Abstract
The role of the purinergic signal has been extensively investigated in many tissues and related organs, including the central and peripheral nervous systems as well as the gastrointestinal, cardiovascular, respiratory, renal, and immune systems. Less attention has been paid to the influence of purines in the oral cavity, which is the first part of the digestive apparatus and also acts as the body’s first antimicrobial barrier. In this review, evidence is provided of the presence and possible physiological role of the purinergic system in the different structures forming the oral cavity including teeth, tongue, hard palate, and soft palate with their annexes such as taste buds, salivary glands, and nervous fibers innervating the oral structures. We also report findings on the involvement of the purinergic signal in pathological conditions affecting the oral apparatus such as Sjögren’s syndrome or following irradiation for the treatment of head and neck cancer, and the use of experimental drugs interfering with the purine system to improve bone healing after damage. Further investigations are required to translate the results obtained so far into the clinical setting in order to pave the way for a wider application of purine-based treatments in oral diseases.
1. Introduction
Purines are ancestral molecules present in virtually all cell types. The discovery of their crucial function in cell biology dates back to 1953, when Watson and Crick published an article with the structure of DNA [1], a molecule that had been isolated in 1869 by Friedrich Miescher [2]. From the second half of the 19th century onwards, a huge number of studies has revealed the role of these compounds as fundamental intracellular constituents involved in numerous biochemical reactions and biological functions [3]. However, in the early 1970s, the pioneering studies carried out by Geoffrey Burnstock [4] pointed out the activity of these compounds as signal molecules. Since then, the influence of purines at extracellular level has been extensively elucidated on a wide variety of physiological and pathological processes in major organ systems including the central and peripheral nervous systems, gastrointestinal, cardiovascular, respiratory, renal, and immune systems [5,6,7,8,9,10,11,12]. However, the role of the purine system in the oral cavity is still not fully elucidated.
In humans, the oral cavity is bounded by teeth, tongue, hard palate, and soft palate [13]. A revision of the literature on the involvement of the purinergic system in the functioning/alterations of the tissues/cells which constitute this apparatus dates back to 2012 [14]. Since then, few other reviews have addressed the activity of purines on specific functions of the oral cavity [15,16] without a unifying framework. Thus, the purpose of this overview is to provide insight into the pathophysiological role of the purinergic signal in the oral cavity, pointing out new potential therapeutic targets in oral diseases.
2. Brief Outline of the Purine System as Intercellular Signal Network
In order to fulfill the large variety of the aforementioned effects [17,18], purines, in particular adenine-based compounds, must be present at extracellular level where they act as signal molecules.
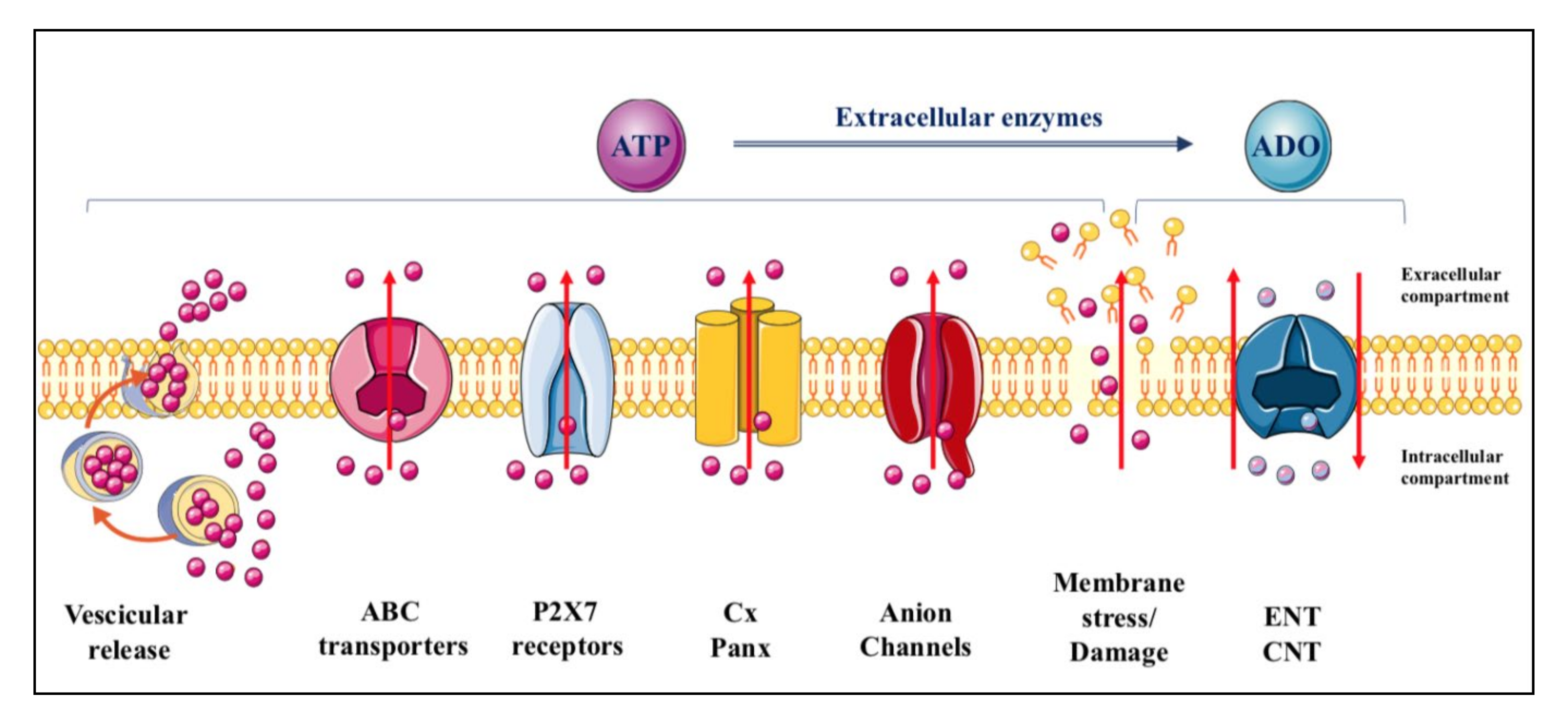
ATP is constitutively released from all cells in physiological conditions and upon different types of stimulation [19], which remarkably increase the extracellular levels of the nucleotide from the basal ones (concentrations in the low nanomolar range) [19,20,21]. The mechanisms through which adenine-based nucleotides are released are numerous, including exocytosis, mainly from nerve terminals and chromaffin cells but also from different types of non-neuronal cells [22,23,24,25], and efflux through connexin/pannexin hemichannels and facilitated diffusion by nucleotide-specific ATP-binding cassette (ABC) transporters and multiple organic anion transporters (Figure 1) [26].
Figure 1.
Mechanisms of purine release. ATP is released from cells in physiological conditions through multiple ways including vesicular exocytosis, connexin/pannexin (Cx/Panx) hemichannels, facilitated diffusion by nucleotide-specific ATP-binding cassette (ABC) transporters, and multiple organic anion transporters. In contrast, while most of the adenosine is formed from the nucleotide metabolism at extracellular level, the principal mechanism of adenosine efflux from cells is assured, under cell stress conditions, by carriers identified as equilibrative (ENT) and concentrative (CNT) nucleoside transporters. The former are bidirectional carriers, the direction of transport depending on the nucleoside concentration gradient across the plasma membrane, while the latter are Na+-dependent, being the nucleoside transport coupled to that of the sodium ion and independent of the nucleoside concentration gradient [27]. Additional transporters such as organic anion and cation transporters and ABC transporter proteins, have been implicated only as carriers of nucleoside-derived drugs, particularly those used as antiviral drugs [28].
Adenosine, mostly derived from ATP metabolism, is also found in extracellular fluid. Occasionally, it can be released under physiological conditions, as it has been reported for neuronal cells, kidney cells, cardiomyocytes and immune cells [29]. However, adenosine release mainly occurs under cell stress conditions via nucleoside transporters, which are ubiquitously expressed in cell membranes but are generally deputed to recover extracellular adenosine to replenish the intracellular purine pool. These molecules are distinguished as equilibrative (ENT) and concentrative (CNT) transporters [27] (Figure 1). In contrast, nucleoside analogues are substrates for other carrier proteins which are mostly deputed to function as efflux transporters [28].
Finally, while there may be a transient ATP efflux under various stimuli perturbing cell membranes without evident damage, which include shear stress, hypotonic swelling, stretching, hydrostatic pressure, brief hypoxia/hypoglycemia/ischemia episodes as well as in response to Ca2+ agonists [30,31], all endogenous nucleotides and nucleosides can be discharged into the extracellular space following persistent membrane injuries. Thus, a massive leakage of these molecules occurs upon cell lysis due to organ injury, traumatic shock or inflammatory conditions [31], but also in the case of endothelial dysfunction with plaque formation, or as a consequence of bacterial and viral infections or intoxication by bacterial toxins, allergen contact or mechanical stimulation [22,29,32].
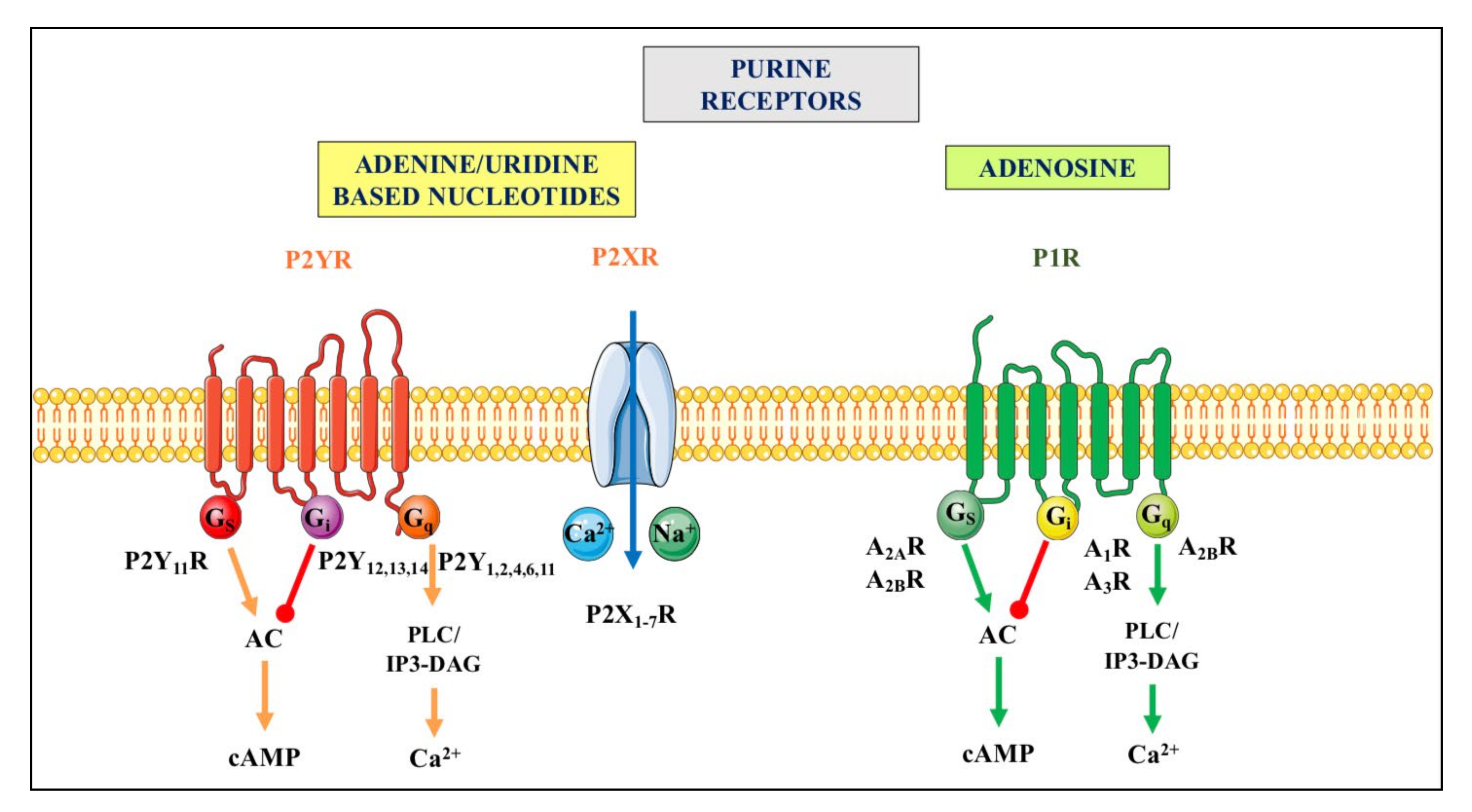
Purine release in the extracellular space represents a critical step for the initiation of purinergic signaling events. Indeed, nucleotides and adenosine activate different types of specific receptors, P1 receptors (primarily activated by adenosine) and P2 receptors (responding to both adenine and/or uracil nucleotides), which are widely but differentially expressed in virtually all cell types [26,32,33]. For more details, see Figure 2.
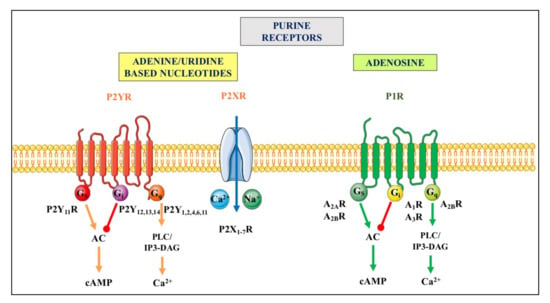
Figure 2.
Purinergic receptors. They are divided in two principal families named P1 and P2. The P1 receptor (P1R) family includes four subtypes (A1R, A2AR, A2BR and A3R), which are all metabotropic G proteins-coupled receptors [34]. The P2 receptor (P2R) family is subdivided into seven ionotropic P2X (P2XR), which are activated by ATP, and eight metabotropic P2Y receptors (P2YR), of which P2Y1R respond to ATP and ADP, P2Y2,4,6R are mainly activated by uridine-based nucleotides, P2Y12,13R respond to ADP, and P2Y14R to UDP-glucose (as recently reviewed by [35,36]). Ionotropic P2XR, when stimulated, allow the entry of cations such as Na+ and or Ca2+ into cells while metabotropic receptors belonging to the P1R family or to the P2YR subtypes are coupled to different G proteins and downstream molecular pathways indicated above. For major details see [37]. Abbreviations: AC, adenylate cyclase; cAMP, cyclic AMP, PLC/IP3-DAG, phospholipase C/inositol triphosphate-diacylglycerol.
Noteworthy, the concentration of extracellular purines and pyrimidines is under the tight control of a multitude of metabolizing enzymes, which mainly function as membrane-bound enzymes (ectoenzymes) [38] and are listed as for names and functions in the Table 1. Altogether, these enzymes modulate the nucleotide signal termination, balancing purines concentration in the extracellular environment.
Thus, all players in this complex network contribute to a fine-tuning regulation of the purinergic signals in physiological conditions. However, these control mechanisms can be altered in several pathological conditions [3,39,40].

Table 1.
Cell membrane-bound purinergic enzymes degrading extracellular adenine-based purines.
Table 1.
Cell membrane-bound purinergic enzymes degrading extracellular adenine-based purines.
| Family | Name | Function | References |
|---|---|---|---|
| Nucleoside triphosphate diphosphohydrolases (NTPDases) | NTPDase 1/CD39 | ATP → AMP | [41] |
| NTPDase 2 | ATP → ADP (sustained accumulation) | ||
| NTPDases 3 and 8 | ATP → ADP (transient accumulation) | ||
| Ectonucleotide pyrophosphatases/phospho- diesterases (ENPPs) | ENPP1 | ATP → AMP and PPi | [42] |
| ENPP3 | ATP → ADP | ||
| Alkaline phosphatases (APs) | Tissue-specific AP (TNAP) | PPi → Pi (mainly in mineralized tissues) | [43] |
| AMP → ADO | [44] | ||
| Acid phosphatases | Prostatic acid phosphatase (PAP) | AMP → ADO | [45] |
| Ecto-5′-nucleotidase | e-5′-NT/CD73 | AMP → ADO | [38] |
| Ecto- adenosine deaminase | e-ADA | ADO → INO | |
| Purine-nucleoside phosphorylase | e-PNP | INO → HYPO |
The principal family of ATP metabolizing enzymes is represented by the nucleoside triphosphate diphosphohydrolases (NTPDases) including eight members. Of these, only NTPDase1, 2, 3, and 8 are cell surface-bound enzymes, with different activities. Extracellular ATP can also be metabolized by enzymes belonging to other families of enzymes such as ENPP and APs. AMP, derived from ATP metabolism, can be converted into adenosine by a member of the acid phosphatase superfamily, known as PAP, or by ecto-5′-nucleotidases (e-5′-NT, also known as CD73). Once formed, adenosine is degraded to inosine and then to hypoxanthine by the combined activity of cell surface-located enzymes, i.e., ADA or PNP, or transported into the cell by specific transporters to replenish adenine nucleotide pool. Arrows indicate the conversion of a compound into another one.
3. Purinergic Signals in the Gustatory System
The sense of taste is due to specialized epithelial cells within taste buds which are present in the tongue, palate and larynx. These cells exhibit different morphological and physiological properties so that they can be distinguished into four groups (Type I–IV cells) with Type II and Type III functioning as taste receptor cells [46]. For a long time, there was a debate on the nature of the neurotransmitters released by these cells, but in 2005 Finger et al. [47] indicated ATP as the main neurotransmitter allowing the communications between taste buds and gustatory nerves. It was also demonstrated that taste cells express a vesicular nucleotide transporter (VNUT) [48] that is responsible for the vesicular storage of ATP [49]. Further studies have demonstrated that ATP is released from taste buds, in particular from Type II cells transducing bitter, sweet and umami perception, and that P2X2/P2X3 receptors are required for the transmission of all taste quality, including those mediated by Type III cells (sour). Moreover, an ecto-ATPase, namely NTPDase2, was identified in the taste buds [50] as deputed to nucleotide metabolism; this enzyme activity also avoids P2X2/3 receptor desensitization [51].
The discovery of the mechanism leading to ATP release from these cells has been even more complex. Since Type II taste cells do not express components of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex, which normally assures a vesicular release of the adenine-based nucleotide, some researchers suggested an ion channel-mediated mechanism. Initially, it was hypothesized an involvement of pannexin 1 (Panx1), which afterwards was shown to be responsible for the ATP release from mitochondria, the latter being the main intracellular source of ATP released from taste bud cells reviewed in [15]. Next studies unequivocally demonstrated that taste-evoked ATP release occurs via calcium homeostasis modulator (CALHM) channels, which are voltage gated channels with large calcium conductance. While the subtype CALHM1 was primitively involved, more recent studies have demonstrated that another channel subtype, namely CALHM3, needs to be co-expressed together with CALHM1 to account for ATP release with appropriate electric characteristics from taste buds [52]. However, these mechanisms have been reported only in Type II taste cells [15].
Many other reports have shown the expression of adenosine receptors in the taste buds, wherein A2B receptors are mainly expressed in sweet sensitive taste cells. This finding has been shown in rodents [53] and indirectly confirmed in humans, in whom caffeine, a known nonselective antagonist of adenosine receptors, mainly A1R and A2AR, selectively inhibited sweet taste perception in long-lasting manner. Indeed, the participants to this study received a solution containing caffeine on the first day and a quinine coffee solution on the second; interestingly, findings showed that caffeine suppressed both the sweetness of the coffee samples and the sucrose subsequently tested. However, the treatment had no effect on bitter, sour, salty, or umami perception [54]. Moreover, in patients with taste dysfunction, the oral treatment with theophylline, another methylxanthine like caffeine, improved this dysfunction [55]. The effect was due to the theophylline-promoted increase in salivary levels of ‘Sonic Hedgehog’ (Shh), which is a protein with important regulatory roles in taste system morphogenesis as well as in taste bud homeostasis and taste perception reviewed in [56]. This protein is present also in parotid glands from which it is released into saliva. Theophylline would act to increase transcription factors, e.g., cyclic AMP and cyclic GMP through the known and often invoked inhibition of cyclic nucleotide phosphodiesterases, enzymes deputed to cyclic nucleotide metabolism. However, there are several limitations in finding interpretation, including the small number of patients with taste dysfunction and the variability in the dysfunction etiology.
Thus, these data provide the proof that both caffeine and theophylline, can influence taste acting at different levels and also with different mechanisms. In this regard, the difference in the molecular structure between caffeine and theophylline, namely three methyl groups for caffeine and only two methyl groups for theophylline, could be accountable for the different mechanisms of action to explain their activity. Indeed, while both compounds modulate GABA receptor action and regulate intracellular calcium levels, besides the antagonism of adenosine receptors and phosphodiesterase inhibition [57], it was recently shown that caffeine has a role distinct from theophylline in gene regulation [58]. Therefore, methylxanthine may produce unpredictable effects on taste perception depending on the use of one or the other compound.
4. Purinergic Signaling in the Salivary Glands
Saliva is the liquid secreted by three pairs of salivary glands located in the oral cavity, which are the parotids with pure serous secretion, the submandibular and sublingual glands with mixed secretion, mainly serous the former and mucous the latter. Salivary glands comprise, in turn, acinar epithelial cells, which mainly secrete water and electrolytes in saliva, and ductal cells, able to control the fluid electrolyte concentrations while myoepithelial cells contribute to secrete saliva from acinar cells [59].
Saliva plays a crucial role in maintaining the health of the structures of the oral cavity, participates in the formation of the food bolus as an active part in the food digestion process and protects the oral cavity from possible infections [60]. In addition, saliva facilitates taste sensation and it is ever more used to determine markers of systemic diseases as well as to monitor drug intake both for therapeutic purposes and in case of some illicit/harmful drug assumption [61,62].
Salivary ATP levels can be increased by ATP released from cells and bacteria therein present and reduced by the activity of ectonucleotidases [63]. A recent review has revised literature about P2 receptor signaling in the salivary glands [16]. Some P2 receptors were initially discovered in rat submandibular glands (SMGs) and included both metabotropic (P2Y1, P2Y2) and ionotropic (P2X4, P2X7) receptor subtypes [64], while a major number of P2 receptors have been detected in acinar cells of mouse parotid glands such as P2X2,4,7 and P2Y1,2,10,12,14 [64]. ATP and adenosine receptors have also been described in human oral glands [14].
The role of ionotropic P2 receptors has been investigated in physiological and pathological conditions as for saliva production. P2X7R, which show a low affinity towards ATP and require high micromolar concentrations of nucleotide to be activated [65], are expressed in salivary ductal cells. Their stimulation regulates ionic currents and saliva volume by a mechanism dependent on the influx of extracellular calcium through the non-selective cation channel associated to the stimulation of P2X7R, which in turn rises [Ca2+] [66]. However, P2X7R activation can also modulate the saliva formation by inhibiting intracellular Ca2+ mobilization caused by muscarinic or substance P receptor agonists [67,68]. These data were obtained in rat submandibular acinar cells and for the inhibitory mechanism it was suggested an interference with binding of the autonomic agonists to their receptors [67]. In agreement with these data, cholinergic effect was significantly increased in parotid acinar cells from P2X7R-null mice [69]. Further experiments have corroborated these observations reviewed in [16].
P2X4R, which are activated by lower concentration of ATP than P2X7R, are also expressed in salivary acinar and ductal cells with a role yet to be fully defined. Some studies on P2X7R null mice have highlighted a weak saliva production attributable to P2X4R activity [70]. Subsequent investigation in salivary epithelium has also pointed out that P2X4R and P2X7R can form heteromeric channels while others have demonstrated a possible modulatory role of P2X4R on the P2X7R function, resulting in a reduction of P2X7R affinity to ATP, with a decreased production of saliva. However, these aspects are still being debated. Conversely, it seems clear that there is a synergism between P2X4R and beta-adrenoreceptors so that their activation enhanced the Ca2+ influx in mouse and human parotid acinar cells, thus increasing saliva production [71,72].
As for metabotropic P2 receptors, studies performed so far have shown that P2Y1R play a marginal role in salivary glands, being active mainly during gland development. Indeed, although they are expressed in adult rat SMGs, there is a decreased coupling by them to the usual G protein αq11 subunit, which leads to a lack of activity by these receptors during the adult life [73]. However, these data should be confirmed in mice and humans. Conversely, activation of P2Y2 receptors, initially identified in a cell line of human salivary gland cells and subsequently in other epithelia, seems to contribute to saliva secretion [74]. However, data are not univocal and further research is warranted to clarify the role of these receptors.
In contrast, the literature on the expression of adenosine receptors in the salivary glands and its activity on the saliva production is very limited. In 1986, the presence of adenosine A1 receptors was reported on rodent salivary glands [75]. Subsequently, it was shown that antagonism for this receptor stimulated mucin secretion caused by isoproterenol in rat submandibular acini [76]. Furthermore, Finkelberg and coll. [77] demonstrated that parotid gland A1 receptor activation induces a stimulatory effect on amylase release associated with increased production of cAMP and inositol phosphate accumulation via phospholipase C activation.
As shown for many other tissues and organs, extracellular nucleotides are metabolized to adenosine by ectonucleotidases. It was previously demonstrated that e-5′NT and NTPDase1, 2 and 3, but not NTPDase8, are expressed in rat SMG, mostly associated to microsomes [78]. Subsequently, this activity was predominantly attributed to substrate inactivation by rat and human NTPDase2 [79]. Furthermore, it has been reported that nanovesicles contained in rat saliva have the ability to hydrolyze ATP. The characterization of these extracellular vesicles (EVs) has shown the presence of NTPDase1, -2, and -3, together with e-5′NT [80]. Of note, since it was demonstrated that NTPDases are present in acinar and not in ductal cells [81,82], it was suggested that EV enzymes can be transported along ducts, contributing to the control of saliva production [83] and, more in general, to the homeostasis of the oral cavity.
5. Purinergic Signaling in Teeth and Periodontium
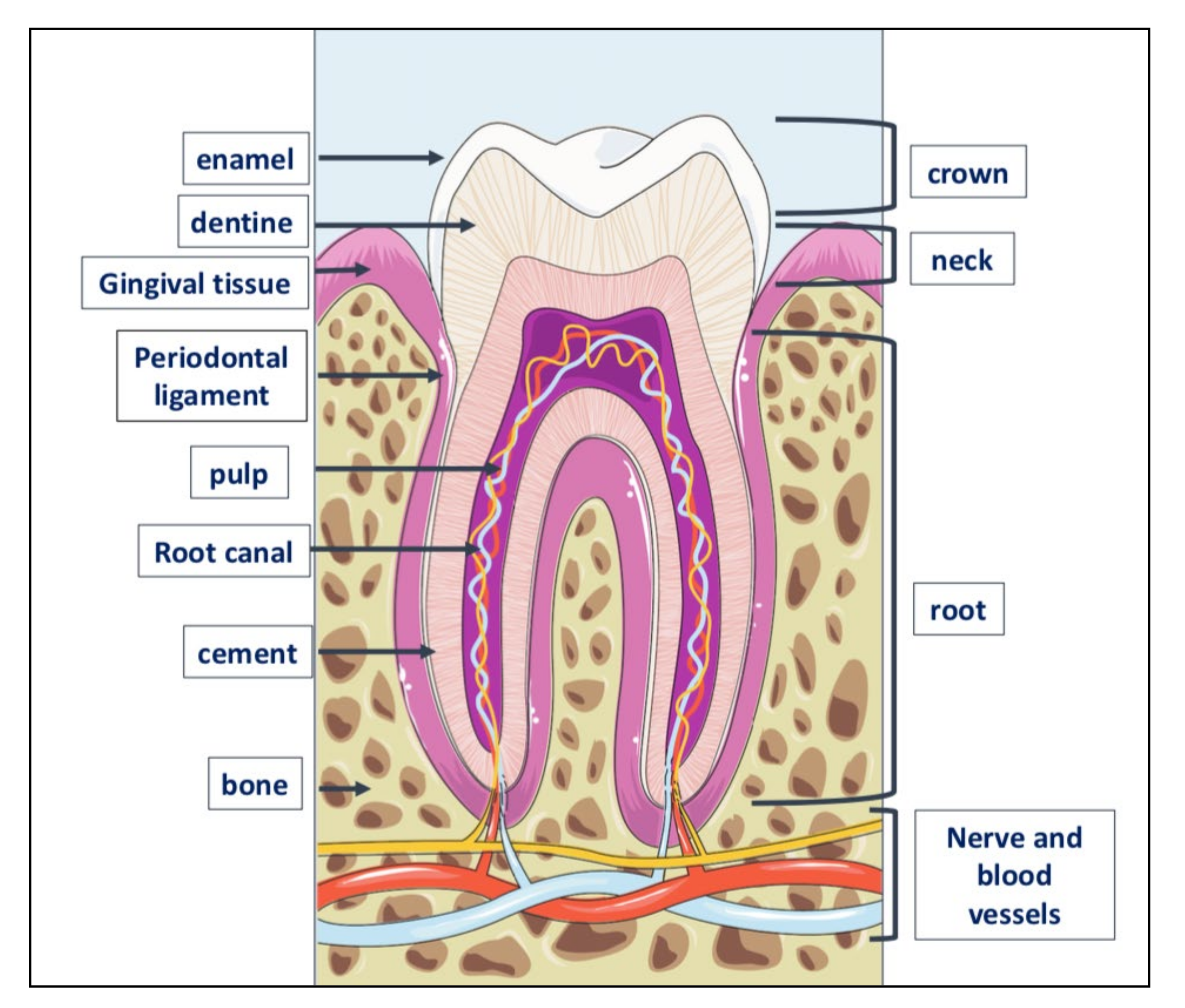
Teeth are very resistant mineralized structures of different shapes and sizes. They are located in the maxillary bones, inside cavities called alveoli (Figure 3).
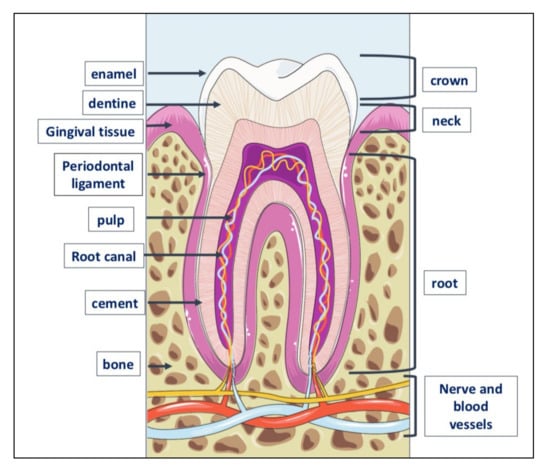
Figure 3.
Tooth anatomy. The tooth consists of three parts: crown, the visible part of the tooth; neck or collar, the part surrounded by the gingiva or gum, and root located inside the alveolar bone to which is linked by the periodontal ligament. The outer part of the crown is covered with a layer of dental enamel, the hardest element in the human body; below the enamel is dentin, the substance that gives the teeth their color and composed of about 70% of inorganic material, the remaining 30% of organic material and water. Dentin is generated by odontoblasts, cells contained in the pulp, which is inside the dentin and includes also the nerve and blood vessels. The periodontium is a set of structures that are located around the tooth and which have a dual function: to keep the tooth firmly in the alveolar bone and to preserve the integrity of the mucous membrane of the oral cavity. It is made up of four tissues: the gum, the periodontal ligament, the root cement and the alveolar bone itself, that is, that bone, also called the hard lamina, which delimits the dental alveolus.
All tooth structures have been investigated for the presence and function of the purinergic signal, even though some of them (mainly dental pulp and periodontal ligament) have gained a major interest.
Starting from the external structures forming periodontium such as gums, adenosine receptors A1, A2A, and A2B (but not A3) are expressed in human gingival epithelial cells (GECs) and fibroblasts therein present. The involvement of adenosine in healthy condition of these cells has not been defined, while its role as anti-inflammatory agent has been circumstantiated, as detailed in the next chapter. However, it should be considered that the expression of adenosine receptors can be remarkably altered by inflammatory processes affecting the gum. Thus, in human gingival tissue with chronic periodontitis, the expression of A1R was decreased by 20%, that of A2AR was more than doubled and that of A2BR increased by about 4-fold compared to healthy gingiva [84]. This implies that the responses to ligands for these receptors may not be the same depending on the inflammatory condition of the tissue. The presence of P2 receptors has also been reported in GECs, mainly the expression of P2X 2, 4, 5, 6, and 7 receptors [85]. In this case too, the role of P2XR, mainly P2X4R and P2X7R subtypes, has been explored in relation to inflammatory conditions [86]. Unfortunately, the release of purines has not been evaluated from the cells mentioned above.
In contrast, for human fibroblasts obtained from the periodontal ligament an increased release of ATP was shown when they were submitted to the application of a mechanical stress, as for example under orthodontic procedures. The mechanisms involved in such a release have not been explored. However, ATP released in this way enhanced extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation, likely acting on P2Y4R or P2Y6R, overexpressed in these cells together with P2Y5R [87]. Additionally, it was recently demonstrated that P2X7R are usually absent in human periodontal ligament and dental tissues, while they can be observed in odontoblasts, which are cells contained in the pulp of the tooth [88].
In these cells, the presence and activity of the purinergic signal have been further investigated. In rat cultured odontoblasts, ATP release was evaluated upon thermal stimulation and it was found that both pulp tissue and odontoblasts expressed VNUT, which was colocalized in intracellular vesicles together with membrane fusion proteins. Further experiments demonstrated that ATP released from odontoblasts by thermal stimulation can interact with P2X3R on axons in the pulp [89].
More recently, the expression of P2Y2R, P2Y4R and all P2XR subtypes was revealed by RT-PCR in dental pulp whole tissue, while in acutely isolated rat odontoblasts the expression of all receptors aforementioned was confirmed in a subset of odontoblasts, except that of some P2XR, namely P2X1,3,5R [90]. As expected, stimulation of these cells with 100 μM ATP caused an increase of [Ca2+]i. This effect was not abolished by thapsigargin-induced depletion of intracellular Ca2+ ions, which suggested ionotropic P2XR activity. Since the ATP effect was maintained in more than half of the cells tested, even in a bath solution without extracellular Ca2+, this finding confirmed the activity of functional Gq/11-coupled metabotropic ATP receptors in a subset of odontoblasts [90].
The presence of purinergic receptors was observed also in human dental pulp cells (HDPCs) by Wang and coll. [91]. These cells express P2X3, P2X4, P2X5, P2X7, and all P2Y receptor subtypes. Interestingly, the stimulation of these cells with low ATP concentration (10 μM) enhanced HDPC proliferation, while higher doses (800 μM) arrested cell proliferation while inducing odontoblastic differentiation, which was coupled to ERK/MAPK activation, and increased expression of specific markers such as dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP). In addition, all adenosine receptors, except A2AR, are expressed by the same cells and concur to their odontoblastic differentiation [92].
The ability of HDPCs to differentiate into odontoblasts and also osteoblasts has been reported in a number of studies and indicates the abundance of stem/progenitor cells among these cells. The evidence of the presence of mesenchymal stromal cells (MSCs) in the dental pulp as well as in various dental structures has emerged in the last ten years. As with those deriving from other tissue/fluid sources, dental MSCs show self-renewal capacity and multi-differentiation potential and a growing attention is now paid to their characteristics for possible therapeutic applications [93]. Among the eight types of dental stem cells (DSCs) so far identified, MSCs from dental pulp (DPSCs), human exfoliated deciduous teeth (SHED), and periodontal ligament (PDLSCs) have more extensively been investigated as autologous stem cells to use for regenerative purposes and tissue engineering therapies reviewed by [94]. Accordingly, the investigation on the purinergic signal has also been performed in these cells. Iwamoto and colleagues [95] reported that ATP release from odontoblast precursors occurred via one of the three members of the pannexin family, pannexin 3 (Panx3), which was not expressed in mature odontoblasts. They also demonstrated that Panx3 ATP hemichannel contributed to the regulation of the odontoblast transition from proliferation to differentiation stimulating the AMP-activated protein kinase (AMPK) signaling pathway, at the same time inducing cell cycle arrest upregulating p21 expression. In contrast the expression of connexin 43 (Cx43) has been recently reported in ameloblasts and not in odontoblasts [96]. The expression and activity of this membrane protein are important for dental development so that Cx43 mutations, revealed in oculodentodigital dysplasia, cause, among others defects, dental abnormalities in humans. However, no connection has been reported about the involvement of Cx43 in the release of ATP and related effects, as shown for other cells [97].
As for the presence of purinergic receptors in DSCs, our group has found the expression of all four adenosine receptors in DPSCs. We also revealed that the stimulation of A1R increased their duplication and mainly their osteogenic differentiation activating the Wnt signal [98]. Another study, comparing the growth characteristics and colony forming efficiency of DPSCs and SHED, found an increased proliferative activity in SHED coupled to a major expression of CD73 and CD146 along the passage number in vitro. Importantly, CD73, in addition to being considered a mesenchymal marker, is also known as the e-5′NT. Thus, it could be conceivable that CD73 activity also as enzyme able to metabolize AMP into adenosine at extracellular level could prolong the proliferative activity of these cells [99]. As for P2 receptors, the expression of P2X4R, P2X6R, and P2X7R and also of P2Y1R and P2Y11 was found in DPSCs. Some of them participated in ATP-induced Ca2+-signaling. In fact, activation of P2Y1R and P2Y11R stimulated the phospholipase C/inositol triphosphate (PLC/IP3) pathway via G protein αq11 with an increased release of Ca2+ form endoplasmic reticulum. This effect was coupled to Ca2+ entry via the stromal interaction molecule 1 (STIM1)-dependent opening of ORAI1 channels and P2X7R-opened channels. Altogether, this complex mechanism led to enhanced cell migration promoted by extracellular ATP [100]. Later on, Zhang et al. [101] found that the same cells expressed also P2X3R as well as P2Y2R and P2Y6R and that all of them likely contribute with the other P2XR and P2YR to ATP-induced inward currents. The authors also suggested that the activity of P2YR was related to the survival and proliferation of DPSCs, while the inhibition of P2XR affected only their proliferation. Furthermore, the activity of ATP in these cells was modulated by the NTPDase3, the blockade of which enhanced both ATP-induced inward ion currents and DPSC survival/proliferation. Again, a Ca2+-permeable Piezo1 channel, recently identified as mechano-sensing ion channel also in dental pulp cells [102], was revealed in human DPSCs and its activation was held responsible for cell migration upon ATP release and activation of P2 receptors (not better identified) coupled to stimulation of the proline-rich tyrosine kinase 2 (PYK2) and mitogen-activated protein kinase (MEK)/ERK pathways [103].
Finally, the oral cavity is widely innervated. In physiological condition, the trigeminal nerve provides important information against irritant and painful stimuli via specialized receptors, which may undergo altered expression and activity under pathological conditions leading to painful syndromes [104]. Fibers from the trigeminal nerve innervate also dental pulp together with sympathetic and parasympathetic neurons, which altogether contribute to the induction and maintenance of the tooth pain [105]. In this network it has to be considered that purinergic receptors are expressed on nervous fibers innervating dental pulp and gingival structures. Their role is to cooperate with the principal sensitive neurons to signal noxious stimuli. Their expression and role are both illustrated in the next section.
6. Purinergic Signal in Pathological Conditions of the Oral Cavity
Cell stress or apoptosis due to traumatic, ischemic or infective events in the oral cavity can trigger the release of ATP, ADP and other nucleotides promoting inflammatory responses via P2 receptors. In particular, high levels of ATP are released from cells during inflammation and they mainly activate P2X7R inducing reactive oxygen species production as well as the release of IL-1β and other cytokines [106]. Subsequent activation of IL-1 receptors by IL-1β in surrounding cells induces P2Y2R upregulation and further downstream responses to ATP and UTP. These nucleotides, being the main agonists of P2Y2R, are able to promote the release of further inflammatory agents (MCP-1, also known as CCL2) from macrophages locally present (i.e., in the dental alveoli thus amplifying the inflammatory environment). In this way, the release of ATP or UTP in response to cellular stress can locally modulate a wide range of signaling pathways to fine-tune the tissue response to inflammatory stimuli. Of course, purine metabolizing enzymes, present at the extracellular level and also in salivary EVs [80], likely lead to a reduction in signaling of P2R, favoring increase of anti-inflammatory events through activation of P1R [14].
However, the most frequent cause of inflammatory changes in the oral cavity are infections caused by bacteria, viruses and even unicellular eukaryotes such as yeasts. Bacterial infections mainly involve the periodontal tissues, therein inducing a host response mediated by immune cells, which are the principal responsible for ATP release. This ATP interacts with P2X7R, whose expression is increased, for example, by acute infection due to Porphyromonas gingivalis, one of the pathogens most frequently associated with periodontitis, and activates the NOD-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome through a canonical pathway. Consequently, the activity of caspase-1 is promoted, which in turn induces the maturation and secretion of interleukin-1β (IL-1β) and other active ILs [107,108,109]. Additionally, ATP, again interacting with P2X7R on local fibroblasts or osteoblasts, also activates the expression of receptor activator of nuclear factor k-B ligand (RANKL), inducing the differentiation and activity of osteoclasts to modify the alveolar bone crest morphology along with cytokines such as IL-1β and tumor necrosis factors α (TNFα), known to promote osteoclastic bone resorption. Therefore, there is a growing interest in the host-pathogenic microorganisms interaction [110], also in relation to the evidence that extracellular ATP from any source is a key modulator that initiates the pathway of alveolar bone resorption and bone loss in patients with periodontal disease [111]. Thus, possible control of extracellular ATP levels should reduce bone loss in periodontitis.
However, in periodontal infection sustained by other pathogens such as Fusobacterium nucleatum, P2X7R stimulation also activates the non-canonical inflammasome pathway with increased activity by caspase-11, in turn causing IL-1β secretion [112]. Just as P. gingivalis is also capable of releasing a nucleoside diphosphate kinase (NDK) homolog, which in turn metabolizes extracellular ATP while preserving GECs and underlying cells from ATP-P2X7R signaling [111], caspase-11 appears to be required not only for inducing cell death during F. nucleatum infection, but also to help limit bacterial load during F. nucleatum infection in bone marrow-derived macrophages and in mouse gingival tissue coinfected with P. gingivalis and F. nucleatum. These data would indicate that the both caspase-1 and caspase-11 pathways activated by P2X7R are involved in the immune response against infections [112].
In this context, although the expression of adenosine receptors can vary as a consequence of pathological conditions of one or more oral tissues [84], a role for this nucleoside should be considered. Indeed, stimulation of the adenosine receptors in GECs increased inducible nitric oxide synthase (iNOS) activity with enhanced production of stable nitric oxide (NO2 and NO3) metabolites, which have anti-inflammatory/antibacterial activity [113]. As well, in gingival fibroblasts, agonists for A1AR and A2AAR were shown to synergistically increase IL-1β-induced IL-6 and IL-8 production, which corroborates the involvement of adenosine signaling in the regulation of inflammatory responses in periodontal tissues [114]. Adenosine also inhibited the action of pro-inflammatory chemokines such as CXCL8, which plays a central role in the inflammatory response driven by periodontal infection. Interestingly, adenosine effect was mediated by an increased activity of the intracellular hemeoxygenase-1 and adenosine monophosphate-activated protein kinase (AMPK), both being key regulators of the host inflammatory responses [115]. Furthermore, studies on A2AR have demonstrated that their stimulation may be used to induce replication of P. gingivalis in GECs [116], which may have a protective effect, as above reported. Even though specific studies on other oral tissues are still lacking, it should be underlined that the signaling of adenosine through its receptors (i.e., A2AR and A2BR) has been proposed as the major immunomodulator stimulus during inflammatory conditions, especially those caused by chronic microbial infections such as those occurring in the periodontium [117].
Accordingly, the activity of purine metabolizing enzymes, which modulates the concentrations of extracellular purines in the oral cavity, must also be considered. ATP hydrolysis was revealed many years ago [118] in rat dental tissues by specific enzymes including alkaline phosphatase, ATPases, 5′-nucleotidase and pyrophosphatases. Further data have shown the activity of these enzymes in pathological conditions of the oral apparatus. For example, an increased secretion of ectonucleotides was demonstrated from saliva nanovesicles in rat SMGs stimulated with histamine, the first agent triggering inflammatory events [119]. Preliminary results from the same group would indicate that in EVs isolated from human saliva ATPase activity was higher in patients with periodontal disease [80]. Thus, enzymes in the saliva EVs could help in local control of nucleotides in sites where they were acutely increased due to injury or inflammation. Again, ATP has been shown to indirectly inhibit IL-1β-induced matrix metalloproteinases (MMPs) through the activity of NTPD1/CD39 corroborated by that of CD73 in primary human gingival fibroblasts (HGF) [120], both leading to an increased formation of adenosine. Another enzyme, the purine nucleoside phosphorylase (PNP), which catalyzes the reversible phosphorolysis of purine nucleosides, i.e., inosine and guanosine into hypoxanthine and guanine, respectively, has resulted to be upregulated in gingival crevicular fluid in periodontal disease [121]. Of note, the inhibition of the activity of this enzyme halted bone loss consequent to periodontal disease in an animal model [122]. Altogether, these results indicate that the increased amount of extracellular ATP released as a consequence of inflammatory environment is metabolized by a chain of extracellular enzymes, whose concerted activity is oriented to reducing potential negative effects of purine compounds and restoring a homeostatic condition.
Clearly, inflammation is tightly coupled to exacerbation of the pain perception. There is a large amount of evidence that an increase in extracellular ATP in oral tissues can exacerbate pain associated with inflammation. Several purinergic receptors are expressed in trigeminal ganglion (TG) nerves, including P2X2R and P2X3R. In particular, it was found that ATP, released from mechanically stimulated odontoblasts via pannexin-1 in response to the activation of transient receptor potential (TRP) channels (which are involved in the painful stimulus detection and transmission) [123], transmits a signal to P2X3R on TG neurons. Thus, odontoblasts behave as sensory receptor cells while ATP released from them acts as a neurotransmitter for dentinal pain [124]. Again, P2X3R expressed in TG appear to be involved in hyperalgesia of the temporomandibular joints and massetere muscle consequent to the placement of an occlusal interference in rats [125]. Kawaguchi et al. [126] observed the expression of other ATP/P2R, specifically the P2Y12R, in TG neurons, while Suguwara et al. [127] have found these receptors in TG satellite cells, which are cells surrounding TG neurons involved in the modulation of TG transmission. The stimulation of P2Y12R on satellite cells leads to an interaction with calcitonin gene-related peptide (CGRP) neurons, thus contributing to enhance the neuropathic pain in the tongue due to lingual nerve injury in rats. Conversely, adenosine has an opposite role, being analgesic, anti-inflammatory and tissue protector [128,129]. Together with purinergic receptor expression, purine enzymes have been detected in nervous fibers innervating dental tissues. While NTPDase3 is expressed in TG nociceptive neurons [130], NTPDase2 is present in the dental pulp odontoblast layer and it likely derives from the Schwann cells that encapsulate the nerve fibers projecting into this layer [131]. The expression of CD73 has also been detected in TG nociceptive neurons [132]. Altogether, these results suggest that ecto-nucleotidases also participate in the modulation of nociceptive signals. All of these aspects are summarized in Table 2.

Table 2.
Purinergic signaling in oral cavity pathologies.
7. Clinical Perspectives
In dentistry, bone repair represents one of the main issues, especially in the presence of large-size bone defects. Regenerative medicine has introduced the experimental use of MSCs, although there are still several limits to overcome linked to accessibility, storage and expansion of these cells. Furthermore, it is even more evident that MSCs contribute to heal injured tissues through the release of factors contained in EVs released from MSCs. In particular, studies on these EVs and their content can facilitate their use in diverse animal models of calvarial and alveolar bone regeneration [136]. Recently, it was demonstrated that EVs derived from PDLSCs can be embedded in a hydrogel scaffold (i.e., matrigel) and then transplanted into a rat model of calvarial defect. The results showed that this device stimulated host cell proliferation and bone repair in vivo and this effect, inhibited in the presence of a nonselective adenosine receptor antagonist, theophylline, could be attributed also to adenosine-mediated activation of AKT and ERK1/2 pathways [137].
Accordingly, other findings obtained by Verma et al. [138] showed that an osteogenic scaffold comprising adenosine/epigallocatechin gallate-N,O-carboxymethyl chitosan/collagen type I (AD/EGCG-g-NOCC@clgn I), exhibited osteo-inductive properties in a calvarial defect model reproduced in BALB/c mice. The ex-vivo findings clearly established the pro-osteogenic potential of adenosine and EGCG, which both stimulated MSCs toward osteoblast differentiation as evaluated by the increased expression of selective osteogenic markers such as alkaline phosphatase and osteocalcin and enhanced calcium deposits. Noteworthy, this type of 3D matrix held some extracellular matrix (ECM) properties, providing a favorable microenvironment and a structural support against mechanical stress. 3D matrix also acted as a reservoir for the sustained release of osteo-inductive molecules such as adenosine. Thus, two different experimental approaches have confirmed/highlighted an important osteo-inductive role for this nucleoside. Of course, further studies are needed in this direction to open the way to new strategies for bone repair.
Interestingly, the use of a regenerative therapy has been attempted also to restore the function of salivary glands after radiation injury. By now, this possibility has been ascribed to the activation of muscarinic receptors through a mechanism dependent on the presence of progenitor cells also in salivary glands [139]. Given the expression of purinergic receptors in this district, it should be of interest to evaluate whether their modulation can play a role also in the regeneration of acinar cells.
However, other experimental findings seem to converge towards a useful application of the purinergic receptor modulation in salivary gland dysfunction, which is usually observed in the Sjögren’s syndrome (SS) and as a consequence of radiotherapy (RT) used for treating head and neck cancer (HNC). In both pathological conditions, hyposalivation is coupled to impairment of muscarinic receptor signal and aquaporin channel activity, which are required for saliva formation and fluid secretion, respectively [140,141]. Noteworthy, an inflammatory environment with an increased ATP release and P2X7R expression are present in both cases and play a fundamental role in the onset and evolution of these conditions [142]. Thus, as for SS and RT applied to HNC treatment, the use of antagonists for these receptors has been evaluated. In particular, inhibition of P2X7R by the competitive antagonist A-438079 significantly reduced sialadenitis, while improving carbachol-induced saliva flow in a murine model of SS-like syndrome [143]. Furthermore, in salivary gland biopsies from SS patients, P2X7R antagonism reduced the expression of upregulated immuno-active molecules such as IL-1β, intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), E-selectin, CD80, and CD86 [143]. As well, in vivo administration of A-438079 in γ-irradiated mice induced a significant radioprotection to salivary glands and maintained saliva flow rates similar to non-irradiated mice for a prolonged period after irradiation. This effect was coupled to a reduction in the secretion of prostaglandin E2 (PGE2), which is considered one of the most inflammatory markers following gamma-radiation therapy [144]. Altogether, these findings highlight the importance of P2X7R for a potential application of their ligands in both diseases. However, besides P2X7R, we cannot neglect the existence and the role of P2Y2R, whose upregulation has been observed under stress/inflammatory condition. Indeed, upregulation of these receptors but not that of other uridine nucleotide receptors such as P2Y4R and P2Y6R is present in SMGs of NOD.B10 mouse model of primary SS as compared to normal C57BL/6 mice [145]. In agreement with these findings, Jasmer et al. [146] have recently shown that inhibition of P2Y2R using the selective antagonist AR-C118925 in another animal model of SS resolved sialadenitis, while improving compromised salivary flow. Likewise, P2Y2R deletion ameliorated sialadenitis induced by IL-14α, an autoimmune syndrome with characteristics similar to SS [147].
Finally, while the research for purine enzyme dysregulation seems to be still in its infancy, the investigation of the presence of receptor polymorphisms looks more realistic and useful. Indeed, recent data support a relationship between four P2X7R genetic variants showing a gain of function and the amplitude of the inflammatory response in patients with aggressive periodontitis [148]. This is consistent with a study reporting a single polymorphism at the minor allele of P2X7R A1405G, which was associated with a gain of receptor function and may represent a risk factor in a subset of SS patients who do not express human leukocyte antigen (HLA) risk alleles [149]. Further findings on the variability of P2X7R gene suggest that this factor could contribute to the etiopathogenesis of post-orthodontic external apical root resorption, as investigated in two different populations of young people [150,151].
8. Conclusions
There is convincing evidence that purinergic receptors are expressed and functioning in oral tissues, contributing with their signals to the homeostatic regulation of the mechanisms that ensure the correct functionality of the oral cavity. Moreover, data so far collected as for therapeutic applications of purinergic compounds in oral pathologies are encouraging, although further studies are needed regarding their efficacy and long-term safety profile. Therefore, a cooperative approach is desirable to bridge the gap between basic and clinical research in the dental field and to hopefully pave the way for new clinical applications of ligands of purine receptors and/or enzymes in the management of diseases of the oral cavity.
Author Contributions
Conceptualization, R.C. and P.D.I.; data curation, M.Z., P.G., M.R., V.C., F.C. and P.D.I.; writing—original draft preparation, R.C.; writing—review and editing, all authors; supervision, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part funded by the University of Chieti-Pescara, grant number AT2020 to P.D.I.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| A1R, A2AR, A2BR, A3R | adenosine receptor subtypes A1, A2A, A2B, A3 |
| ABC | ATP binding cassette |
| AD/EGCG-g-NOCC@clgn I | adenosine/epigallocatechingallate-N,O-carboxymethylchitosan/collagen type I |
| AMPK | adenosine monophosphate-activated protein kinase |
| APs | alkaline phosphatases |
| CALHM | calcium homeostasis modulator |
| CNT | concentrative nucleoside transporters |
| CGRP | calcitonin gene-related peptide |
| Cx | connexin |
| DMP1 | dentin matrix protein 1 |
| DPSCs | dental pulp stem cells |
| DSPP | dental sialophosphoprotein |
| e-ADA | ecto-adenosine deaminase |
| ECM | extracellular matrix |
| ENPPs | ectonucleotide pyrophosphatases/phosphodiesterases |
| ENT | equilibrative nucleoside transporters |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| EVs | extracellular vesicles |
| GECs | gingival epithelial cells |
| HDPCs | human dental pulp cells |
| HLA | human leukocyte antigen |
| HGF | human gingival fibroblasts |
| HNC | head and neck cancer |
| ICAM | intercellular adhesion molecule |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| MEK | mitogen-activated protein kinase |
| MMPs | matrix metalloproteinases |
| MSCs | mesenchymal stem cells |
| NDK | nucleoside diphosphate kinase |
| NLRP3 | NOD-like receptor pyrin domain-containing protein 3 |
| NO | nitric oxide |
| e-5′NT/CD73 | 5′-ectonucleotidase |
| NTPDase | ecto-nucleotide diphosphohydrolase |
| P2R | P2 receptors |
| Panx | pannexin |
| PAP | prostatic acid phosphatase |
| PDLSCs | periodontal ligament stem cells |
| PGE2 | prostaglandin E2 |
| PLC/IP3-DAG | phospholipase C/inositol triphosphate-diacylglycerol |
| PNP | purine nucleoside phosphorylase |
| PYK2 | proline-rich tyrosine kinase 2 |
| RANKL | receptor activator of nuclear factor kappa-B ligand |
| RT | radiotherapy |
| RT-PCR | retro-transcription protein chain polymerase |
| SHEDs | human exfoliated deciduous teeth |
| Shh | sonic hedgehog |
| SMG | submandibular gland |
| SNARE | soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| STIM1 | stromal interaction molecule 1 |
| SS | Sjögren’s syndrome |
| TG | trigeminal ganglion |
| TNAP | tissue-specific AP |
| TRP | activation of transient receptor potential |
| VCAM | vascular cell adhesion molecule |
| VNUT | vesicular nucleotide transporter |
References
- Watson, J.D.; Crick, F.H.C. A Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Dahm, R. Friedrich Miescher and the discovery of DNA. Dev. Biol. 2005, 278, 274–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic receptors. J. Theor. Biol. 1976, 62, 491–503. [Google Scholar] [CrossRef]
- Burnstock, G. Introduction to Purinergic Signalling in the Brain. Adv. Exp. Med. Biol. 2020, 1202, 1–12. [Google Scholar] [CrossRef]
- Ben, D.D.; Antonioli, L.; Lambertucci, C.; Spinaci, A.; Fornai, M.; D’Antongiovanni, V.; Pellegrini, C.; Blandizzi, C.; Volpini, R. Approaches for designing and discovering purinergic drugs for gastrointestinal diseases. Expert Opin. Drug Discov. 2020, 15, 687–703. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Haskó, G. Adenosine and inflammation: It’s time to (re)solve the problem. Trends Pharmacol. Sci. 2022, 43, 43–55. [Google Scholar] [CrossRef]
- Jain, S.; Jacobson, K.A. Purinergic Signaling in Liver Pathophysiology. Front. Endocrinol. 2021, 12, 718429. [Google Scholar] [CrossRef]
- Jain, S.; Jacobson, K.A. Purinergic signaling in diabetes and metabolism. Biochem. Pharmacol. 2021, 187, 114393. [Google Scholar] [CrossRef]
- Monaghan, M.-L.T.; Bailey, M.A.; Unwin, R.J. Purinergic signalling in the kidney: In physiology and disease. Biochem. Pharmacol. 2021, 187, 114389. [Google Scholar] [CrossRef]
- Zhang, Y.; Wernly, B.; Cao, X.; Mustafa, S.J.; Tang, Y.; Zhou, Z. Adenosine and adenosine receptor-mediated action in coronary microcirculation. Basic Res. Cardiol. 2021, 116, 22. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Boucher, R.C. Purinergic receptors in airway hydration. Biochem. Pharmacol. 2021, 187, 114387. [Google Scholar] [CrossRef]
- Dotiwala, A.K.; Samra, N.S. Anatomy: Head and Neck, Tongue; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lim, J.C.; Mitchell, C.H. Inflammation, Pain, and Pressure—Purinergic Signaling in Oral Tissues. J. Dent. Res. 2012, 91, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Finger, T.; Kinnamon, S. Purinergic neurotransmission in the gustatory system. Auton. Neurosci. 2021, 236, 102874. [Google Scholar] [CrossRef]
- Khalafalla, M.G.; Woods, L.T.; Jasmer, K.J.; Forti, K.M.; Camden, J.M.; Jensen, J.L.; Limesand, K.H.; Galtung, H.K.; Weisman, G.A. P2 Receptors as Therapeutic Targets in the Salivary Gland: From Physiology to Dysfunction. Front. Pharmacol. 2020, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Muñoz, M.; Hernández-Muñoz, R.; Butanda-Ochoa, A. Structure–activity features of purines and their receptors: Implications in cell physiopathology. Mol. Biomed. 2022, 3, 5. [Google Scholar] [CrossRef]
- Nelson, K.L.; Voruganti, V.S. Purine metabolites and complex diseases: Role of genes and nutrients. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 296–302. [Google Scholar] [CrossRef]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef] [Green Version]
- Helenius, M.; Jalkanen, S.; Yegutkin, G.G. Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim. Biophys. Acta 2012, 1823, 1967–1975. [Google Scholar] [CrossRef] [Green Version]
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef]
- Burnstock, G.; Verkhratsky, A. (Eds.) Mechanisms of ATP release and inactivation. In Purinergic Signaling and the Nervous System; Springer: Berlin/Heidelberg, Germany, 2012; pp. 79–118. [Google Scholar]
- Mielnicka, A.; Michaluk, P. Exocytosis in Astrocytes. Biomolecules 2021, 11, 1367. [Google Scholar] [CrossRef]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef] [Green Version]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Kaebisch, C.; Schipper, D.; Babczyk, P.; Tobiasch, E. The role of purinergic receptors in stem cell differentiation. Comput. Struct. Biotechnol. J. 2014, 13, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Wright, N.J.; Lee, S.-Y. Toward a Molecular Basis of Cellular Nucleoside Transport in Humans. Chem. Rev. 2021, 121, 5336–5358. [Google Scholar] [CrossRef]
- Cano-Soldado, P.; Pastor-Anglada, M. Transporters that translocate nucleosides and structural similar drugs: Structural requirements for substrate recognition. Med. Res. Rev. 2012, 32, 428–457. [Google Scholar] [CrossRef]
- Ferrari, D.; McNamee, E.N.; Idzko, M.; Gambari, R.; Eltzschig, H.K. Purinergic Signaling During Immune Cell Trafficking. Trends Immunol. 2016, 37, 399–411. [Google Scholar] [CrossRef]
- Burnstock, G.; Ralevic, V. Purinergic Signaling and Blood Vessels in Health and Disease. Pharmacol. Rev. 2014, 66, 102–192. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 2008, 1783, 673–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnstock, G. Purinergic signalling: From discovery to current developments. Exp. Physiol. 2014, 99, 16–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnstock, G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Ijzerman, A.P.; Jacobson, K.A.; Müller, C.E.; Cronstein, B.N.; Cunha, R.A. International Union of Basic and Clinical Pharmacology. CXII: Adenosine Receptors: A Further Update. Pharmacol. Rev. 2022, 74, 340–372. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
- Patritti-Cram, J.; Coover, R.A.; Jankowski, M.P.; Ratner, N. Purinergic signaling in peripheral nervous system glial cells. Glia 2021, 69, 1837–1851. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef]
- Losenkova, K.; Zuccarini, M.; Karikoski, M.; Laurila, J.; Boison, D.; Jalkanen, S.; Yegutkin, G.G. Compartmentalization of adenosine metabolism in cancer cells and its modulation during acute hypoxia. J. Cell Sci. 2020, 133, jcs241463. [Google Scholar] [CrossRef]
- Vultaggio-Poma, V.; Falzoni, S.; Salvi, G.; Giuliani, A.L.; Di Virgilio, F. Signalling by extracellular nucleotides in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119237. [Google Scholar] [CrossRef]
- Kukulski, F.; Lévesque, S.A.; Lavoie, É.G.; Lecka, J.; Bigonnesse, F.; Knowles, A.F.; Robson, S.C.; Kirley, T.L.; Sévigny, J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005, 1, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, H. Ectonucleotidases: Some recent developments and a note on nomenclature. Drug Dev. Res. 2001, 52, 44–56. [Google Scholar] [CrossRef]
- Patel, J.J.; Zhu, D.; Opdebeeck, B.; D’Haese, P.; Millán, J.L.; Bourne, L.E.; Wheeler-Jones, C.P.; Arnett, T.R.; Macrae, V.E.; Orriss, I.R. Inhibition of arterial medial calcification and bone mineralization by extracellular nucleotides: The same functional effect mediated by different cellular mechanisms. J. Cell. Physiol. 2018, 233, 3230–3243. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.L. Alkaline Phosphatases: Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006, 2, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, H. Prostatic acid phosphatase, a neglected ectonucleotidase. Purinergic Signal. 2009, 5, 273–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, R.; Ninomiya, Y. New Insights into the Signal Transmission from Taste Cells to Gustatory Nerve Fibers. Int. Rev. Cell Mol. Biol. 2010, 279, 101–134. [Google Scholar] [CrossRef] [PubMed]
- Finger, T.E.; Danilova, V.; Barrows, J.; Bartel, D.L.; Vigers, A.J.; Stone, L.; Hellekant, G.; Kinnamon, S.C. ATP Signaling Is Crucial for Communication from Taste Buds to Gustatory Nerves. Science 2005, 310, 1495–1499. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Ichikawa, R.; Hiasa, M.; Moriyama, Y.; Torii, K.; Uneyama, H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem. Biophys. Res. Commun. 2009, 388, 1–5. [Google Scholar] [CrossRef]
- Moriyama, Y.; Hiasa, M.; Sakamoto, S.; Omote, H.; Nomura, M. Vesicular nucleotide transporter (VNUT): Appearance of an actress on the stage of purinergic signaling. Purinergic Signal. 2017, 13, 387–404. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.L.; Sullivan, S.L.; Lavoie, E.G.; Sévigny, J.; Finger, T.E. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J. Comp. Neurol. 2006, 497, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vandenbeuch, A.; Anderson, C.B.; Parnes, J.; Enjyoji, K.; Robson, S.C.; Finger, T.E.; Kinnamon, S.C. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc. Natl. Acad. Sci. USA 2013, 110, 14789–14794. [Google Scholar] [CrossRef] [Green Version]
- Taruno, A.; Nomura, K.; Kusakizako, T.; Ma, Z.; Nureki, O.; Foskett, J.K. Taste transduction and channel synapses in taste buds. Pflügers Arch. Eur. J. Physiol. 2021, 473, 3–13. [Google Scholar] [CrossRef]
- Nishida, K.; Dohi, Y.; Yamanaka, Y.; Miyata, A.; Tsukamoto, K.; Yabu, M.; Ohishi, A.; Nagasawa, K. Expression of adenosine A2b receptor in rat type II and III taste cells. Histochem. Cell Biol. 2014, 141, 499–506. [Google Scholar] [CrossRef]
- Choo, E.; Picket, B.; Dando, R. Caffeine May Reduce Perceived Sweet Taste in Humans, Supporting Evidence That Adenosine Receptors Modulate Taste. J. Food Sci. 2017, 82, 2177–2182. [Google Scholar] [CrossRef]
- Henkin, R.I.; Knöppel, A.B.; Abdelmeguid, M.; Stateman, W.A.; Hosein, S. Theophylline increases saliva sonic hedgehog and improves taste dysfunction. Arch. Oral Biol. 2017, 82, 263–270. [Google Scholar] [CrossRef]
- Mistretta, C.M.; Kumari, A. Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation. Int. J. Mol. Sci. 2019, 20, 1341. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef] [Green Version]
- Janitschke, D.; Lauer, A.A.; Bachmann, C.M.; Seyfried, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Unique Role of Caffeine Compared to Other Methylxanthines (Theobromine, Theophylline, Pentoxifylline, Propentofylline) in Regulation of AD Relevant Genes in Neuroblastoma SH-SY5Y Wild Type Cells. Int. J. Mol. Sci. 2020, 21, 9015. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Proctor, G.B.; Shaalan, A.M. Disease-Induced Changes in Salivary Gland Function and the Composition of Saliva. J. Dent. Res. 2021, 100, 1201–1209. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Bielas, M.; Zalewska, A.; Gerreth, K. Salivary Biomarkers of Oxidative Stress and Inflammation in Stroke Patients: From Basic Research to Clinical Practice. Oxid. Med. Cell. Longev. 2021, 2021, 5545330. [Google Scholar] [CrossRef]
- Langman, L.J. The Use of Oral Fluid for Therapeutic Drug Management: Clinical and Forensic Toxicology. Ann. N. Y. Acad. Sci. 2007, 1098, 145–166. [Google Scholar] [CrossRef]
- Gallez, F.; Fadel, M.; Scruel, O.; Cantraine, F.; Courtois, P. Salivary biomass assessed by bioluminescence ATP assay related to (bacterial and somatic) cell counts. Cell Biochem. Funct. 2000, 18, 103–108. [Google Scholar] [CrossRef]
- Turner, J.T.; Weisman, G.A.; Landon, L.A.; Park, M.; Camden, J.M. Salivary gland nucleotide receptors: Evidence for functional expression of both P2X and P2Y subtypes. Eur. J. Morphol. 1998, 36, 170–175. [Google Scholar] [PubMed]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Dehaye, J.P. ATP4− Increases the intracellular calcium concentration in rat submandibular glands. Gen. Pharmacol. 1993, 24, 1097–1100. [Google Scholar] [CrossRef]
- Hurley, T.W.; Shoemaker, D.D.; Ryan, M.P. Extracellular ATP prevents the release of stored Ca2+ by autonomic agonists in rat submandibular gland acini. Am. J. Physiol. 1993, 265, C1472–C1478. [Google Scholar] [CrossRef]
- Métioui, M.; Amsallem, H.; Alzola, E.; Chaib, N.; Elyamani, A.; Moran, A.; Marino, A.; Dehaye, J.P. Low affinity purinergic receptor modulates the response of rat submandibular glands to carbachol and substance P. J. Cell. Physiol. 1996, 168, 462–475. [Google Scholar] [CrossRef]
- Novak, I.; Jans, I.M.; Wohlfahrt, L. Effect of P2X(7) receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J. Physiol. 2010, 588, 3615–3627. [Google Scholar] [CrossRef]
- Nakamoto, T.; Brown, D.A.; Catalán, M.A.; Gonzalez-Begne, M.; Romanenko, V.G.; Melvin, J.E. Purinergic P2X7 Receptors Mediate ATP-Induced Saliva Secretion by the Mouse Submandibular Gland. J. Biol. Chem. 2009, 284, 4815–4822. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Imbery, J.F.; Ampem, P.T.; Giovannucci, D.R. Crosstalk between purinergic receptors and canonical signaling pathways in the mouse salivary gland. Cell Calcium 2015, 58, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.A.; Bruce, J.I.E.; Straub, S.V.; Yule, D.I. cAMP Potentiates ATP-Evoked Calcium Signaling in Human Parotid Acinar Cells. J. Biol. Chem. 2004, 279, 39485–39494. [Google Scholar] [CrossRef] [Green Version]
- Baker, O.J.; Camden, J.M.; Ratchford, A.M.; Seye, C.I.; Erb, L.; Weisman, G.A. Differential coupling of the P2Y1 receptor to Gα14 and Gαq/11 proteins during the development of the rat salivary gland. Arch. Oral Biol. 2006, 51, 359–370. [Google Scholar] [CrossRef]
- Moriguchi-Mori, K.; Higashio, H.; Isobe, K.; Kumagai, M.; Sasaki, K.; Satoh, Y.-I.; Kuji, A.; Saino, T. P2Y purinoceptors mediate ATP-induced changes in intracellular calcium and amylase release in acinar cells of mouse parotid glands. Biomed. Res. 2016, 37, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Putney, J.W., Jr. Identification of Cellular Activation Mechanisms Associated with Salivary Secretion. Annu. Rev. Physiol. 1986, 48, 75–88. [Google Scholar] [CrossRef]
- Pereira, M.M.C.; Mills, C.L.; Dormer, R.L.; McPherson, M.A. Actions of adenosine A1 and A2 receptor antagonists on CFTR antibody-inhibited β-adrenergic mucin secretion response. Br. J. Pharmacol. 1998, 125, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Finkelberg, A.; Busch, L.; Reina, S.; Sterin-Borda, L.; Borda, E. Endogenous signalling system involved in parotid gland adenosine A(1) receptor-amylase release. Acta Physiol. 2006, 186, 29–36. [Google Scholar] [CrossRef]
- Ostuni, M.A.; Egido, P.; Peranzi, G.; Alonso, G.L.; Lacapere, J.-J.; Gonzalez, D.A. Characterization of a functional NTPDase in the endoplasmic reticulum of rat submandibular salivary gland. Physiol. Res. 2009, 58, 843–854. [Google Scholar] [CrossRef]
- González, D.A.; Egido, P.; Balcarcel, N.B.; Hattab, C.; van Haaster, M.M.B.; Pelletier, J.; Sévigny, J.; Ostuni, M.A. Rat submandibular glands secrete nanovesicles with NTPDase and 5′-nucleotidase activities. Purinergic Signal. 2015, 11, 107–116. [Google Scholar] [CrossRef] [Green Version]
- González, D.A.; van Haaster, M.M.B.; Villarruel, E.Q.; Hattab, C.; Ostuni, M.A.; Orman, B. Salivary extracellular vesicles can modulate purinergic signalling in oral tissues by combined ectonucleoside triphosphate diphosphohydrolases and ecto-5′-nucleotidase activities. Mol. Cell. Biochem. 2020, 463, 1–11. [Google Scholar] [CrossRef]
- Kittel, A.; Pelletier, J.; Bigonnesse, F.; Guckelberger, O.; Kordás, K.; Braun, N.; Robson, S.C.; Sévigny, J. Localization of Nucleoside Triphosphate Diphosphohydrolase-1 (NTPDase1) and NTPDase2 in Pancreas and Salivary Gland. J. Histochem. Cytochem. 2004, 52, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, E.G.; Gulbransen, B.D.; Martín-Satué, M.; Aliagas, E.; Sharkey, K.A.; Sévigny, J. Ectonucleotidases in the digestive system: Focus on NTPDase3 localization. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, 608–620. [Google Scholar] [CrossRef]
- Ishibashi, K.; Okamura, K.; Yamazaki, J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl− reabsorption in rat submandibular gland. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1729–R1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.-X.; Wall, N.R.; Angelov, N.; Ririe, C.; Chen, J.W.; Boskovic, D.S.; Henkin, J.M. Changes in mRNA expression of adenosine receptors in human chronic periodontitis. Chin. J. Dent. Res. 2011, 14, 113–120. [Google Scholar] [PubMed]
- Yilmaz, Ö.; Yao, L.; Maeda, K.; Rose, T.M.; Lewis, E.L.; Duman, M.; Lamont, R.J.; Ojcius, D.M. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell. Microbiol. 2008, 10, 863–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasova, K.R.; Yilmaz, Ö. Inflammasome Activation in Gingival Epithelial Cells. Methods Mol. Biol. 2022, 2459, 149–167. [Google Scholar] [CrossRef]
- Ito, M.; Arakawa, T.; Okayama, M.; Shitara, A.; Mizoguchi, I.; Takuma, T. Gravity loading induces adenosine triphosphate release and phosphorylation of extracellular signal-regulated kinases in human periodontal ligament cells. J. Investig. Clin. Dent. 2014, 5, 266–274. [Google Scholar] [CrossRef]
- Menéndez-Diaz, I.; Muriel, J.D.; García-Suárez, O.; Obaya, A.; Cal, S.; Cobo, J.; Vega, J.A.; Cobo, T. Periostin, dentin matrix protein 1 and P2rx7 ion channel in human teeth and periodontal ligament. Ann. Anat. 2018, 216, 103–111. [Google Scholar] [CrossRef]
- Ikeda, E.; Goto, T.; Gunjigake, K.; Kuroishi, K.; Ueda, M.; Kataoka, S.; Toyono, T.; Nakatomi, M.; Seta, Y.; Kitamura, C.; et al. Expression of Vesicular Nucleotide Transporter in Rat Odontoblasts. Acta Histochem. Cytochem. 2016, 49, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.M.; Jo, H.; Park, G.; Kim, Y.H.; Park, C.K.; Jung, S.J.; Chung, G.; Oh, S.B. Extracellular ATP Induces Calcium Signaling in Odontoblasts. J. Dent. Res. 2017, 96, 200–207. [Google Scholar] [CrossRef]
- Wang, W.; Yi, X.; Ren, Y.; Xie, Q. Effects of Adenosine Triphosphate on Proliferation and Odontoblastic Differentiation of Human Dental Pulp Cells. J. Endod. 2016, 42, 1483–1489. [Google Scholar] [CrossRef]
- Yi, X.; Wang, W.; Xie, Q. Adenosine receptors enhance the ATP-induced odontoblastic differentiation of human dental pulp cells. Biochem. Biophys. Res. Commun. 2018, 497, 850–856. [Google Scholar] [CrossRef]
- Yamagishi, H.; Shigematsu, K. Perspectives on Stem Cell-Based Regenerative Medicine with a Particular Emphasis on Mesenchymal Stem Cell Therapy. JMA J. 2022, 5, 36–43. [Google Scholar] [CrossRef]
- Smojver, I.; Katalinić, I.; Bjelica, R.; Gabrić, D.; Matišić, V.; Molnar, V.; Primorac, D. Mesenchymal Stem Cells Based Treatment in Dental Medicine: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 1662. [Google Scholar] [CrossRef]
- Iwamoto, T.; Nakamura, T.; Ishikawa, M.; Yoshizaki, K.; Sugimoto, A.; Ida-Yonemochi, H.; Ohshima, H.; Saito, M.; Yamada, Y.; Fukumoto, S. Pannexin 3 regulates proliferation and differentiation of odontoblasts via its hemichannel activities. PLoS ONE 2017, 12, e0177557. [Google Scholar] [CrossRef] [Green Version]
- Yamada, A.; Yoshizaki, K.; Ishikawa, M.; Saito, K.; Chiba, Y.; Fukumoto, E.; Hino, R.; Hoshikawa, S.; Chiba, M.; Nakamura, T.; et al. Connexin 43-Mediated Gap Junction Communication Regulates Ameloblast Differentiation via ERK1/2 Phosphorylation. Front. Physiol. 2021, 12, 748574. [Google Scholar] [CrossRef]
- Peng, B.; Xu, C.; Wang, S.; Zhang, Y.; Li, W. The Role of Connexin Hemichannels in Inflammatory Diseases. Biology 2022, 11, 237. [Google Scholar] [CrossRef]
- D’Alimonte, I.; Nargi, E.; Lannutti, A.; Marchisio, M.; Pierdomenico, L.; Costanzo, G.; Di Iorio, P.; Ballerini, P.; Giuliani, P.; Caciagli, F.; et al. Adenosine A1 receptor stimulation enhances osteogenic differentiation of human dental pulp-derived mesenchymal stem cells via WNT signaling. Stem Cell Res. 2013, 11, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Sivasankar, V.; Ranganathan, K. Growth characteristics and expression of CD73 and CD146 in cells cultured from dental pulp. J. Investig. Clin. Dent. 2016, 7, 278–285. [Google Scholar] [CrossRef]
- Peng, H.; Hao, Y.; Mousawi, F.; Roger, S.; Li, J.; Sim, J.A.; Ponnambalam, S.; Yang, X.; Jiang, L.-H. Purinergic and Store-Operated Ca2+ Signaling Mechanisms in Mesenchymal Stem Cells and Their Roles in ATP-Induced Stimulation of Cell Migration. Stem Cells 2016, 34, 2102–2114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Ye, D.; Ma, L.; Ren, Y.-F.; Dirksen, R.T.; Liu, X. Purinergic Signaling Modulates Survival/Proliferation of Human Dental Pulp Stem Cells. J. Dent. Res. 2019, 98, 242–249. [Google Scholar] [CrossRef]
- Gao, Q.; Cooper, P.R.; Walmsley, A.D.; Scheven, B.A. Role of Piezo Channels in Ultrasound-Stimulated Dental Stem Cells. J. Endod. 2017, 43, 1130–1136. [Google Scholar] [CrossRef] [Green Version]
- Mousawi, F.; Peng, H.; Li, J.; Ponnambalam, S.; Roger, S.; Zhao, H.; Yang, X.; Jiang, L.-H. Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells 2020, 38, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.H. The orotrigeminal system. Handb. Clin. Neurol. 2019, 164, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. Dental nerves: A neglected mediator of pulpitis. Int. Endod. J. 2021, 54, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.T.; Camden, J.M.; Batek, J.M.; Petris, M.J.; Erb, L.; Weisman, G.A. P2X7 receptor activation induces inflammatory responses in salivary gland epithelium. Am. J. Physiol. Cell Physiol. 2012, 303, C790–C801. [Google Scholar] [CrossRef] [Green Version]
- Soare, A.Y.; Freeman, T.L.; Min, A.K.; Malik, H.S.; Osota, E.O.; Swartz, T.H. P2RX7 at the Host-Pathogen Interface of Infectious Diseases. Microbiol. Mol. Biol. Rev. 2021, 85, e00055-20. [Google Scholar] [CrossRef]
- Ramos-Junior, E.S.; Morandini, A.C.; Almeida-da-Silva, C.L.; Franco, E.J.; Potempa, J.; Nguyen, K.A.; Oliveira, A.C.; Zamboni, D.S.; Ojcius, D.M.; Scharfstein, J.; et al. A Dual Role for P2X7 Receptor during Porphyromonas gingivalis Infection. J. Dent. Res. 2015, 94, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Almeida-da-Silva, C.L.C.; Ramos-Junior, E.S.; Morandini, A.C.; Rocha, G.D.C.; Marinho, Y.; Tamura, A.S.; de Andrade, K.Q.; Bellio, M.; Savio, L.E.B.; Scharfstein, J.; et al. P2X7 receptor-mediated leukocyte recruitment and Porphyromonas gingivalis clearance requires IL-1β production and autocrine IL-1 receptor activation. Immunobiology 2019, 224, 50–59. [Google Scholar] [CrossRef]
- Spari, D.; Beldi, G. Extracellular ATP as an Inter-Kingdom Signaling Molecule: Release Mechanisms by Bacteria and Its Implication on the Host. Int. J. Mol. Sci. 2020, 21, 5590. [Google Scholar] [CrossRef]
- Binderman, I.; Gadban, N.; Yaffe, A. Extracellular ATP is a key modulator of alveolar bone loss in periodontitis. Arch. Oral Biol. 2017, 81, 131–135. [Google Scholar] [CrossRef]
- De Andrade, K.Q.; Almeida-da-Silva, C.L.C.; Ojcius, D.M.; Coutinho-Silva, R. Differential involvement of the canonical and noncanonical inflammasomes in the immune response against infection by the periodontal bacteria Porphyromonas gingivalis and Fusobacterium nucleatum. Curr. Res. Microb. Sci. 2021, 2, 100023. [Google Scholar] [CrossRef]
- Nathan, C.F.; Hibbs, J.B. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991, 3, 65–70. [Google Scholar] [CrossRef]
- Murakami, S.; Hashikawa, T.; Saho, T.; Takedachi, M.; Nozaki, T.; Shimabukuro, Y.; Okada, H. Adenosine regulates the IL-1β-induced cellular functions of human gingival fibroblasts. Int. Immunol. 2001, 13, 1533–1540. [Google Scholar] [CrossRef]
- Ramos-Junior, E.S.; Pedram, M.; Lee, R.E.; Exstrom, D.; Yilmaz, Ö.; Coutinho-Silva, R.; Ojcius, D.M.; Morandini, A.C. CD73-dependent adenosine dampens interleukin-1β-induced CXCL8 production in gingival fibroblasts: Association with heme oxygenase-1 and adenosine monophosphate-activated protein kinase. J. Periodontol. 2020, 91, 253–262. [Google Scholar] [CrossRef]
- Spooner, R.; DeGuzman, J.; Lee, K.L.; Yilmaz, Ö. Danger signal adenosine via adenosine 2a receptor stimulates growth of Porphyromonas gingivalis in primary gingival epithelial cells. Mol. Oral Microbiol. 2014, 29, 67–78. [Google Scholar] [CrossRef]
- Lee, J.S.; Yilmaz, Ö. Unfolding Role of a Danger Molecule Adenosine Signaling in Modulation of Microbial Infection and Host Cell Response. Int. J. Mol. Sci. 2018, 19, 199. [Google Scholar] [CrossRef] [Green Version]
- Mörnstad, H.; Sundström, B. Adenosine triphosphate hydrolysis in rat dental tissues. A histochemical study to differentiate the enzymes involved. Histochem. Cell Biol. 1976, 47, 303–314. [Google Scholar] [CrossRef]
- González, D.A.; van Haaster, M.M.B.; Villarruel, E.Q.; Brandt, M.; Benítez, M.B.; Stranieri, G.M.; Orman, B. Histamine stimulates secretion of extracellular vesicles with nucleotidase activity in rat submandibular gland. Arch. Oral Biol. 2018, 85, 201–206. [Google Scholar] [CrossRef]
- Nemoto, E.; Gotoh, K.; Tsuchiya, M.; Sakisaka, Y.; Shimauchi, H. Extracellular ATP inhibits IL-1-induced MMP-1 expression through the action of CD39/nucleotidase triphosphate dephosphorylase-1 on human gingival fibroblasts. Int. Immunopharmacol. 2013, 17, 513–518. [Google Scholar] [CrossRef]
- Batista, E.L.B., Jr.; Deves, C.; Ayub, L.; da Silva, R.G.; Filho, L.C.C.; Basso, L.A.; Santos, D.S. Purine nucleoside phosphorylase activity and expression are upregulated in sites affected by periodontal disease. J. Periodontal Res. 2010, 45, 664–671. [Google Scholar] [CrossRef]
- Deves, C.; de Assunção, T.M.; Ducati, R.G.; Campos, M.M.; Basso, L.A.; Santos, D.S.; Batista, E.L., Jr. The transition state analog inhibitor of Purine Nucleoside Phosphorylase (PNP) Immucillin-H arrests bone loss in rat periodontal disease models. Bone 2013, 52, 167–175. [Google Scholar] [CrossRef]
- González-Ramírez, R.; Chen, Y.; Liedtke, W.B.; Morales-Lázaro, S.L. TRP Channels and Pain. In Neurobiology of TRP Channels; Emir, T.L.R., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; Chapter 8. [Google Scholar]
- Shibukawa, Y.; Sato, M.; Kimura, M.; Sobhan, U.; Shimada, M.; Nishiyama, A.; Kawaguchi, A.; Soya, M.; Kuroda, H.; Katakura, A.; et al. Odontoblasts as sensory receptors: Transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transduction. Pflugers Arch. Eur. J. Physiol. 2015, 467, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Yang, Y.; Ji, P.; Kong, J.; Wu, Q.; Si, H. Upregulation of the Purinergic Receptor Subtype P2X3 in the Trigeminal Ganglion Is Involved in Orofacial Pain Induced by Occlusal Interference in Rats. J. Oral Facial Pain Headache 2016, 30, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A.; Sato, M.; Kimura, M.; Ichinohe, T.; Tazaki, M.; Shibukawa, Y. Expression and function of purinergic P2Y12 receptors in rat trigeminal ganglion neurons. Neurosci. Res. 2015, 98, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, S.; Okada, S.; Katagiri, A.; Saito, H.; Suzuki, T.; Komiya, H.; Kanno, K.; Ohara, K.; Iinuma, T.; Toyofuku, A.; et al. Interaction between calcitonin gene-related peptide-immunoreactive neurons and satellite cells via P2Y12 R in the trigeminal ganglion is involved in neuropathic tongue pain in rats. Eur. J. Oral Sci. 2017, 125, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Sowa, N.A.; Taylor-Blake, B.; Zylka, M.J. Ecto-5′-Nucleotidase (CD73) Inhibits Nociception by Hydrolyzing AMP to Adenosine in Nociceptive Circuits. J. Neurosci. 2010, 30, 2235–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawynok, J. Adenosine receptor targets for pain. Neuroscience 2015, 338, 1–18. [Google Scholar] [CrossRef]
- Ma, L.; Trinh, T.; Ren, Y.; Dirksen, R.T.; Liu, X. Neuronal NTPDase3 Mediates Extracellular ATP Degradation in Trigeminal Nociceptive Pathway. PLoS ONE 2016, 11, e0164028. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Wang, Q.; Pelletier, J.; Fausther, M.; Sévigny, J.; Malmström, H.S.; Dirksen, R.T.; Ren, Y.-F. Expression of Ecto-ATPase NTPDase2 in Human Dental Pulp. J. Dent. Res. 2012, 91, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ma, L.; Zhang, S.; Ren, Y.-F.; Dirksen, R.T. CD73 Controls Extracellular Adenosine Generation in the Trigeminal Nociceptive Nerves. J. Dent. Res. 2017, 96, 671–677. [Google Scholar] [CrossRef]
- Ahn, J.S.; Camden, J.M.; Schrader, A.M.; Redman, R.S.; Turner, J.T. Reversible regulation of P2Y(2) nucleotide receptor expression in the duct-ligated rat submandibular gland. Am. J. Physiol. Cell Physiol. 2000, 279, C286–C294. [Google Scholar] [CrossRef]
- Hung, S.-C.; Choi, C.H.; Said-Sadier, N.; Johnson, L.; Atanasova, K.R.; Sellami, H.; Yilmaz, Ö.; Ojcius, D.M. P2X4 Assembles with P2X7 and Pannexin-1 in Gingival Epithelial Cells and Modulates ATP-Induced Reactive Oxygen Species Production and Inflammasome Activation. PLoS ONE 2013, 8, e70210. [Google Scholar] [CrossRef] [Green Version]
- Binderman, I.; Bahar, H.; Jacob-Hirsch, J.; Zeligson, S.; Amariglio, N.; Rechavi, G.; Shoham, S.; Yaffe, A. P2X4 Is Up-Regulated in Gingival Fibroblasts after Periodontal Surgery. J. Dent. Res. 2007, 86, 181–185. [Google Scholar] [CrossRef]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Q.; Zhao, L.; Mao, J.; Huang, W.; Han, X.; Liu, Y. Periodontal Ligament Stem Cell-Derived Small Extracellular Vesicles Embedded in Matrigel Enhance Bone Repair through the Adenosine Receptor Signaling Pathway. Int. J. Nanomed. 2022, 17, 519–536. [Google Scholar] [CrossRef]
- Verma, N.K.; Kar, A.K.; Singh, A.; Jagdale, P.; Satija, N.K.; Ghosh, D.; Patnaik, S. Control Release of Adenosine Potentiate Osteogenic Differentiation within a Bone Integrative EGCG-g-NOCC/Collagen Composite Scaffold toward Guided Bone Regeneration in a Critical-Sized Calvarial Defect. Biomacromolecules 2021, 22, 3069–3083. [Google Scholar] [CrossRef]
- Emmerson, E.; May, A.J.; Berthoin, L.; Cruz-Pacheco, N.; Nathan, S.; Mattingly, A.J.; Chang, J.L.; Ryan, W.R.; Tward, A.D.; Knox, S.M. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol. Med. 2018, 10, e8051. [Google Scholar] [CrossRef]
- Yu, X.; Riemekasten, G.; Petersen, F. Autoantibodies against muscarinic acetylcholine receptor M3 in Sjogren’s syndrome and corresponding mouse models. Front. Biosci. 2018, 23, 2053–2064. [Google Scholar] [CrossRef]
- D’Agostino, C.; Elkashty, O.A.; Chivasso, C.; Perret, J.; Tran, S.D.; Delporte, C. Insight into Salivary Gland Aquaporins. Cells 2020, 9, 1547. [Google Scholar] [CrossRef]
- Jasmer, K.J.; Gilman, K.E.; Forti, K.M.; Weisman, G.A.; Limesand, K.H. Radiation-Induced Salivary Gland Dysfunction: Mechanisms, Therapeutics and Future Directions. J. Clin. Med. 2020, 9, 4095. [Google Scholar] [CrossRef]
- Khalafalla, M.G.; Woods, L.T.; Camden, J.M.; Khan, A.A.; Limesand, K.H.; Petris, M.J.; Erb, L.; Weisman, G.A. P2X7 receptor antagonism prevents IL-1β release from salivary epithelial cells and reduces inflammation in a mouse model of autoimmune exocrinopathy. J. Biol. Chem. 2017, 292, 16626–16637. [Google Scholar] [CrossRef] [Green Version]
- Gilman, K.E.; Camden, J.M.; Klein, R.R.; Zhang, Q.; Weisman, G.A.; Limesand, K.H. P2X7 receptor deletion suppresses γ-radiation-induced hyposalivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R687–R696. [Google Scholar] [CrossRef]
- Schrader, A.M.; Camden, J.M.; Weisman, G.A. P2Y2 nucleotide receptor up-regulation in submandibular gland cells from the NOD.B10 mouse model of Sjögren’s syndrome. Arch. Oral Biol. 2005, 50, 533–540. [Google Scholar] [CrossRef]
- Jasmer, K.J.; Woods, L.T.; Forti, K.M.; Martin, A.L.; Camden, J.M.; Colonna, M.; Weisman, G.A. P2Y2 receptor antagonism resolves sialadenitis and improves salivary flow in a Sjögren’s syndrome mouse model. Arch. Oral Biol. 2021, 124, 105067. [Google Scholar] [CrossRef]
- Woods, L.T.; Camden, J.M.; Khalafalla, M.G.; Petris, M.J.; Erb, L.; Ambrus, J.L., Jr.; Weisman, G.A. P2Y2 R deletion ameliorates sialadenitis in IL-14α-transgenic mice. Oral Dis. 2018, 24, 761–771. [Google Scholar] [CrossRef]
- Harris, T.H.; Wallace, M.R.; Huang, H.; Li, H.; Mohiuddeen, A.; Gong, Y.; Kompotiati, T.; Harrison, P.; Aukhil, I.; Shaddox, L.M. Association of P2RX7 functional variants with localized aggressive periodontitis. J. Periodontal Res. 2020, 55, 32–40. [Google Scholar] [CrossRef]
- Lester, S.; Stokes, L.; Skarratt, K.; Gu, B.J.; Sivils, K.L.; Lessard, C.J.; Wiley, J.S.; Rischmueller, M. Epistasis with HLA DR3 implicates the P2X7 receptor in the pathogenesis of primary Sjögren’s syndrome. Arthritis Res. Ther. 2013, 15, R71. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; Lavado, N.; Nogueira, L.; Lopez, M.; Abreu, J.; Silva, H. Polymorphisms of genes encoding P2X7R, IL-1B, OPG and RANK in orthodontic-induced apical root resorption. Oral Dis. 2014, 20, 659–667. [Google Scholar] [CrossRef]
- Linhartova, P.B.; Cernochova, P.; Kastovsky, J.; Vrankova, Z.; Sirotkova, M.; Holla, L.I. Genetic determinants and post orthodontic external apical root resorption in Czech children. Oral Dis. 2017, 23, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).