Antifungal Potential of Bacillus velezensis CE 100 for the Control of Different Colletotrichum Species through Isolation of Active Dipeptide, Cyclo-(D-phenylalanyl-D-prolyl)
Abstract
:1. Introduction
2. Results
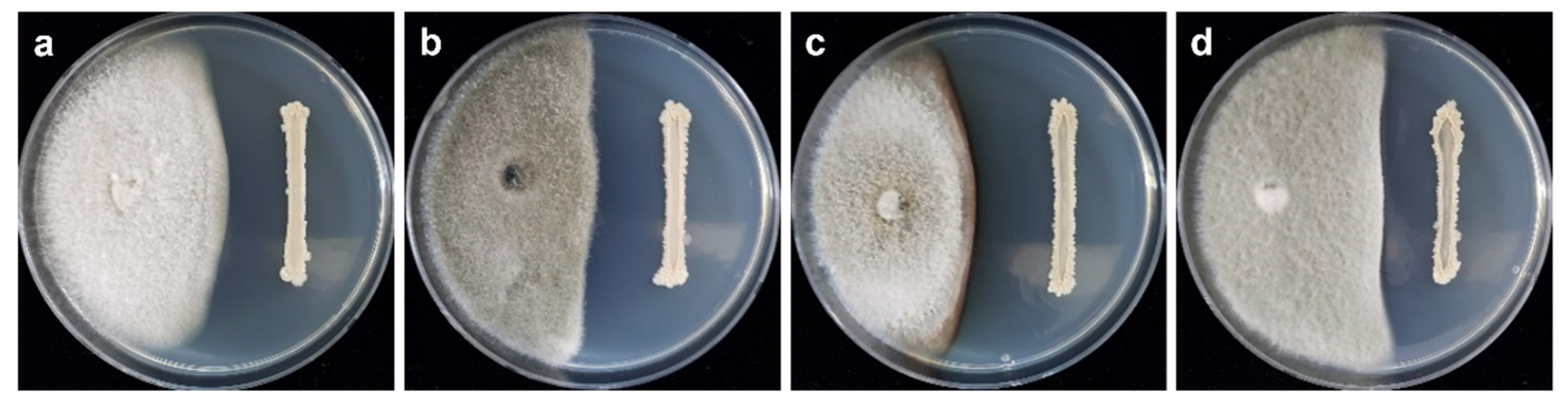
2.1. Antagonistic Activity of B. velezensis CE 100 against Phytopathogenic Fungi
2.2. Inhibitory Effect of Volatile Organic Compounds (VOCs) Released by B. velezensis CE 100 on the Growth of Phytopathogenic Colletotrichum Species
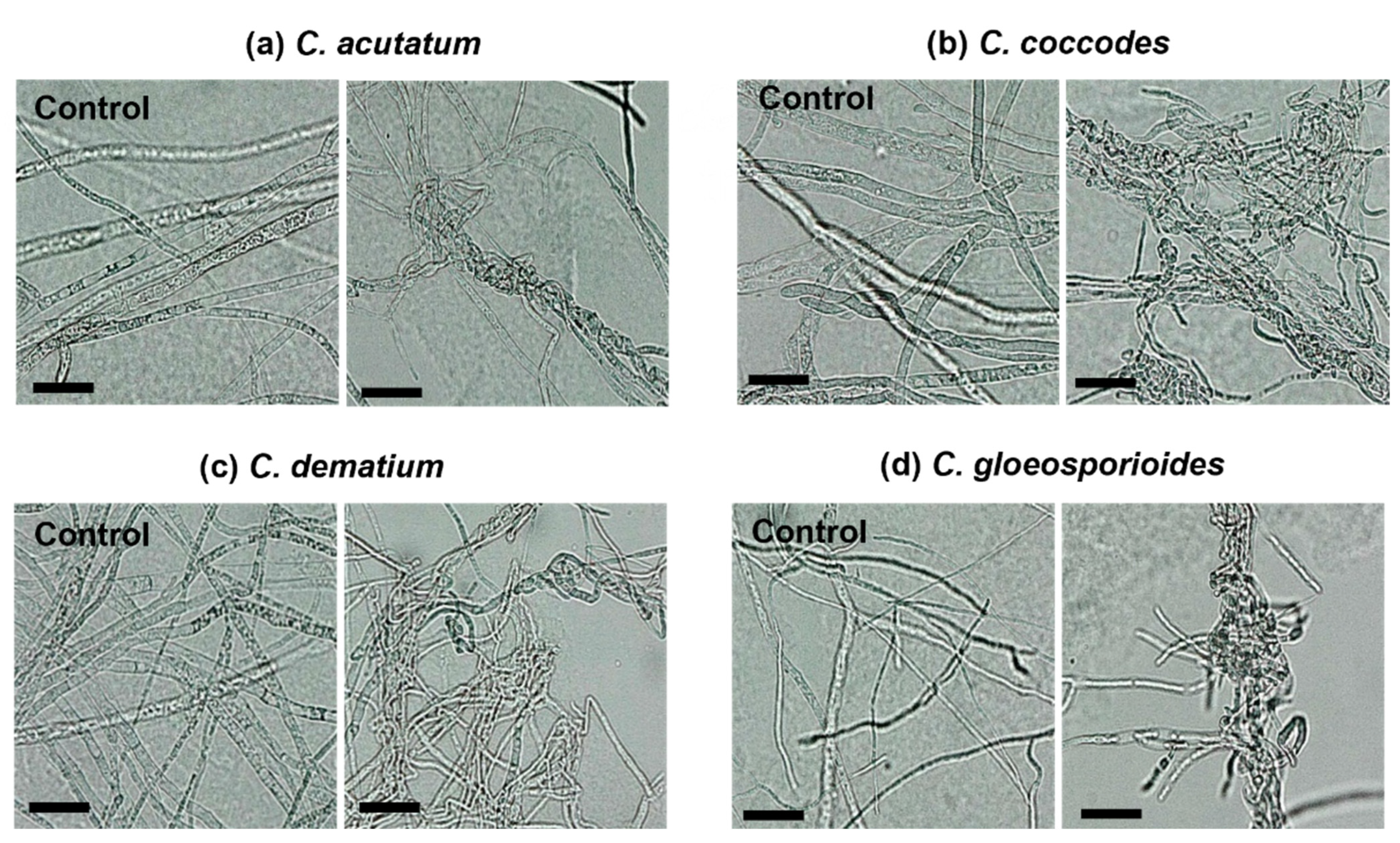
2.3. Effect of Chloroform Crude Extract on Mycelial Growth and Hyphal Morphology of the Colletotrichum Species
2.4. Identification of the Antifungal Cyclic Dipeptide
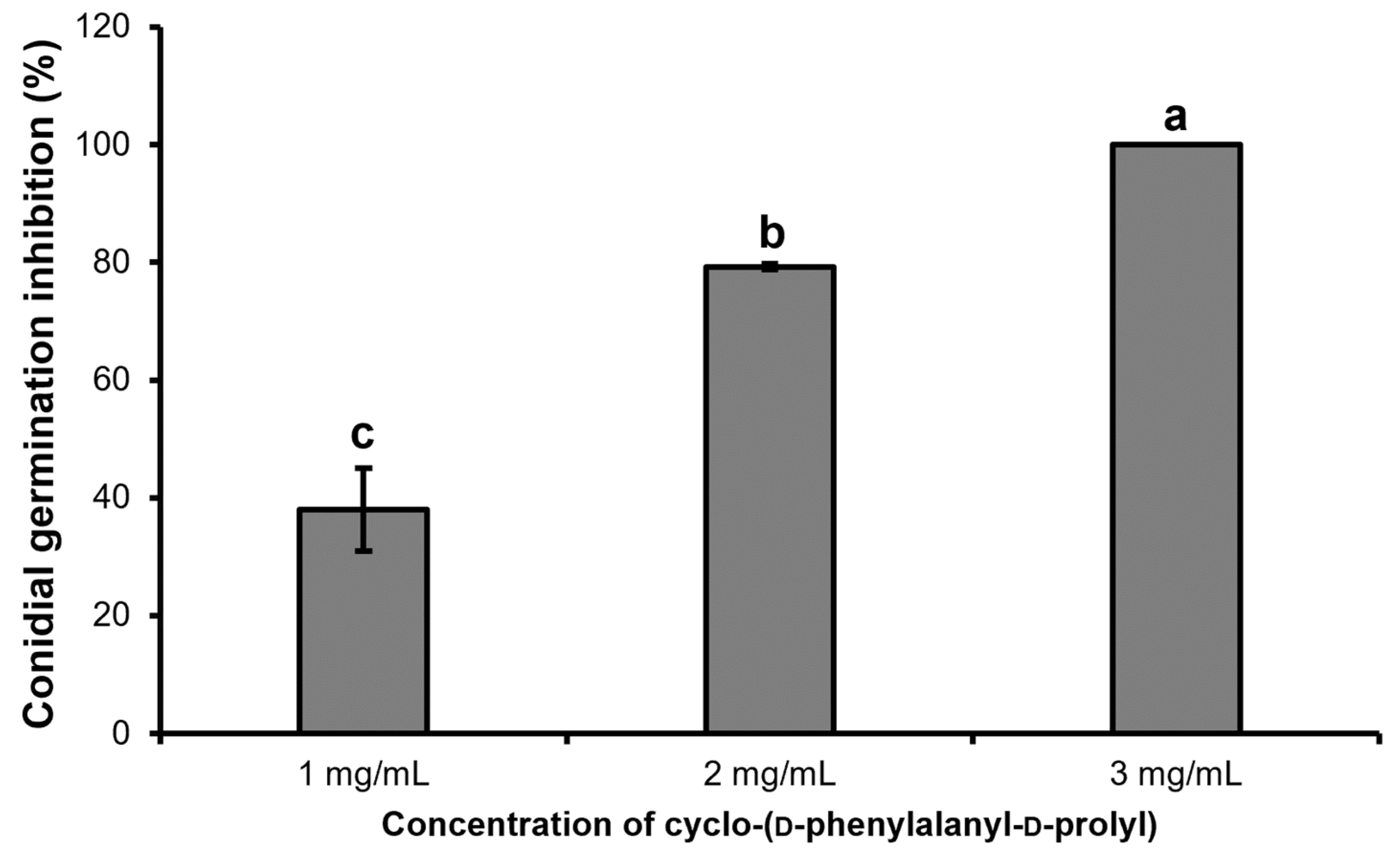
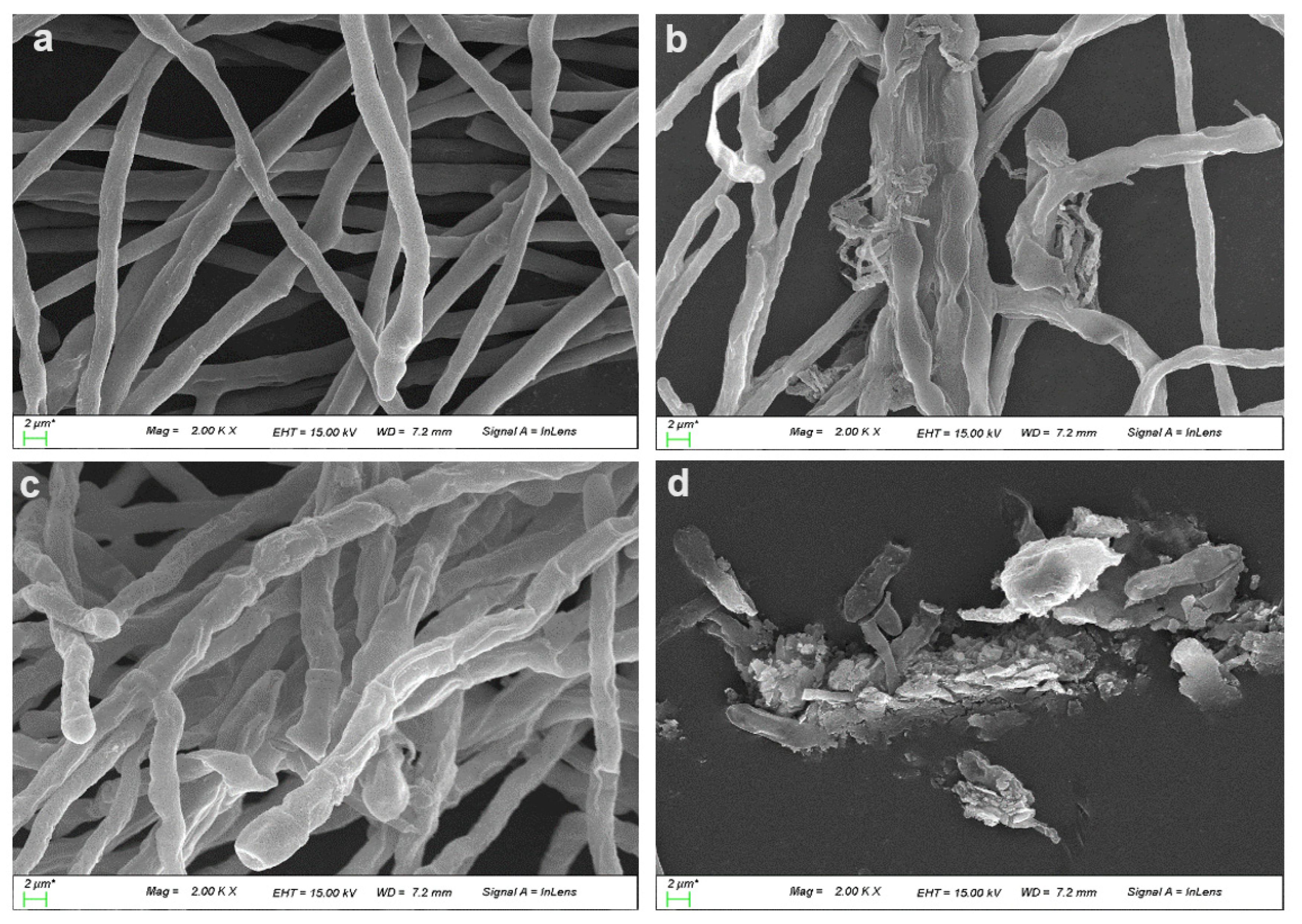
2.5. Inhibitory Effect of Purified Dipeptide on Conidial Germination of C. gloeosporioides and Hyphal Deformation by Scanning Electron Microscopy (SEM)
3. Discussion
4. Materials and Methods
4.1. Culture Conditions for Bacterial and Fungal Strains
4.2. Antagonistic Activity of B. velezensis CE 100 against Fungal Phytopathogens
4.2.1. Dual-Culture Assay
4.2.2. Volatile Organic Compound (VOC) Inhibition Assay
4.2.3. Antifungal Activities of Chloroform Crude Extract Compounds against Phytopathogens
4.3. Isolation and Identification of an Antifungal Compound from Chloroform Crude Extract
4.4. Inhibition of C. gloeosporioides Conidial Germination by the Purified Compound
4.5. Preparation of Samples for Analysis of Scanning Electron Microscopy (SEM)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dowling, M.; Peres, N.; Villani, S.; Schnabel, G. Managing Colletotrichum on fruit crops: A “complex” challenge. Plant Dis. 2020, 104, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Peres, N.A.; Timmer, L.W.; Adaskaveg, J.E.; Correll, J.C. Lifestyles of Colletotrichum acutatum. Plant Dis. 2005, 89, 784–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riolo, M.; Aloi, F.; Pane, A.; Cara, M.; Cacciola, S.O. Twig and shoot dieback of citrus, a new disease caused by Colletotrichum species. Cells 2021, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacciola, S.O.; Gilardi, G.; Faedda, R.; Schena, L.; Pane, A.; Garibaldi, A.; Gullino, M.L. Characterization of Colletotrichum ocimi population associated with black spot of sweet basil (Ocimum basilicum) in Northern Italy. Plants 2020, 9, 654. [Google Scholar] [CrossRef]
- Sewedy, M.E.; Atia, M.M.; Zayed, M.A.; Ghonim, M.I. Molecular detection and controlling of seed-borne Colletotrichum spp. in common bean and soybean. Zagazig J. Agric. Res. 2019, 46, 1919–1935. [Google Scholar] [CrossRef]
- Nicholson, R.L. Survival of Colletotrichum graminicola: Importance of the spore matrix. Phytopathology 1980, 70, 255. [Google Scholar] [CrossRef]
- Deising, H.B.; Werner, S.; Wernitz, M. The role of fungal appressoria in plant infection. Microbes Infect. 2000, 2, 1631–1641. [Google Scholar] [CrossRef]
- Yu, S.-M.; Ramkumar, G.; Lee, Y.H. Light quality influences the virulence and physiological responses of Colletotrichum acutatum causing anthracnose in pepper plants. J. Appl. Microbiol. 2013, 115, 509–516. [Google Scholar] [CrossRef]
- Zakaria, L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Prusky, D. Pathogen quiescence in postharvest diseases. Annu. Rev. Phytopathol. 1996, 34, 413–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, M.W.; Emmanuel, C.J.; Emilda, D.; Terhem, R.B.; Shafia, A.; Tsamaidi, D.; Emblow, M.; van Kan, J.A.L. Analysis of cryptic, systemic Botrytis infections in symptomless hosts. Front. Plant Sci. 2016, 7, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talhinhas, P.; Mota-Capitão, C.; Martins, S.; Ramos, A.P.; Neves-Martins, J.; Guerra-Guimarães, L.; Várzea, V.; Silva, M.C.; Sreenivasaprasad, S.; Oliveira, H. Epidemiology, histopathology and aetiology of olive anthracnose caused by Colletotrichum acutatum and C. gloeosporioides in Portugal: Olive anthracnose in Portugal. Plant Pathol. 2011, 60, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Harp, T.L.; Pernezny, K.; Ivey, M.L.L.; Miller, S.A.; Kuhn, P.J.; Datnoff, L. The etiology of recent pepper anthracnose outbreaks in Florida. Crop Prot. 2008, 27, 1380–1384. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Yang, S.H.; Cheng, J.H.; Meng, L.; Suh, J.-W. Biological control of anthracnose (Colletotrichum gloeosporioides) in yam by Streptomyces sp.MJM5763: Biocontrol of yam anthracnose. J. Appl. Microbiol. 2011, 111, 443–455. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The Evolution of fungicide resistance. Adv. Appl. Microbiol 2015, 90, 29–92. [Google Scholar] [CrossRef]
- McGrath, M.T. Fungicides and other chemical approaches for use in plant disease control. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 412–421. [Google Scholar] [CrossRef]
- Onyeka, T.J.; Pétro, D.; Ano, G.; Etienne, S.; Rubens, S. Resistance in water yam (Dioscorea alata) cultivars in the French West Indies to anthracnose disease based on tissue culture-derived whole-plant assay. Plant Pathol. 2006, 55, 671–678. [Google Scholar] [CrossRef]
- Rändler-Kleine, M.; Wolfgang, A.; Dietel, K.; Junge, H.; Cernava, T.; Berg, G. How microbiome approaches can assist industrial development of biological control products. In Integrative Biological Control; Gao, Y., Hokkanen, H.M.T., Menzler-Hokkanen, I., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Volume 20, pp. 201–215. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Carter, G.R. Bacillus. In Diagnostic Procedure in Veterinary Bacteriology and Mycology; Elsevier: Amsterdam, The Netherlands, 1990; pp. 221–228. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Logan, N.A.; Vos, P.D. Bacillus. In Bergey’s Manual of Systematics of Archaea and Bacteria, 1st ed.; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–163. [Google Scholar] [CrossRef]
- Nicholson, W.L. Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. (CMLS) 2002, 59, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Sumi, C.D.; Yang, B.W.; Yeo, I.-C.; Hahm, Y.T. Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can. J. Microbiol. 2015, 61, 93–103. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Z.; Han, Y.; Wang, Z.; Fan, J.; Xiao, H. Isolation and identification of antifungal peptides from Bacillus BH072, a novel bacterium isolated from honey. Microbiol. Res. 2013, 168, 598–606. [Google Scholar] [CrossRef]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. Biomed. Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Choub, V.; Ajuna, H.B.; Won, S.-J.; Moon, J.-H.; Choi, S.-I.; Maung, C.E.H.; Kim, C.-W.; Ahn, Y.S. Antifungal activity of Bacillus velezensis CE 100 against anthracnose disease (Colletotrichum gloeosporioides) and growth promotion of walnut (Juglans regia L.) trees. Int. J. Mol. Sci. 2021, 22, 10438. [Google Scholar] [CrossRef]
- Maung, C.E.H.; Lee, H.G.; Cho, J.-Y.; Kim, K.Y. Antifungal compound, methyl hippurate from Bacillus velezensis CE 100 and its inhibitory effect on growth of Botrytis cinerea. World J. Microbiol. Biotechnol. 2021, 37, 159. [Google Scholar] [CrossRef]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. DD-Diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, X.; Ng, T.B.; Zhang, W. Study on the biocontrol potential of antifungal peptides produced by Bacillus velezensis against Fusarium solani that infects the passion fruit Passiflora edulis. J. Agric. Food Chem. 2021, 69, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Yoon, M.-Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Shin, T.S.; Park, H.W.; Yu, N.H.; Kim, Y.H.; Kim, J.-C. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol. J. 2017, 33, 488–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.F.; Meng, J.F.; Tian, T.; Xiao, X.Q.; Zhang, B.; Xiao, Y.N. Endophytic Bacillus velezensis strain B-36 is a potential biocontrol agent against lotus rot caused by Fusarium oxysporum. J. Appl. Microbiol. 2020, 128, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Liu, X.; Si, Z.; Li, X.; Bi, J.; Dong, W.; Chen, M.; Wang, S.; Zhang, J.; Song, A.; et al. Volatile organic compounds from rice rhizosphere bacteria inhibit growth of the pathogen Rhizoctonia solani. Agriculture 2021, 11, 368. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Martínez-Checa, F.; Montaño, A.; Cortés-Delgado, A.; Smolinska, A.; Llamas, I.; Sampedro, I. Identification of volatile organic compounds in extremophilic bacteria and their effective use in biocontrol of postharvest fungal phytopathogens. Front. Microbiol. 2021, 12, 773092. [Google Scholar] [CrossRef]
- Gao, H.; Li, P.; Xu, X.; Zeng, Q.; Guan, W. Research on volatile organic compounds from Bacillus subtilis CF-3: Biocontrol effects on fruit fungal pathogens and dynamic changes during fermentation. Front. Microbiol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Rajaofera, M.J.N.; Wang, Y.; Dahar, G.Y.; Jin, P.; Fan, L.; Xu, L.; Liu, W.; Miao, W. Volatile organic compounds of Bacillus atrophaeus HAB-5 inhibit the growth of Colletotrichum gloeosporioides. Pestic. Biochem. Phys. 2019, 156, 170–176. [Google Scholar] [CrossRef]
- Prapagdee, B.; Tharasaithong, L.; Nanthaphot, R.; Paisitwiroj, C. Efficacy of crude extract of antifungal compounds produced from Bacillus subtilis on prevention of anthracnose disease in Dendrobium orchid. EnvironmentAsia 2012, 5, 32–38. [Google Scholar] [CrossRef]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Bacillus-based biological control of plant diseases. In Pesticides in the Modern World—Pesticides Use and Management; Stoytcheva, M., Ed.; InTech: Vienna, Austria, 2011. [Google Scholar] [CrossRef] [Green Version]
- Mondol, M.A.M.; Shin, H.J.; Islam, M.T. Diversity of secondary metabolites from marine Bacillus species: Chemistry and biological activity. Mar. Drugs 2013, 11, 2846–2872. [Google Scholar] [CrossRef] [Green Version]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Luna-Bulbarela, A.; Tinoco-Valencia, R.; Corzo, G.; Kazuma, K.; Konno, K.; Galindo, E.; Serrano-Carreón, L. Effects of bacillomycin D homologues produced by Bacillus amyloliquefaciens 83 on growth and viability of Colletotrichum gloeosporioides at different physiological stages. Biol. Control 2018, 127, 145–154. [Google Scholar] [CrossRef]
- Jumpathong, W.; Intra, B.; Euanorasetr, J.; Wanapaisan, P. Biosurfactant-producing Bacillus velezensis PW192 as an anti-fungal biocontrol agent against Colletotrichum gloeosporioides and Colletotrichum musae. Microorganisms 2022, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, C.; Ye, X.; Lian, Y.; Wu, Y.; Wang, X. Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against Colletotrichum gloeosporioides in loquat fruits. Biol. Control 2020, 146, 104281. [Google Scholar] [CrossRef]
- Osherov, N.; May, G.S. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 2001, 199, 153–160. [Google Scholar] [CrossRef]
- Kumar, S.N.; Nambisan, B.; Mohandas, C. Purification and identification of two antifungal cyclic dipeptides from Bacillus cereus subsp. thuringiensis associated with a rhabditid entomopathogenic nematode especially against Fusarium oxysporum. J. Enzyme Inhib. Med. Chem. 2014, 29, 190–197. [Google Scholar] [CrossRef] [Green Version]





| Position | δH (Int., Multi., J in Hz) | δC |
|---|---|---|
| 1 | - | 171.0 |
| 2 | 4.07 (1H, ddd, 11.5, 6.0 2.0) | 60.2 |
| 3a 3b | 2.07–2.13 (1H, m) 1.17–1.26 (1H, m) | 22.9 |
| 4 | 1.78–1.84 (2H, m) | 29.5 |
| 5a 5b | 3.52–3.38 (1H, m) 3.56–3.40 (1H, m) | 46.1 |
| 1′ | - | 137.4 |
| 2′,6′ | 7.22–7.31 (5H, m) | 131.2 |
| 3′,5′ | 129.6 | |
| 4′ | 128.2 | |
| 7′ | 3.11–3.21 (2H, m) | 38.4 |
| 8′ | 4.46 (1H, t, 5.0) | 57.8 |
| 9′ | - | 167.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.Y.; Hwang, S.H.; Noh, J.S.; Cho, J.-Y.; Maung, C.E.H. Antifungal Potential of Bacillus velezensis CE 100 for the Control of Different Colletotrichum Species through Isolation of Active Dipeptide, Cyclo-(D-phenylalanyl-D-prolyl). Int. J. Mol. Sci. 2022, 23, 7786. https://doi.org/10.3390/ijms23147786
Kim TY, Hwang SH, Noh JS, Cho J-Y, Maung CEH. Antifungal Potential of Bacillus velezensis CE 100 for the Control of Different Colletotrichum Species through Isolation of Active Dipeptide, Cyclo-(D-phenylalanyl-D-prolyl). International Journal of Molecular Sciences. 2022; 23(14):7786. https://doi.org/10.3390/ijms23147786
Chicago/Turabian StyleKim, Tae Yoon, Seo Hyun Hwang, Jun Su Noh, Jeong-Yong Cho, and Chaw Ei Htwe Maung. 2022. "Antifungal Potential of Bacillus velezensis CE 100 for the Control of Different Colletotrichum Species through Isolation of Active Dipeptide, Cyclo-(D-phenylalanyl-D-prolyl)" International Journal of Molecular Sciences 23, no. 14: 7786. https://doi.org/10.3390/ijms23147786






