Control of Directed Cell Migration after Tubular Cell Injury by Nucleotide Signaling
Abstract
1. Introduction
2. Results
2.1. Adenosine Receptors Support the Repair of Laser-Induced Zebrafish Pronephros Injuries
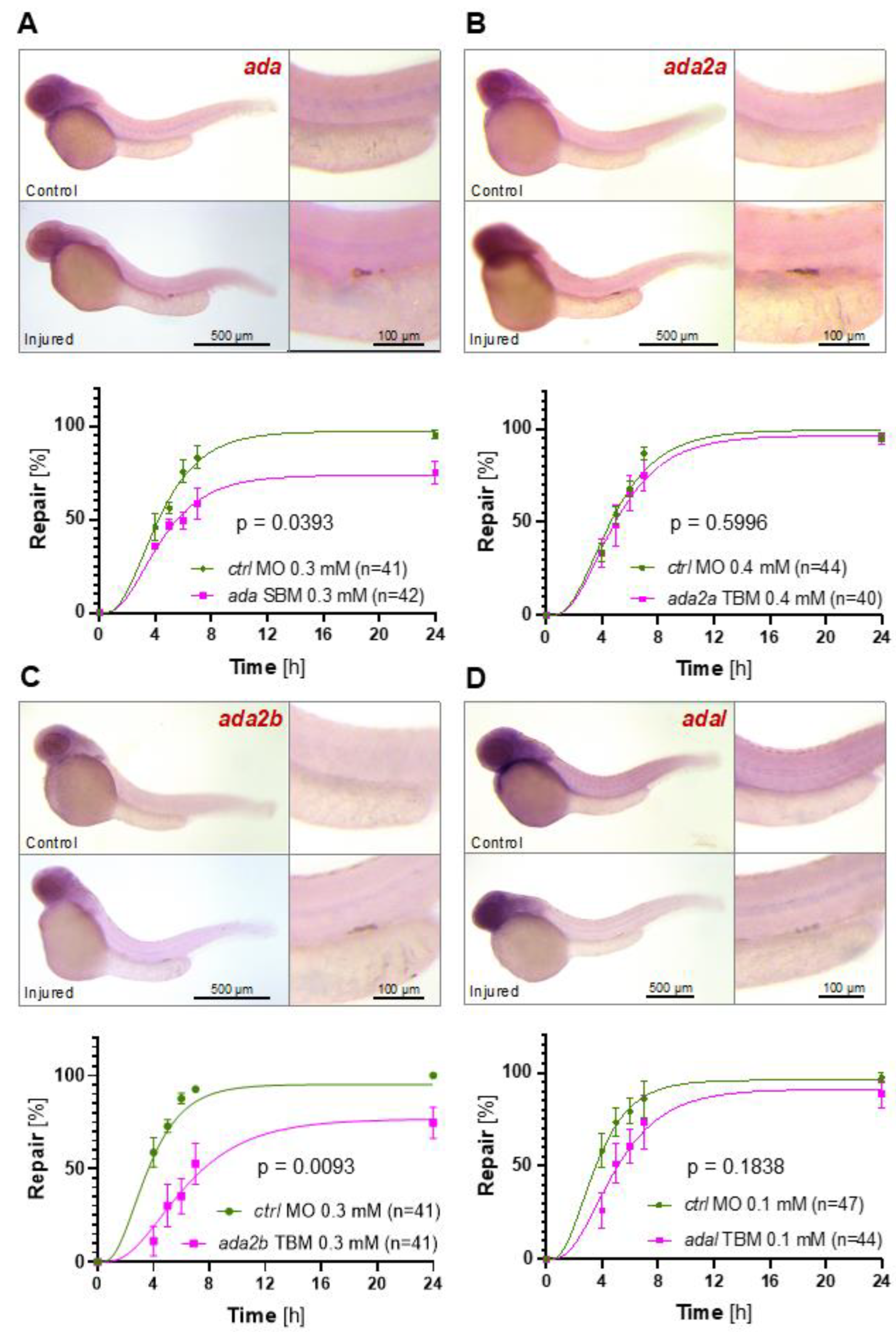
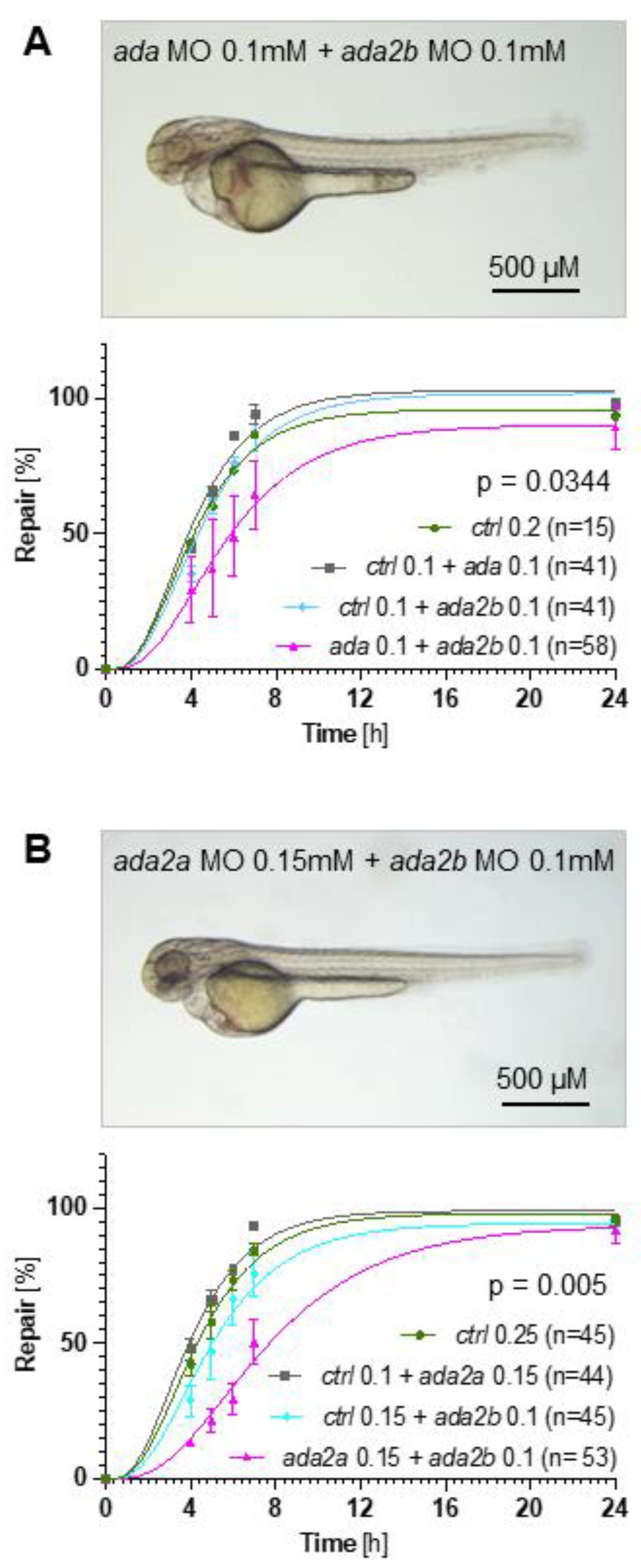
2.2. Adenosine Degradation Represents an Essential Component of the Repair Process
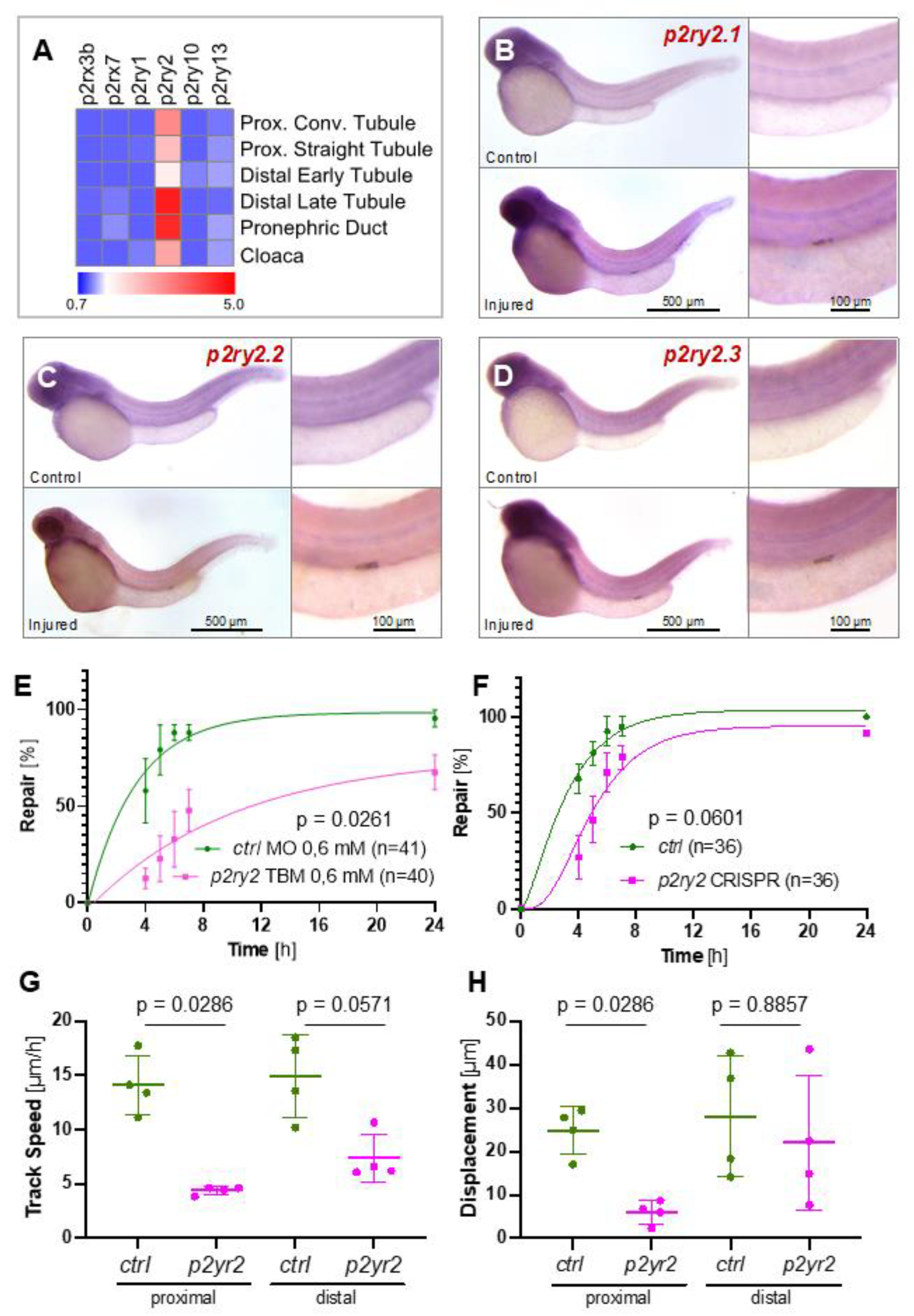
2.3. The ATP-Sensing Purinergic P2RY2 Receptor Is Required for a Normal Migratory Response after Pronephros Injury
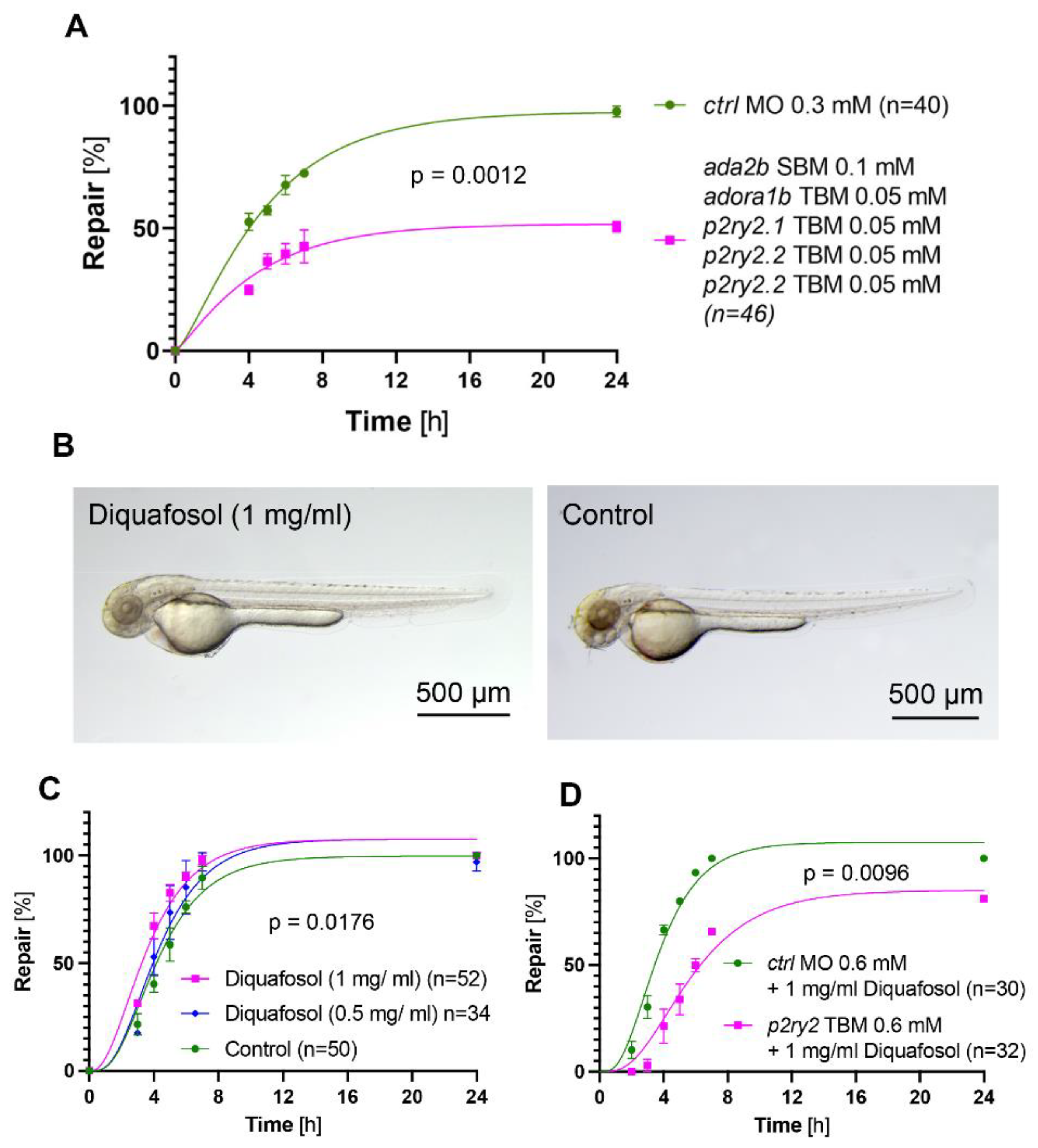
2.4. The Adenosine Pathway Is Required for the Pronephros Repair Process
2.5. Activation of Purinergic Signaling Promotes Pronephros Repair
3. Discussion
4. Materials and Methods
4.1. Zebrafish Lines Maintenance and Treatment
4.2. Data Analysis and Visualization
4.3. Laser Ablation, Image Acquisition and Migration Quantification
4.4. Whole-Mount In Situ Hybridization (WISH)
4.5. CRISPR/Cas9 Gene Targeting
4.6. Morpholino Oligonucleotides (MOs) Gene Targeting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noble, R.A.; Lucas, B.J.; Selby, N.M. Long-Term Outcomes in Patients with Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2020, 15, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Czerniak, S.; DiRocco, D.P.; Hasnain, W.; Cheema, R.; Bonventre, J.V. Repair of Injured Proximal Tubule Does Not Involve Specialized Progenitors. Proc. Natl. Acad. Sci. USA 2011, 108, 9226–9231. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated Kidney Epithelial Cells Repair Injured Proximal Tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Kusaba, T.; Humphreys, B.D. Who Regenerates the Kidney Tubule? Nephrol. Dial. Transplant. 2015, 30, 903–910. [Google Scholar] [CrossRef]
- Chang-Panesso, M.; Humphreys, B.D. Cellular Plasticity in Kidney Injury and Repair. Nat. Rev. Nephrol. 2017, 13, 39–46. [Google Scholar] [CrossRef]
- Palmyre, A.; Lee, J.; Ryklin, G.; Camarata, T.; Selig, M.K.; Duchemin, A.-L.; Nowak, P.; Arnaout, M.A.; Drummond, I.A.; Vasilyev, A. Collective Epithelial Migration Drives Kidney Repair after Acute Injury. PLoS ONE 2014, 9, e101304. [Google Scholar] [CrossRef]
- Yakulov, T.A.; Todkar, A.P.; Slanchev, K.; Wiegel, J.; Bona, A.; Groß, M.; Scholz, A.; Hess, I.; Wurditsch, A.; Grahammer, F.; et al. CXCL12 and MYC Control Energy Metabolism to Support Adaptive Responses after Kidney Injury. Nat. Commun. 2018, 9, 3660. [Google Scholar] [CrossRef]
- Grygorczyk, R.; Boudreault, F.; Ponomarchuk, O.; Tan, J.J.; Furuya, K.; Goldgewicht, J.; Kenfack, F.D.; Yu, F. Lytic Release of Cellular ATP: Physiological Relevance and Therapeutic Applications. Life 2021, 11, 700. [Google Scholar] [CrossRef]
- Linden, J. Cell Biology. Purinergic Chemotaxis. Science 2006, 314, 1689–1690. [Google Scholar] [CrossRef]
- Bauerle, J.D.; Grenz, A.; Kim, J.-H.; Lee, H.T.; Eltzschig, H.K. Adenosine Generation and Signaling during Acute Kidney Injury. J. Am. Soc. Nephrol. 2011, 22, 14–20. [Google Scholar] [CrossRef]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine Receptors: Therapeutic Aspects for Inflammatory and Immune Diseases. Nat. Rev. Drug. Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Grenz, A.; Homann, D.; Eltzschig, H.K. Extracellular Adenosine: A Safety Signal That Dampens Hypoxia-Induced Inflammation during Ischemia. Antioxid. Redox. Signal. 2011, 15, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.I.; Tam, F.W.; Unwin, R.J.; Bailey, M.A. Purinergic Signaling in Kidney Disease. Kidney. Int. 2017, 91, 315–323. [Google Scholar] [CrossRef]
- Vallon, V.; Unwin, R.; Inscho, E.W.; Leipziger, J.; Kishore, B.K. Extracellular Nucleotides and P2 Receptors in Renal Function. Physiol. Rev. 2020, 100, 211–269. [Google Scholar] [CrossRef] [PubMed]
- Weihofen, W.A.; Liu, J.; Reutter, W.; Saenger, W.; Fan, H. Crystal Structure of CD26/Dipeptidyl-Peptidase IV in Complex with Adenosine Deaminase Reveals a Highly Amphiphilic Interface. J. Biol. Chem. 2004, 279, 43330–43335. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zajac, B.; Mierzejewska, P.; Slominska, E.M.; Smolenski, R.T. Therapeutic Perspectives of Adenosine Deaminase Inhibition in Cardiovascular Diseases. Molecules. 2020, 25, 4652. [Google Scholar] [CrossRef]
- Flinn, A.M.; Gennery, A.R. Adenosine Deaminase Deficiency: A Review. Orphanet. J. Rare. Dis. 2018, 13, 65. [Google Scholar] [CrossRef]
- El-Brolosy, M.A.; Kontarakis, Z.; Rossi, A.; Kuenne, C.; Günther, S.; Fukuda, N.; Kikhi, K.; Boezio, G.L.M.; Takacs, C.M.; Lai, S.-L.; et al. Genetic Compensation Triggered by Mutant MRNA Degradation. Nature 2019, 568, 193–197. [Google Scholar] [CrossRef]
- Peng, J. Gene Redundancy and Gene Compensation: An Updated View. J. Genet. Genom. 2019, 46, 329–333. [Google Scholar] [CrossRef]
- Vasilyev, A.; Liu, Y.; Mudumana, S.; Mangos, S.; Lam, P.-Y.; Majumdar, A.; Zhao, J.; Poon, K.-L.; Kondrychyn, I.; Korzh, V.; et al. Collective Cell Migration Drives Morphogenesis of the Kidney Nephron. PLoS Biol. 2009, 7, e9. [Google Scholar] [CrossRef]
- Schoels, M.; Zhuang, M.; Fahrner, A.; Küchlin, S.; Sagar; Franz, H.; Schmitt, A.; Walz, G.; Yakulov, T.A. Single-Cell MRNA Profiling Reveals Changes in Solute Carrier Expression and Suggests a Metabolic Switch during Zebrafish Pronephros Development. Am. J. Physiol. Renal Physiol. 2021, 320, F826–F837. [Google Scholar] [CrossRef] [PubMed]
- von Kügelgen, I. Pharmacology of P2Y Receptors. Brain Res. Bull. 2019, 151, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.; Amor, S.; Montoya, J.J.; Monge, L.; Fernández, N.; García-Villalón, Á.L. Altered Expression of P2Y2 and P2X7 Purinergic Receptors in the Isolated Rat Heart Mediates Ischemia-Reperfusion Injury. Vascul. Pharmacol. 2015, 73, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.-X.; Chen, S.-F.; Xue, S.-X.; Zhang, X.-Z.; Lian, Y.-J. P2RY2 Alleviates Cerebral Ischemia-Reperfusion Injury by Inhibiting YAP Phosphorylation and Reducing Mitochondrial Fission. Neuroscience 2022, 480, 155–166. [Google Scholar] [CrossRef]
- Thadhani, R.; Pascual, M.; Bonventre, J.V. Acute Renal Failure. N. Engl. J. Med. 1996, 334, 1448–1460. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular Pathophysiology of Ischemic Acute Kidney Injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute Kidney Injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute Kidney Injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 Initiative for Acute Kidney Injury (Zero Preventable Deaths by 2025): A Human Rights Case for Nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic Epithelial Cells Repair the Kidney after Injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, E.; Angelotti, M.L.; Peired, A.; Conte, C.; Marschner, J.A.; Maggi, L.; Mazzinghi, B.; Lombardi, D.; Melica, M.E.; Nardi, S.; et al. Endocycle-Related Tubular Cell Hypertrophy and Progenitor Proliferation Recover Renal Function after Acute Kidney Injury. Nat. Commun. 2018, 9, 1344. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Gusella, G.L.; He, J.C. Epithelial Proliferation and Cell Cycle Dysregulation in Kidney Injury and Disease. Kidney Int. 2021, 100, 67–78. [Google Scholar] [CrossRef]
- Kitching, A.R.; Hickey, M.J. Immune Cell Behaviour and Dynamics in the Kidney-Insights from in Vivo Imaging. Nat. Rev. Nephrol. 2022, 18, 22–37. [Google Scholar] [CrossRef]
- Franco, R.; Cordomí, A.; Llinas Del Torrent, C.; Lillo, A.; Serrano-Marín, J.; Navarro, G.; Pardo, L. Structure and Function of Adenosine Receptor Heteromers. Cell. Mol. Life Sci. 2021, 78, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Rivas-Santisteban, R.; Reyes-Resina, I.; Navarro, G. The Old and New Visions of Biased Agonism Through the Prism of Adenosine Receptor Signaling and Receptor/Receptor and Receptor/Protein Interactions. Front. Pharmacol. 2020, 11, 628601. [Google Scholar] [CrossRef] [PubMed]
- Rabadi, M.; Kim, M.; Li, H.; Han, S.J.; Choi, Y.; D’Agati, V.; Lee, H.T. ATP Induces PAD4 in Renal Proximal Tubule Cells via P2X7 Receptor Activation to Exacerbate Ischemic AKI. Am. J. Physiol. Renal. Physiol. 2018, 314, F293–F305. [Google Scholar] [CrossRef]
- Han, S.J.; Lovaszi, M.; Kim, M.; D’Agati, V.; Haskó, G.; Lee, H.T. P2X4 Receptor Exacerbates Ischemic AKI and Induces Renal Proximal Tubular NLRP3 Inflammasome Signaling. FASEB J. 2020, 34, 5465–5482. [Google Scholar] [CrossRef]
- Breton, S.; Brown, D. Novel Proinflammatory Function of Renal Intercalated Cells. Ann. Nutr. Metab. 2018, 72 (Suppl. 2), 11–16. [Google Scholar] [CrossRef]
- Potthoff, S.A.; Stegbauer, J.; Becker, J.; Wagenhaeuser, P.J.; Duvnjak, B.; Rump, L.C.; Vonend, O. P2Y2 Receptor Deficiency Aggravates Chronic Kidney Disease Progression. Front. Physiol. 2013, 4, 234. [Google Scholar] [CrossRef]
- Cohen, R.; Shainberg, A.; Hochhauser, E.; Cheporko, Y.; Tobar, A.; Birk, E.; Pinhas, L.; Leipziger, J.; Don, J.; Porat, E. UTP Reduces Infarct Size and Improves Mice Heart Function after Myocardial Infarct via P2Y2 Receptor. Biochem. Pharmacol. 2011, 82, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio); University of Oregon: Eugene, OR, USA, 2007. [Google Scholar]
- Zhou, Q.; Yang, D.; Ombrello, A.K.; Zavialov, A.V.; Toro, C.; Zavialov, A.V.; Stone, D.L.; Chae, J.J.; Rosenzweig, S.D.; Bishop, K.; et al. Early-Onset Stroke and Vasculopathy Associated with Mutations in ADA2. N. Engl. J. Med. 2014, 370, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-Y.; Ching, Y.-H.; Chang, T.-Y.; Hu, L.-S.; Yong, Y.S.; Keak, P.Y.; Mustika, I.; Lin, M.-D.; Liao, B.-Y. Novel Eye Genes Systematically Discovered through an Integrated Analysis of Mouse Transcriptomes and Phenome. Comput. Struct. Biotechnol. J. 2020, 18, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.P.; Machado Torresini, F.; Nery, L.R.; da Silva, R.S. Transient Disruption of Adenosine Signaling During Embryogenesis Triggers a Pro-Epileptic Phenotype in Adult Zebrafish. Mol. Neurobiol. 2018, 55, 6547–6557. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Tamplin, O.J.; Chen, M.J.; Deng, Q.; Patterson, S.; Kim, P.G.; Durand, E.M.; McNeil, A.; Green, J.M.; Matsuura, S.; et al. Adenosine Signaling Promotes Hematopoietic Stem and Progenitor Cell Emergence. J. Exp. Med. 2015, 212, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Andersson, O.; Adams, B.A.; Yoo, D.; Ellis, G.C.; Gut, P.; Anderson, R.M.; German, M.S.; Stainier, D.Y.R. Adenosine Signaling Promotes Regeneration of Pancreatic β Cells in Vivo. Cell Metab. 2012, 15, 885–894. [Google Scholar] [CrossRef]
- Fontenas, L.; Welsh, T.G.; Piller, M.; Coughenour, P.; Gandhi, A.V.; Prober, D.A.; Kucenas, S. The Neuromodulator Adenosine Regulates Oligodendrocyte Migration at Motor Exit Point Transition Zones. Cell Rep. 2019, 27, 115–128.e5. [Google Scholar] [CrossRef]
- Robu, M.E.; Larson, J.D.; Nasevicius, A.; Beiraghi, S.; Brenner, C.; Farber, S.A.; Ekker, S.C. P53 Activation by Knockdown Technologies. PLoS Genet. 2007, 3, e78. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gessler, S.; Guthmann, C.; Schuler, V.; Lilienkamp, M.; Walz, G.; Yakulov, T.A. Control of Directed Cell Migration after Tubular Cell Injury by Nucleotide Signaling. Int. J. Mol. Sci. 2022, 23, 7870. https://doi.org/10.3390/ijms23147870
Gessler S, Guthmann C, Schuler V, Lilienkamp M, Walz G, Yakulov TA. Control of Directed Cell Migration after Tubular Cell Injury by Nucleotide Signaling. International Journal of Molecular Sciences. 2022; 23(14):7870. https://doi.org/10.3390/ijms23147870
Chicago/Turabian StyleGessler, Sabrina, Clara Guthmann, Vera Schuler, Miriam Lilienkamp, Gerd Walz, and Toma Antonov Yakulov. 2022. "Control of Directed Cell Migration after Tubular Cell Injury by Nucleotide Signaling" International Journal of Molecular Sciences 23, no. 14: 7870. https://doi.org/10.3390/ijms23147870
APA StyleGessler, S., Guthmann, C., Schuler, V., Lilienkamp, M., Walz, G., & Yakulov, T. A. (2022). Control of Directed Cell Migration after Tubular Cell Injury by Nucleotide Signaling. International Journal of Molecular Sciences, 23(14), 7870. https://doi.org/10.3390/ijms23147870





