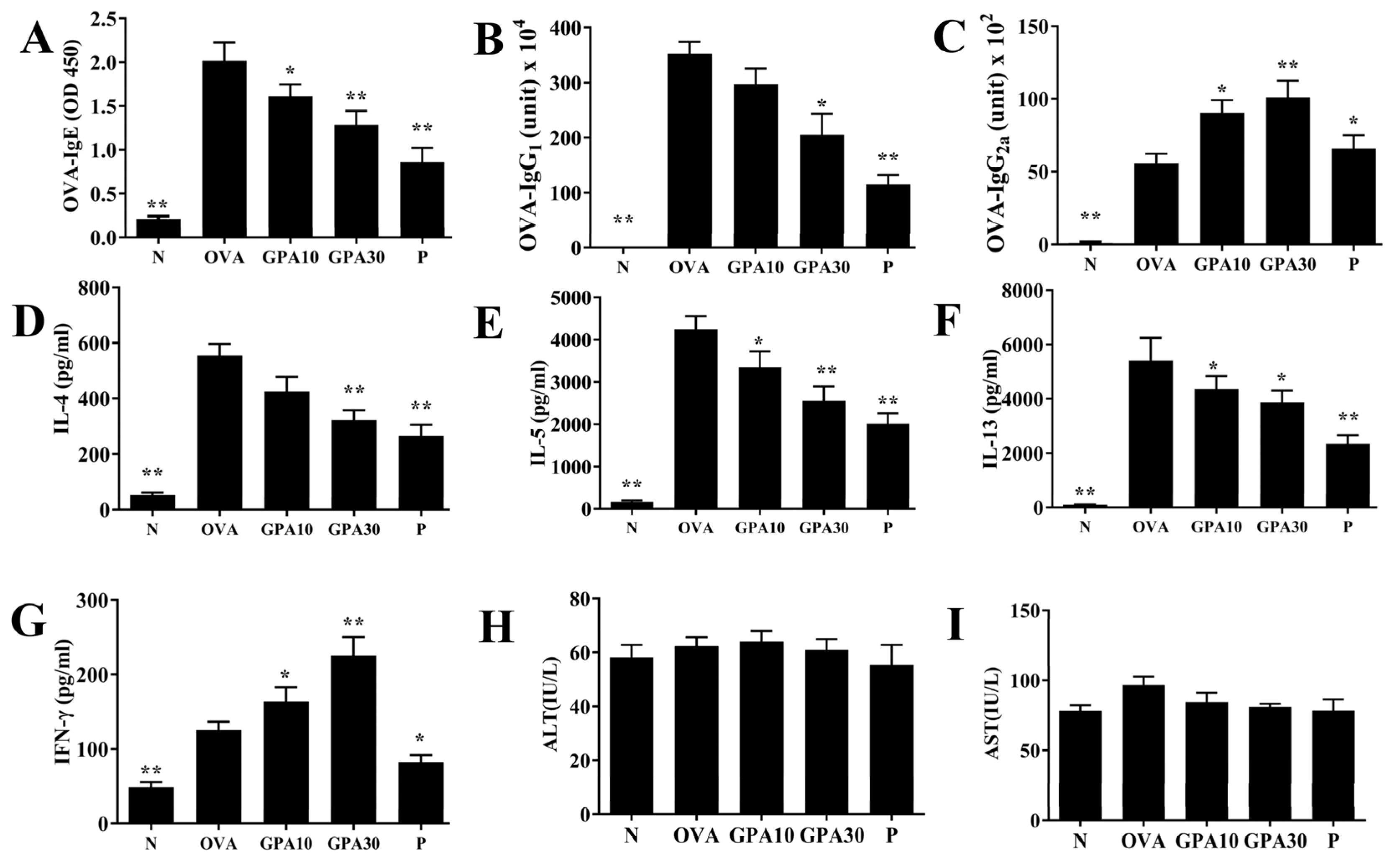
2.4. Gypenoside A Regulates Serum Antibodies and Splenocyte Cytokine Levels
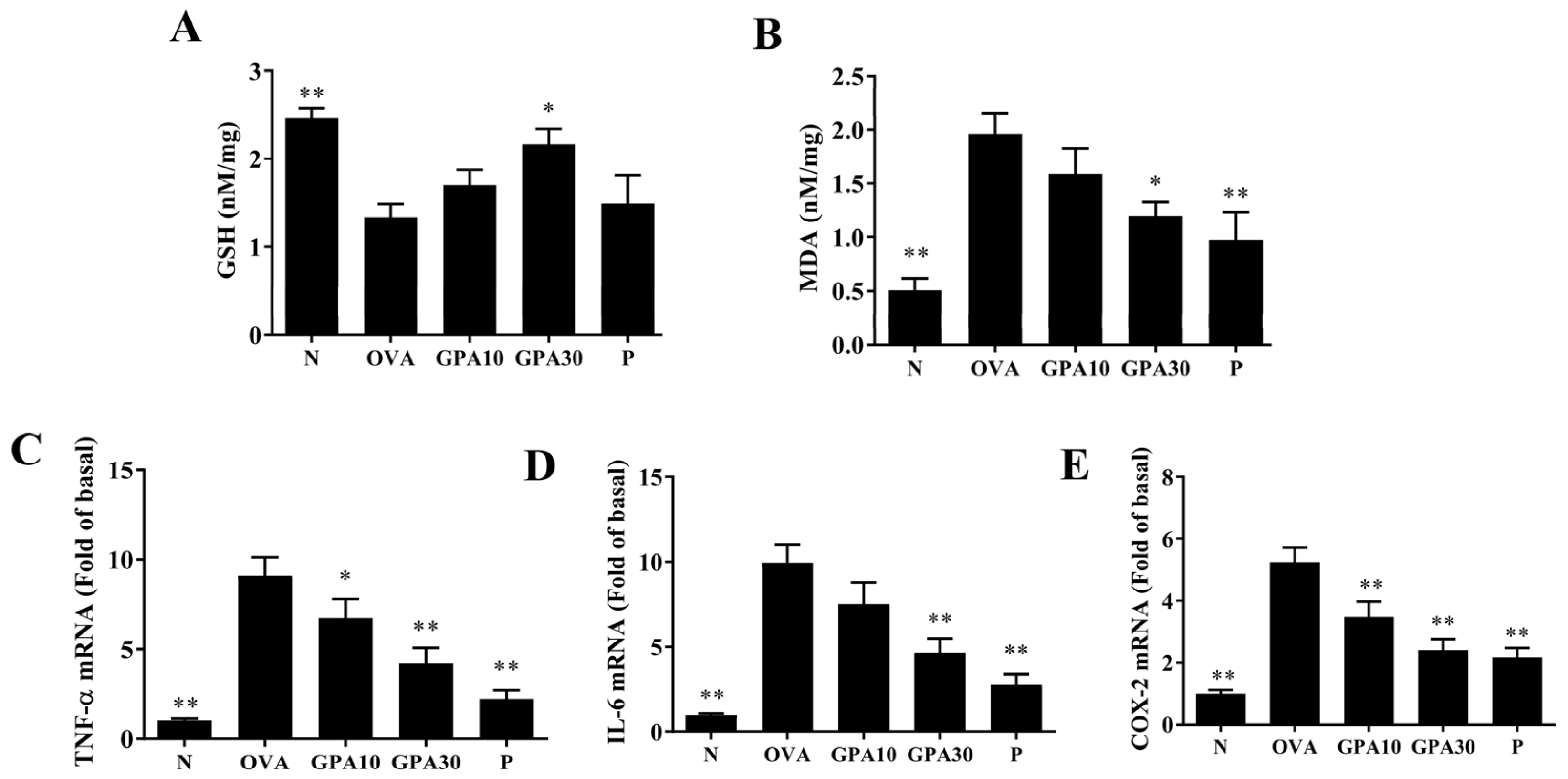
2.5. Gypenoside A Modulates Antioxidant Enzyme and Inflammatory Gene Expression in Lung
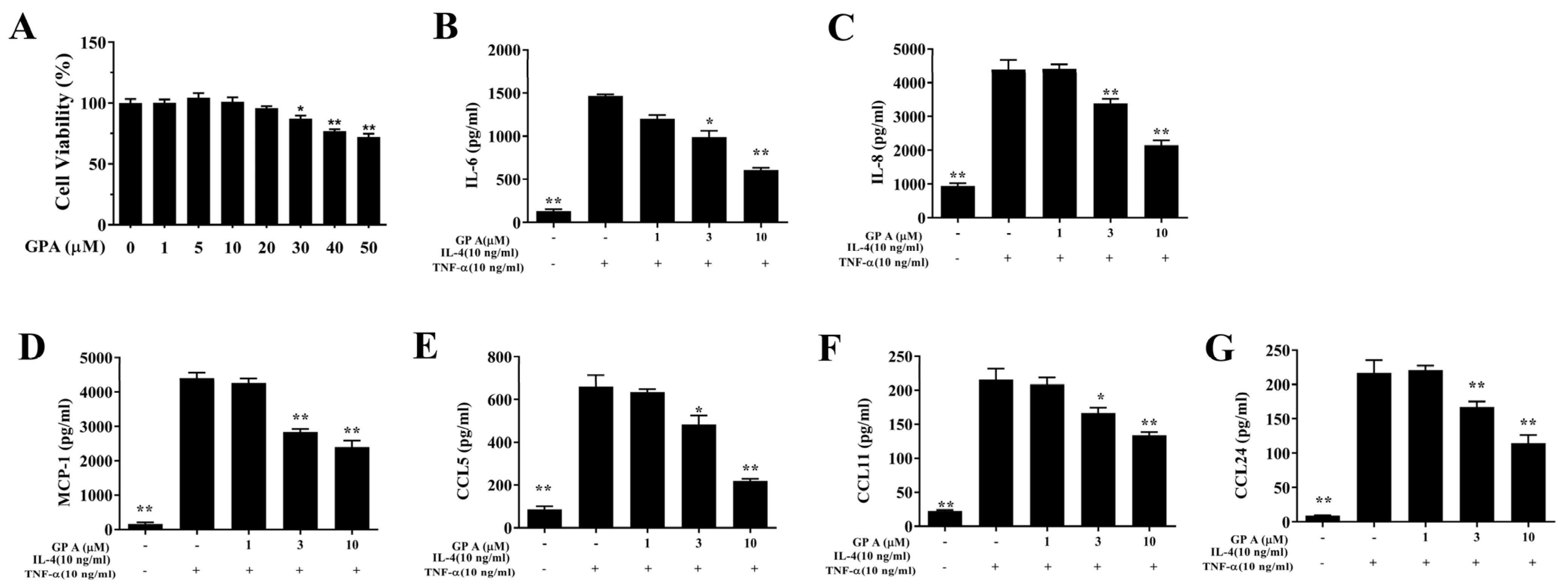
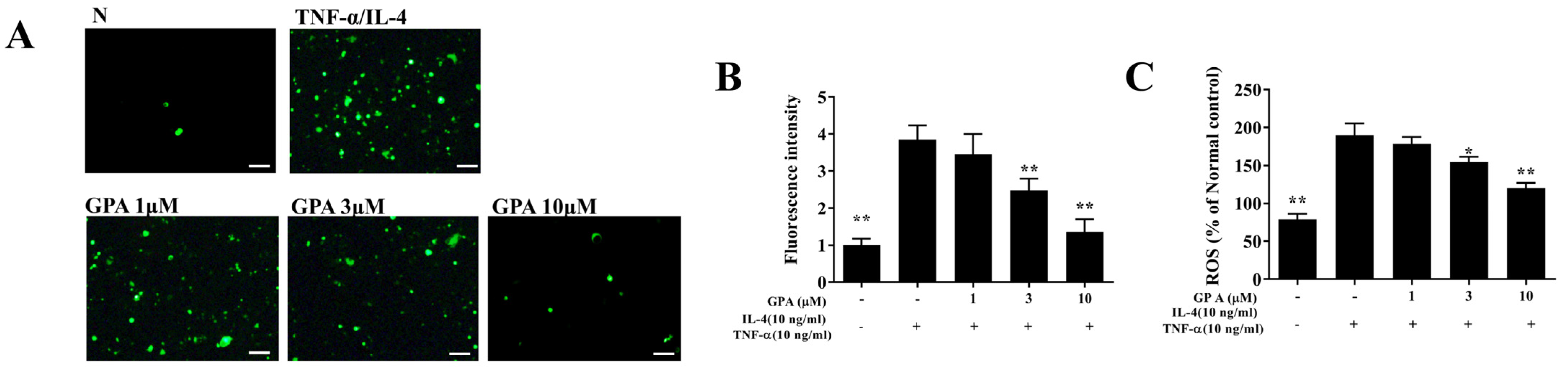
2.6. Gypenoside A Mitigates Inflammation and the ROS Response in BEAS-2B Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Mouse Experimental Procedure
4.4. Measurement of Airway Hyperresponsiveness
4.5. Bronchoalveolar Lavage Fluid
4.6. Histopathological Analysis of Lung
4.7. Serum Analysis and Splenocyte Culture
4.8. BEAS-2B Cell Culture and Gypenoside A Treatment
4.9. ELISA Assay
4.10. Reactive Oxygen Species Detection
4.11. Quantitative Real-Time PCR Analysis
4.12. MDA Activity and Glutathione Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brusselle, G.G.; Koppelman, G.H. Biologic therapies for severe asthma. N. Engl. J. Med. 2022, 386, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Koefoed, H.J.L.; Zwitserloot, A.M.; Vonk, J.M.; Koppelman, G.H. Asthma, bronchial hyperresponsiveness, allergy and lung function development until early adulthood: A systematic literature review. Pediatr. Allergy Immunol. 2021, 32, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pansare, M. New treatments for asthma. Immunol. Allergy Clin. N. Am. 2021, 41, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.F. Treatment approaches for the patient with T2 low asthma. Ann. Allergy Asthma Immunol. 2021, 127, 530–535. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The cytokines of asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Ha, T.K.Q.; Yang, J.L.; Pham, H.T.T.; Oh, W.K. Triterpenoids from the genus gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Wang, M.; Zhao, C. Profiling analysis of amino acids from hyperlipidaemic rats treated with Gynostemma pentaphyllum and atorvastatin. Pharm. Biol. 2016, 54, 2254–2263. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the medicinal value, bioactive compounds, and pharmacological activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef]
- Li, Y.; Lin, W.; Huang, J.; Xie, Y.; Ma, W. Anti-cancer effects of Gynostemma pentaphyllum (thunb.) makino (jiaogulan). Chin. Med. 2016, 11, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wang, F.; Wang, Y.; Ma, X.; Zhao, M.; Zhao, C. Metabonomics study of the therapeutic mechanism of Gynostemma pentaphyllum and atorvastatin for hyperlipidemia in rats. PLoS ONE 2013, 8, e78731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, C.J.; Huang, W.C.; Kuo, M.L.; Yang, R.C.; Shen, J.J. Long-term oral administration of Gynostemma pentaphyllum extract attenuates airway inflammation and Th2 cell activities in ovalbumin-sensitized mice. Food Chem. Toxicol. 2010, 48, 2592–2598. [Google Scholar] [CrossRef]
- Huang, W.C.; Kuo, M.L.; Li, M.L.; Yang, R.C.; Liou, C.J.; Shen, J.J. Gynostemma pentaphyllum decreases allergic reactions in a murine asthmatic model. Am. J. Chin. Med. 2008, 36, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Shi, R.; Wang, X.; Bao, Y. Gypenoside a protects ischemia/reperfusion injuries by suppressing mir-143-3p level via the activation of AMPK/FOXO1 pathway. Biofactors 2020, 46, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Huang, W.C.; Cheng, C.Y.; Wang, M.C.; Cheng, S.C.; Liou, C.J. Fisetin suppresses the inflammatory response and oxidative stress in bronchial epithelial cells. Nutrients 2022, 14, 1841. [Google Scholar] [CrossRef] [PubMed]
- Circosta, C.; De Pasquale, R.; Palumbo, D.R.; Occhiuto, F. Bronchodilatory effects of the aqueous extract of Gynostemma pentaphyllum and gypenosides III and VIII in anaesthetized guinea-pigs. J. Pharm. Pharmacol. 2005, 57, 1053–1058. [Google Scholar] [CrossRef]
- Ji, X.; Shen, Y.; Guo, X. Isolation, structures, and bioactivities of the polysaccharides from Gynostemma pentaphyllum (thunb.) makino: A review. BioMed Res. Int. 2018, 2018, 6285134. [Google Scholar] [CrossRef] [Green Version]
- Ntontsi, P.; Papathanassiou, E.; Loukides, S.; Bakakos, P.; Hillas, G. Targeted anti-IL-13 therapies in asthma: Current data and future perspectives. Expert Opin. Investig. Drugs 2018, 27, 179–186. [Google Scholar] [CrossRef]
- Bonser, L.R.; Erle, D.J. The airway epithelium in asthma. Adv. Immunol. 2019, 142, 1–34. [Google Scholar]
- Alagha, K.; Bourdin, A.; Vernisse, C.; Garulli, C.; Tummino, C.; Charriot, J.; Vachier, I.; Suehs, C.; Chanez, P.; Gras, D. Goblet cell hyperplasia as a feature of neutrophilic asthma. Clin. Exp. Allergy 2019, 49, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Pavord, I.D. Anti-IL-4/IL-13 for the treatment of asthma: The story so far. Expert Opin. Biol. Ther. 2020, 20, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Yancey, S.W.; Keene, O.N.; Albers, F.C.; Ortega, H.; Bates, S.; Bleecker, E.R.; Pavord, I. Biomarkers for severe eosinophilic asthma. J. Allergy Clin. Immunol. 2017, 140, 1509–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, Ö. Oxidative stress in asthma: Part of the puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.G.; Lebold, K.M.; Roth-Carter, Q.R.; Pincus, A.B.; Blum, E.D.; Proskocil, B.J.; Jacoby, D.B.; Fryer, A.D.; Nie, Z. Eosinophil and airway nerve interactions in asthma. J. Leukoc. Biol. 2018, 104, 61–67. [Google Scholar] [CrossRef]
- Jesenak, M.; Zelieskova, M.; Babusikova, E. Oxidative stress and bronchial asthma in children-causes or consequences? Front. Pediatr. 2017, 5, 162. [Google Scholar] [CrossRef]
- Liou, C.J.; Chen, Y.L.; Yu, M.C.; Yeh, K.W.; Shen, S.C.; Huang, W.C. Sesamol alleviates airway hyperresponsiveness and oxidative stress in asthmatic mice. Antioxidants 2020, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Liou, C.J.; Cheng, C.Y.; Yeh, K.W.; Wu, Y.H.; Huang, W.C. Protective effects of casticin from Vitex trifolia alleviate eosinophilic airway inflammation and oxidative stress in a murine asthma model. Front. Pharmacol. 2018, 9, 635. [Google Scholar] [CrossRef]
- Huang, W.C.; Huang, T.H.; Yeh, K.W.; Chen, Y.L.; Shen, S.C.; Liou, C.J. Ginsenoside Rg3 ameliorates allergic airway inflammation and oxidative stress in mice. J. Ginseng Res. 2021, 45, 654–664. [Google Scholar] [CrossRef]
- Huang, W.C.; Liu, C.Y.; Shen, S.C.; Chen, L.C.; Yeh, K.W.; Liu, S.H.; Liou, C.J. Protective effects of licochalcone A improve airway hyper-responsiveness and oxidative stress in a mouse model of asthma. Cells 2019, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.J.; Liou, C.J.; Chen, Y.L.; Cheng, S.C.; Huang, W.C. Fucoxanthin ameliorates oxidative stress and airway inflammation in tracheal epithelial cells and asthmatic mice. Cells 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chan, C.C.; Wu, S.J.; Chen, L.C.; Shen, J.J.; Kuo, M.L.; Chen, M.C.; Liou, C.J. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J. Ethnopharmacol. 2014, 151, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lai, C.L.; Liang, Y.T.; Hung, H.C.; Liu, H.C.; Liou, C.J. Phloretin attenuates LPS-induced acute lung injury in mice via modulation of the NF-κB and MAPK pathways. Int. Immunopharmacol. 2016, 40, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Lai, Y.R.; Chen, Y.L.; Chang, Y.H.; Li, Z.Y.; Huang, W.C. Matrine attenuates COX-2 and ICAM-1 expressions in human lung epithelial cells and prevents acute lung injury in LPS-induced mice. Mediat. Inflamm. 2016, 2016, 3630485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Primer | 5′–3′ Sequence |
|---|---|---|
| COX-2 | F R | ACCAGCAGTTCCAGTATCAGA CAGGAGGATGGAGTTGTTGTAG |
| IL-6 | F | AGGACCAAGACCATCCAATTCA |
| R | GCTTAGGCATAACGCACTAGG | |
| Muc5AC | F R | AATGCTGGTGCCTGTGTCTCAGAGGGA CCTCCTATGCCATCTGTTGTG |
| TNF-α | F | GCACCACCATCAAGGACTC |
| R | AGGCAACCTGACCACTCTC | |
| β-actin | F | AAGACCTCTATGCCAACACAGT |
| R | AGCCAGAGCAGTAATCTCCTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Wu, S.-J.; Yeh, K.-W.; Liou, C.-J. Gypenoside A from Gynostemma pentaphyllum Attenuates Airway Inflammation and Th2 Cell Activities in a Murine Asthma Model. Int. J. Mol. Sci. 2022, 23, 7699. https://doi.org/10.3390/ijms23147699
Huang W-C, Wu S-J, Yeh K-W, Liou C-J. Gypenoside A from Gynostemma pentaphyllum Attenuates Airway Inflammation and Th2 Cell Activities in a Murine Asthma Model. International Journal of Molecular Sciences. 2022; 23(14):7699. https://doi.org/10.3390/ijms23147699
Chicago/Turabian StyleHuang, Wen-Chung, Shu-Ju Wu, Kuo-Wei Yeh, and Chian-Jiun Liou. 2022. "Gypenoside A from Gynostemma pentaphyllum Attenuates Airway Inflammation and Th2 Cell Activities in a Murine Asthma Model" International Journal of Molecular Sciences 23, no. 14: 7699. https://doi.org/10.3390/ijms23147699
APA StyleHuang, W.-C., Wu, S.-J., Yeh, K.-W., & Liou, C.-J. (2022). Gypenoside A from Gynostemma pentaphyllum Attenuates Airway Inflammation and Th2 Cell Activities in a Murine Asthma Model. International Journal of Molecular Sciences, 23(14), 7699. https://doi.org/10.3390/ijms23147699






