Distinct Chemokine Receptor Expression Profiles in De Novo DLBCL, Transformed Follicular Lymphoma, Richter’s Trans-Formed DLBCL and Germinal Center B-Cells
Abstract
1. Introduction
2. Results
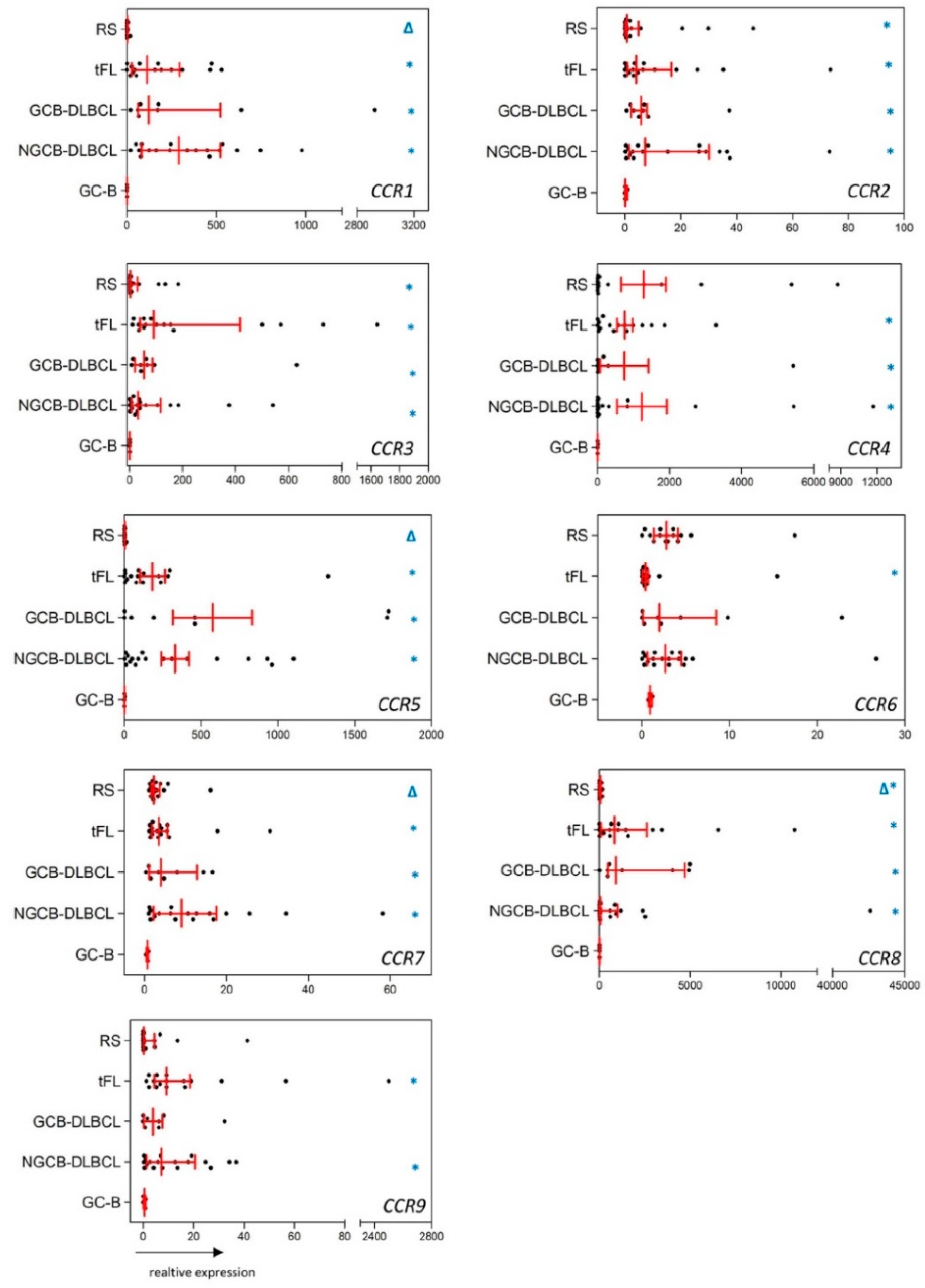
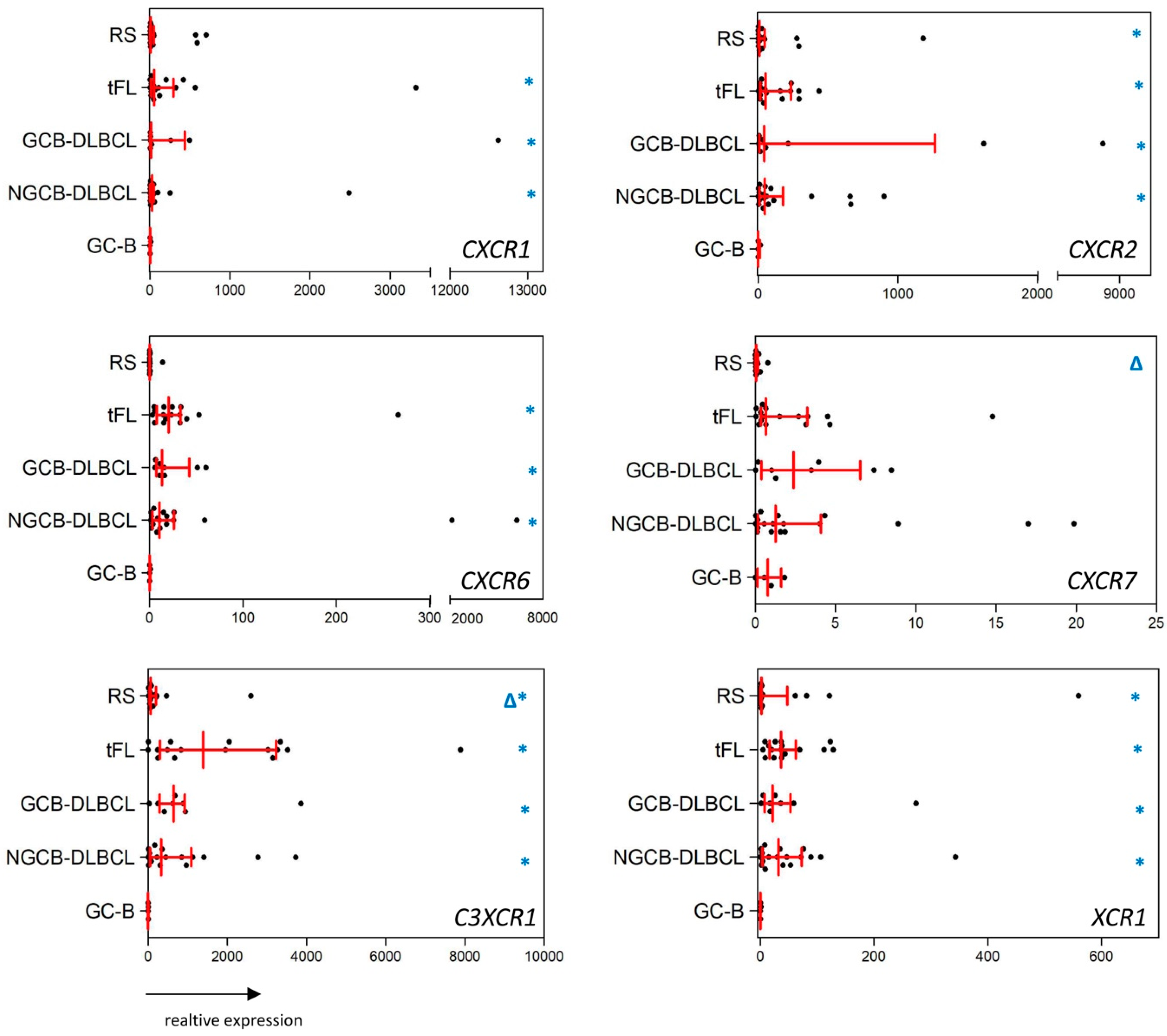
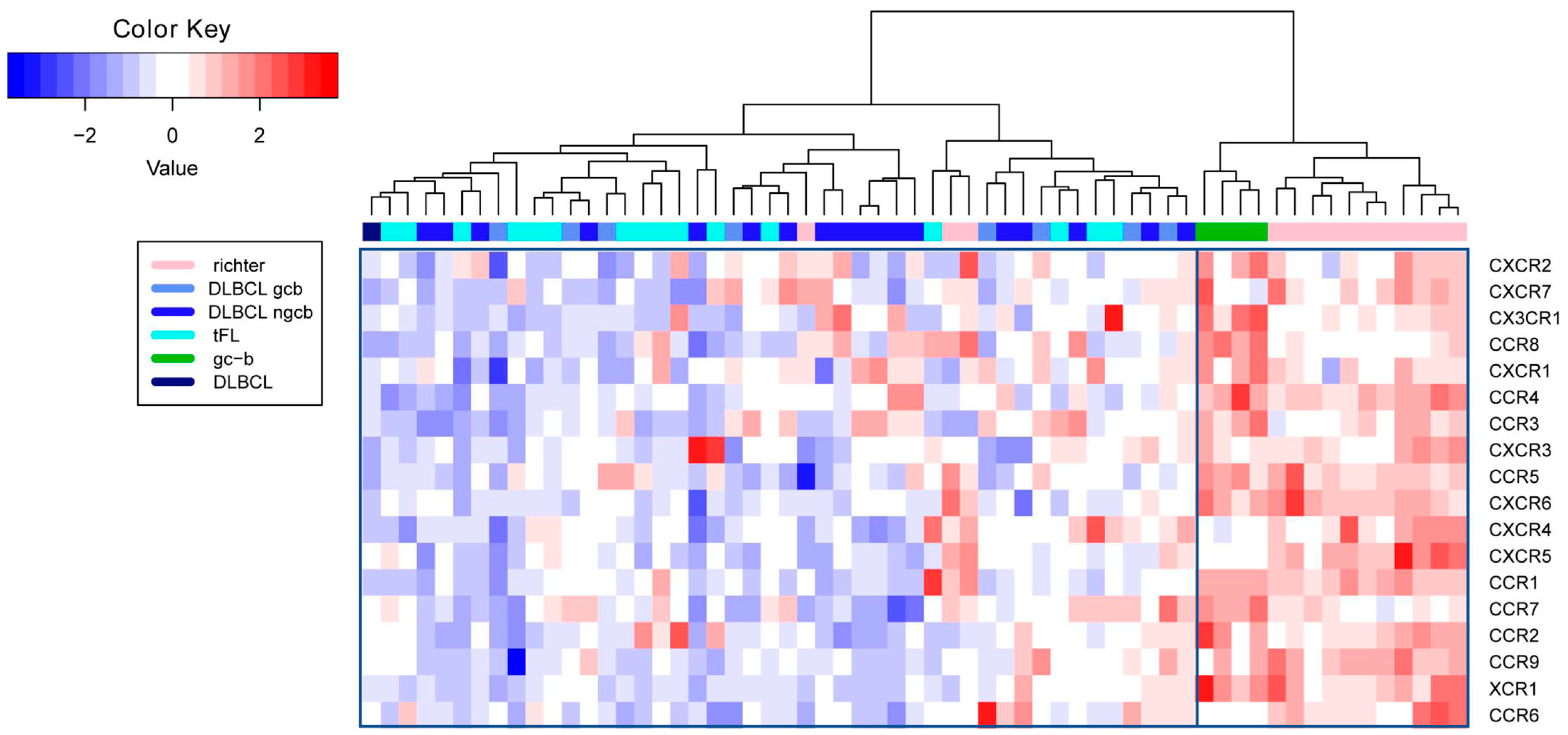
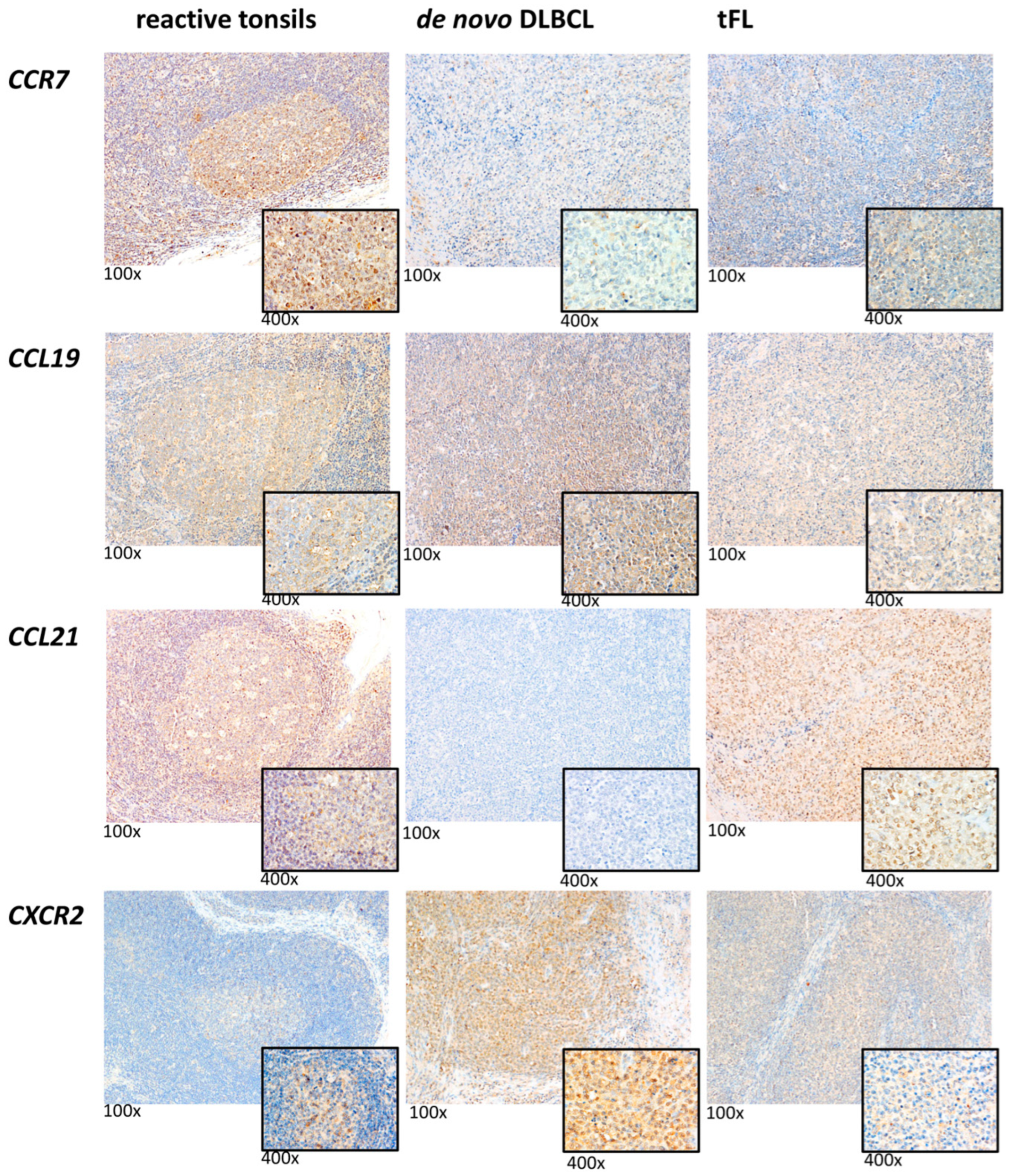
2.1. Substantial Differences in the CCR and CXCR Expression Patterns in DLBCL, tFL, RS and GC-B
2.2. The Chemokine Receptor Expression Pattern Is not Influenced by Intratumoral Inflammatory Cells
2.3. Reduced CCR5, CCR6, and CCR8 Protein Content in RS Compared with De Novo DLBCL and tFL
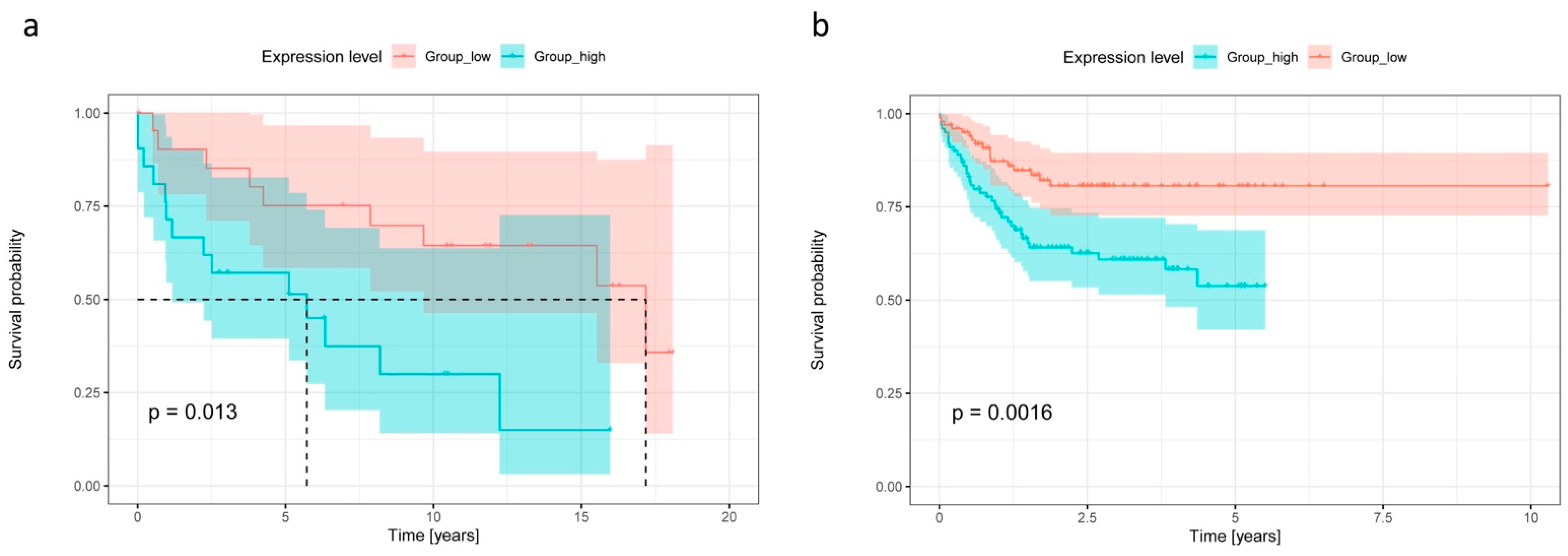
2.4. High CCR7 Expression Is Associated with Poor Overall Survival and High CXCR2 Content in De Novo DLBCL and tFL
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. RNA Isolation, cDNA Synthesis and Real-Time PCR
4.3. Immunohistochemical Analyses for CCR1, CCR4, CCR5, CCR6, CCR7, CCR8, CXCR2, CCL19, and CCL21
4.4. Multicolor-Immunofluorescence Staining of T-Cells and Subpopulations
4.5. Statistics
4.6. Survival Analysis and Heatmap
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Jong, D.; Ponz, O.B. The molecular background of aggressive B cell lymphomas as a basis for targeted therapy. J. Pathol. 2010, 223, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.P.; Pittaluga, S.; Jaffe, E.S. The Histological and Biological Spectrum of Diffuse Large B-Cell Lymphoma in the World Health Organization Classification. Cancer J. 2012, 18, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Müller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kridel, R.; Sehn, L.H.; Gascoyne, R.D. Can histologic transformation of follicular lymphoma be predicted and prevented? Blood 2017, 130, 258–266. [Google Scholar] [CrossRef]
- Parikh, S.A.; Rabe, K.G.; Call, T.G.; Zent, C.S.; Habermann, T.M.; Ding, W.; Leis, J.F.; Schwager, S.M.; Hanson, C.A.; Macon, W.R.; et al. Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): A cohort study of newly diagnosed patients. Br. J. Haematol. 2013, 162, 774–782. [Google Scholar] [CrossRef]
- Godfrey, J.; Leukam, M.; Smith, S.M. An update in treating transformed lymphoma. Best Pract. Res. Clin. Haematol. 2018, 31, 251–261. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; O’Brien, S.; Khouri, I.; Giles, F.J.; Kantarjian, H.M.; Champlin, R.; Wen, S.; Do, K.-A.; Smith, S.C.; Lerner, S.; et al. Clinical Outcomes and Prognostic Factors in Patients with Richter’s Syndrome Treated with Chemotherapy or Chemoimmunotherapy with or without Stem-Cell Transplantation. J. Clin. Oncol. 2006, 24, 2343–2351. [Google Scholar] [CrossRef]
- Campbell, D.J.; Kim, C.H.; Butcher, E.C. Chemokines in the systemic organization of immunity. Immunol. Rev. 2003, 195, 58–71. [Google Scholar] [CrossRef]
- Laurence, A.D.J. Location, movement and survival: The role of chemokines in haematopoiesis and malignancy. Br. J. Haematol. 2005, 132, 255–267. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Bowman, E.P.; Campbell, J.; Soler, D.; Dong, Z.; Manlongat, N.; Picarella, D.; Hardy, R.R.; Butcher, E.C. Developmental Switches in Chemokine Response Profiles during B Cell Differentiation and Maturation. J. Exp. Med. 2000, 191, 1303–1318. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G. Chemokines, Sphingosine-1-Phosphate, and Cell Migration in Secondary Lymphoid Organs. Annu. Rev. Immunol. 2005, 23, 127–159. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Höpken, U.E.; Lipp, M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol. Rev. 2003, 195, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Trentin, L.; Agostini, C.; Facco, M.; Piazza, F.; Perin, A.; Siviero, M.; Gurrieri, C.; Galvan, S.; Adami, F.; Zambello, R.; et al. The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J. Clin. Investig. 1999, 104, 115–121. [Google Scholar] [CrossRef][Green Version]
- Trentin, L.; Cabrelle, A.; Facco, M.; Carollo, D.; Miorin, M.; Tosoni, A.; Pizzo, P.; Binotto, G.; Nicolardi, L.; Zambello, R.; et al. Homeostatic chemokines drive migration of malignant B cells in patients with non-Hodgkin lymphomas. Blood 2004, 104, 502–508. [Google Scholar] [CrossRef]
- Suefuji, H.; Ohshima, K.; Karube, K.; Kawano, R.; Nabeshima, K.; Suzumiya, J.; Hayabuchi, N.; Kikuchi, M. CXCR3-positive B cells found at elevated frequency in the peripheral blood of patients with MALT lymphoma are attracted by MIG and belong to the lymphoma clone. Int. J. Cancer 2005, 114, 896–901. [Google Scholar] [CrossRef]
- Deutsch, A.J.A.; Steinbauer, E.; Hofmann, N.A.; Strunk, D.; Gerlza, T.; Beham-Schmid, C.; Schaider, H.; Neumeister, P. Chemokine receptors in gastric MALT lymphoma: Loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod. Pathol. 2012, 26, 182–194. [Google Scholar] [CrossRef]
- Pansy, K.; Feichtinger, J.; Ehall, B.; Uhl, B.; Sedej, M.; Roula, D.; Pursche, B.; Wolf, A.; Zoidl, M.; Steinbauer, E.; et al. The CXCR4–CXCL12-Axis Is of Prognostic Relevance in DLBCL and Its Antagonists Exert Pro-Apoptotic Effects In Vitro. Int. J. Mol. Sci. 2019, 20, 4740. [Google Scholar] [CrossRef]
- Golay, J.T.; Introna, M. Chemokines and antagonists in non-Hodgkin’s lymphoma. Expert Opin. Ther. Targets 2008, 12, 621–635. [Google Scholar] [CrossRef]
- Smith, S. Transformed lymphoma: What should I do now? Hematology 2020, 2020, 306–311. [Google Scholar] [CrossRef]
- Lenz, G.; Wright, G.; Dave, S.S.; Xiao, W.; Powell, J.; Zhao, H.; Xu, W.; Tan, B.; Goldschmidt, N.; Iqbal, J.; et al. Stromal Gene Signatures in Large-B-Cell Lymphomas. N. Engl. J. Med. 2008, 359, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Comerford, I.; Milasta, S.; Morrow, V.; Milligan, G.; Nibbs, R. The chemokine receptor CCX-CKR mediates effective scavenging of CCL19in vitro. Eur. J. Immunol. 2006, 36, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, M.; Zawieja, D.C.; Brook, B.S.; Nibbs, R.J.B.; Moore, J.E. A Novel Computational Model Predicts Key Regulators of Chemokine Gradient Formation in Lymph Nodes and Site-Specific Roles for CCL19 and ACKR4. J. Immunol. 2017, 199, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Leick, M.; Catusse, J.; Follo, M.; Nibbs, R.J.; Hartmann, T.N.; Veelken, H.; Burger, M. CCL19 is a specific ligand of the constitutively recycling atypical human chemokine receptor CRAM-B. Immunology 2009, 129, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Townson, J.R.; Nibbs, R.J.B. Characterization of mouse CCX-CKR, a receptor for the lymphocyte-attracting chemokines TECK/mCCL25, SLC/mCCL21 and MIP-3 I/mCCL19: Comparison to human CCX-CKR. Eur. J. Immunol. 2002, 32, 1230–1241. [Google Scholar] [CrossRef]
- Yoshida, R.; Imai, T.; Hieshima, K.; Kusuda, J.; Baba, M.; Kitaura, M.; Nishimura, M.; Kakizaki, M.; Nomiyama, H.; Yoshie, O. Molecular Cloning of a Novel Human CC Chemokine EBI1-ligand Chemokine That Is a Specific Functional Ligand for EBI1, CCR7. J. Biol. Chem. 1997, 272, 13803–13809. [Google Scholar] [CrossRef]
- Yoshida, R.; Nagira, M.; Kitaura, M.; Imagawa, N.; Imai, T.; Yoshie, O. Secondary Lymphoid-tissue Chemokine Is a Functional Ligand for the CC Chemokine Receptor CCR7. J. Biol. Chem. 1998, 273, 7118–7122. [Google Scholar] [CrossRef]
- Rodig, S.J.; Jones, D.; Shahsafaei, A.; Dorfman, D.M. CCR6 is a functional chemokine receptor that serves to identify select B-cell non-hodgkin’s lymphomas. Hum. Pathol. 2002, 33, 1227–1233. [Google Scholar] [CrossRef]
- Müller, G.; Reiterer, P.; Höpken, U.E.; Golfier, S.; Lipp, M. Role of Homeostatic Chemokine and Sphingosine-1-Phosphate Receptors in the Organization of Lymphoid Tissue. Ann. N. Y. Acad. Sci. 2003, 987, 107–116. [Google Scholar] [CrossRef]
- Anderson, M.W.; Zhao, S.; Ai, W.Z.; Tibshirani, R.; Levy, R.; Lossos, I.S.; Natkunam, Y. C-C Chemokine Receptor 1 Expression in Human Hematolymphoid Neoplasia. Am. J. Clin. Pathol. 2010, 133, 473–483. [Google Scholar] [CrossRef]
- Li, Y.-L.; Shi, Z.-H.; Wang, X.; Gu, K.-S.; Zhai, Z.-M. Prognostic significance of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in diffuse large B cell lymphoma. Ann. Hematol. 2018, 98, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, T.; Lan, Q.; Lerro, C.; Zhao, N.; Qin, Q.; Hu, X.; Huang, H.; Liang, J.; Holford, T.; et al. Single-Nucleotide Polymorphisms in Genes Encoding for CC Chemokines Were Not Associated with the Risk of Non-Hodgkin Lymphoma. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Nannini, P.R.; Borge, M.; Mikolaitis, V.C.; Abreu, C.; Morande, P.E.; Zanetti, S.R.; Oppezzo, P.; Palacios, F.; Ledesma, I.; Bezares, R.F.; et al. CCR4 expression in a case of cutaneous Richter’s transformation of chronic lymphocytic leukemia (CLL) to diffuse large B-cell lymphoma (DLBCL) and in CLL patients with no skin manifestations. Eur. J. Haematol. 2011, 87, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, F.; Fu, H.; Shen, J. Epigenetic regulation of miR-518a-5p-CCR6 feedback loop promotes both proliferation and invasion in diffuse large B cell lymphoma. Epigenetics 2020, 16, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, L.; Li, G.; Liu, W.; Tang, W.; Zhang, H.; Luan, J.; Gao, L.; Wang, X. CXCR4 and CCR7 Expression in Primary Nodal Diffuse Large B-Cell Lymphoma—A Clinical and Immunohistochemical Study. Am. J. Med. Sci. 2019, 357, 302–310. [Google Scholar] [CrossRef]
- Wu, W.; Doan, N.; Said, J.; Karunasiri, D.; Pullarkat, S.T. Strong expression of chemokine receptor CCR9 in diffuse large B-cell lymphoma and follicular lymphoma strongly correlates with gastrointestinal involvement. Hum. Pathol. 2014, 45, 1451–1458. [Google Scholar] [CrossRef]
- Butrym, A.; Kryczek, I.; Dlubek, D.; Jaskula, E.; Lange, A.; Jurczyszyn, A.; Mazur, G. High expression of CC chemokine receptor 5 (CCR5) promotes disease progression in patients with B-cell non-Hodgkin lymphomas. Curr. Probl. Cancer 2018, 42, 268–275. [Google Scholar] [CrossRef]
- Seifert, M.; Sellmann, L.; Bloehdorn, J.; Wein, F.; Stilgenbauer, S.; Dürig, J.; Küppers, R. Cellular origin and pathophysiology of chronic lymphocytic leukemia. J. Exp. Med. 2012, 209, 2183–2198. [Google Scholar] [CrossRef]
- Seifert, M.; Scholtysik, R.; Küppers, R. Origin and Pathogenesis of B Cell Lymphomas. Methods Mol. Biol. 2012, 971, 1–25. [Google Scholar] [CrossRef]
- Küppers, R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Cancer 2005, 5, 251–262. [Google Scholar] [CrossRef]
- Rossi, D.; Spina, V.; Deambrogi, C.; Rasi, S.; Laurenti, L.; Stamatopoulos, K.; Arcaini, L.; Lucioni, M.; Rocque, G.B.; Xu-Monette, Z.Y.; et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood 2011, 117, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tschautscher, M.A.; Rabe, K.G.; Call, T.G.; Leis, J.F.; Kenderian, S.S.; Kay, N.E.; Muchtar, E.; Van Dyke, D.L.; Koehler, A.B.; et al. Clinical characteristics and outcomes of Richter transformation: Experience of 204 patients from a single center. Haematologica 2019, 105, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Cerri, M.; Capello, D.; Deambrogi, C.; Rossi, F.M.; Zucchetto, A.; De Paoli, L.; Cresta, S.; Rasi, S.; Spina, V.; et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br. J. Haematol. 2008, 142, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Autio, M.; Leivonen, S.-K.; Brück, O.; Karjalainen-Lindsberg, M.-L.; Pellinen, T.; Leppä, S. Clinical Impact of Immune Cells and Their Spatial Interactions in Diffuse Large B-Cell Lymphoma Microenvironment. Clin. Cancer Res. 2021, 28, 781–792. [Google Scholar] [CrossRef]
- Korbecki, J.; Grochans, S.; Gutowska, I.; Barczak, K.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020, 21, 7619. [Google Scholar] [CrossRef]
- Rehm, A.; Mensen, A.; Schradi, K.; Gerlach, K.; Wittstock, S.; Winter, S.; Büchner, G.; Dörken, B.; Lipp, M.; Höpken, U.E. Cooperative function of CCR7 and lymphotoxin in the formation of a lymphoma-permissive niche within murine secondary lymphoid organs. Blood 2011, 118, 1020–1033. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, R.; Wang, X.; Hu, K.; Huang, L.; Lu, M.; Hu, Q. CCL19 and CCR7 Expression, Signaling Pathways, and Adjuvant Functions in Viral Infection and Prevention. Front. Cell Dev. Biol. 2019, 7, 212. [Google Scholar] [CrossRef]
- Moreno, M.J.; Gallardo, A.; Novelli, S.; Mozos, A.; Aragó, M.; Pavon, M.A.; Céspedes, M.V.; Pallarès, V.; Falgàs, A.; Alcoceba, M.; et al. CXCR7 expression in diffuse large B-cell lymphoma identifies a subgroup of CXCR4+ patients with good prognosis. PLoS ONE 2018, 13, e0198789. [Google Scholar] [CrossRef]
- Moreno, M.J.; Bosch, R.; Dieguez-Gonzalez, R.; Novelli, S.; Mozos, A.; Gallardo, A.; Pavón, M.A.; Céspedes, M.V.; Grañena, A.; Alcoceba, M.; et al. CXCR4 expression enhances diffuse large B cell lymphoma dissemination and decreases patient survival. J. Pathol. 2014, 235, 445–455. [Google Scholar] [CrossRef]
- Manfroi, B.; McKee, T.; Mayol, J.F.; Tabruyn, S.; Moret, S.; Villiers, C.; Righini, C.; Dyer, M.; Callanan, M.; Schneider, P.; et al. CXCL-8/IL8 produced by diffuse large B-cell lymphomas recruits neutrophils expressing a prolifera-tion-inducing ligand April. Cancer Res. 2017, 77, 1097–1107. [Google Scholar] [CrossRef]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Deutsch, A.; Aigelsreiter, A.; Steinbauer, E.; Frühwirth, M.; Kerl, H.; Beham-Schmid, C.; Schaider, H.; Neumeister, P.; Deutsch, A. Distinct signatures of B-cell homeostatic and activation-dependent chemokine receptors in the development and progression of extragastric MALT lymphomas. J. Pathol. 2008, 215, 431–444. [Google Scholar] [CrossRef]
- McCall, M.N.; McMurray, H.; Land, H.; Almudevar, A. On non-detects in qPCR data. Bioinformatics 2014, 30, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M. How to Analyze Real Time qPCR Data? Biochem. Physiol. 2013, 2, e114. [Google Scholar] [CrossRef]
- Zhuang, L.; Lee, C.S.; Scolyer, R.A.; McCarthy, S.W.; Zhang, X.D.; Thompson, J.F.; Screaton, G.; Hersey, P. Progression in melanoma is associated with decreased expression of death receptors for tumor necrosis factor–related apoptosis-inducing ligand. Hum. Pathol. 2006, 37, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, P.; Iliopoulos, A.C.; Parsonidis, P.; Papasotiriou, I. Gene expression profiling as a potential predictor between normal and cancer samples in gastrointestinal carcinoma. Oncotarget 2019, 10, 3328–3338. [Google Scholar] [CrossRef][Green Version]
- Asztalos, S.; Pham, T.N.; Gann, P.H.; Hayes, M.K.; Deaton, R.; Wiley, E.L.; Emmadi, R.; Kajdacsy-Balla, A.; Banerji, N.; McDonald, W.; et al. High incidence of triple negative breast cancers following pregnancy and an associated gene expression signature. Springerplus 2015, 4, 710. [Google Scholar] [CrossRef]
- Doan, T.B.; Eriksson, N.A.; Graham, D.; Funder, J.W.; Simpson, E.R.; Kuczek, E.S.; Clyne, C.; Leedman, P.J.; Tilley, W.; Fuller, P.J.; et al. Breast cancer prognosis predicted by nuclear receptor-coregulator networks. Mol. Oncol. 2014, 8, 998–1013. [Google Scholar] [CrossRef]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. Various R Programming Tools for Plotting Data. (gplots 3.1.1). 2020. Available online: https://github.com/talgalili/gplots (accessed on 1 July 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing, R version 3.6.3/3.5.1; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 1 July 2022).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data; Springer: New York, NY, USA; London, UK, 2000. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0.4.7. (R package version 0.4.7). 2020. Available online: https://CRAN.R-project.org/package=survminer (accessed on 1 July 2022).
- Carvalho, B.S.; Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]

| Type of Lymphoma | CD3+ (Cells/mm²) | p-Value | CD3+CD4+ (Cells/mm²) | p-Value | CD3+CD8+ (Cells/mm²) | p-Value | CD68+ (%) * | p-Value |
|---|---|---|---|---|---|---|---|---|
| GCB-DLBCL | 1491.7 | >0.45 | 909.5 | >0.8 | 499.7 | >0.64 | 18.75 | NGCB-DLBCL vs. tFL: 0.035 |
| Range: | (760.6–2557.6) | (476.2–1828.4) | (275.3–1003.1) | (10–40) | ||||
| NGCB-DLBCL | 1365.8 | 790.3 | 507.2 | 20 | ||||
| Range: | (201.8–3493.3) | (60.1–2510.6) | (105.2–1184.5) | (5–40) | GCB-DLBCL vs. tFL: >0.38 | |||
| tFL | 1035.2 | 560.8 | 545.9 | 12.1 | ||||
| Range: | (347.3–1605.3) | (121.8–1198.8) | (103.1–1138.5) | (5–20) |
| CCR1 | CCR4 | CCR5 | CCR6 | CCR8 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Cases (+) | % | IRS *-Mean | % Cases (+) | % | IRS *-Mean | % Cases (+) | % | IRS *-Mean | % Cases (+) | % | IRS *-Mean | % Cases (+) | % | IRS *-Mean | |
| de novo DLBCL | 30 | 7.1 (0–50) | 0.7 | 30 | 19 (0–90) | 2.4 | 100 | 90 (85–95) | 16.2 | 80 | 57 (0–90) | 6.6 | 90 | 67 (0–90) | 11.7 |
| GCB-DLBCL | 0 | 0 (<0) | 0 | 0 | 0 (<0) | 0 | 100 | 90 (85–95) | 18 | 50 | 25 (0–50) | 2.5 | 100 | 70 (50–90) | 7 |
| NGCB-DLBCL | 38 | 8.9 (0–50) | 0.9 | 38 | 23.7 (0–5) | 3 | 100 | 90 (85–95) | 15.8 | 88 | 65 (0–90) | 7.6 | 88 | 66.3 (0–90) | 12.9 |
| tFL | 7 | 0.1 (0–1) | 0.1 | 60 | 43.3 (0–90) | 6.1 | 100 | 83.3 (30–90) | 16.1 | 87 | 51.7 (0–90) | 7 | 73 | 56.7 (0–90) | 10.8 |
| RS | 0 | 0 (<0) | 0 | 50 | 45 (0–90) | 4.5 | 100 | 70 (30–90) | 13 | 100 | 51.7 (0–90) | 5.2 | 33 | 30 (0–90) | 3 |
| CCR7 | CCL19 | CCL21 | CXCR2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Cases (+) | % | IRS-Mean | % Cases (+) | % | IRS-Mean | % Cases (+) | % | IRS-Mean | % Cases (+) | % | IRS-Mean | |
| de novo DLBCL | 100 | 74.5 (5–100) | 12.63 | 100 | 75.2 (20–100) | 9.36 | 32 | 24.7 (0–100) | 3.69 | 88 | 72.5 (0–95) | 5.44 |
| GCB-DLBCL | 100 | 75 (20–100) | 13.5 | 100 | 85 (80–100) | 7 | 17 | 13.3 (0–80) | 2.67 | 92 | 63.78 (0–95) | 2 |
| NGCB-DLBCL | 100 | 74.4 (5–100) | 12.42 | 100 | 72.8 (20–100) | 9.92 | 36 | 27.4 (0–100) | 3.94 | 100 | 75.4 (40–95) | 6.5 |
| tFL | 100 | 87.7 (20–100) | 15.91 | 100 | 73.9 (10–100) | 7.78 | 23 | 19.3 (0–100) | 2.23 | 100 | 83.3 (60–95) | 6.67 |
| De Novo DLBCL | tFL | RS | ||||
|---|---|---|---|---|---|---|
| Clinicopathologic Parameters | Patients (n: 27) | Proportion (%) | Patients (n: 16) | Proportion (%) | Patients (n: 14) | Proportion (%) |
| Gender | ||||||
| Male | 15 | 55.6 | 5 | 31.25 | 10 | 71.4 |
| Female | 12 | 44.4 | 11 | 68.75 | 4 | 28.6 |
| Age | ||||||
| <=60 | 6 | 22.2 | 7 | 43.75 | 5 | 35.7 |
| Male | 5 | 18.5 | 4 | 25.0 | 4 | 28.6 |
| Female | 1 | 3.7 | 3 | 18.75 | 1 | 7.1 |
| >60 | 21 | 77.8 | 9 | 56.25 | 9 | 64.3 |
| Male | 10 | 37.0 | 1 | 6.25 | 6 | 42.8 |
| Female | 11 | 40.7 | 8 | 50.0 | 3 | 21.4 |
| Ann Arbor Stage | ||||||
| 1 | 7 | 25.9 | 2 | 12.5 | n/a * | |
| 2 | 8 | 29.6 | 2 | 12.5 | ||
| 3 | 6 | 22.2 | 7 | 43.75 | ||
| 4 | 4 | 14.8 | 4 | 25.0 | ||
| Not classifiable | 2 | 7.4 | 1 | 6.25 | ||
| Immunophenotype of DLBCL (Hans algorithm) | ||||||
| GCB | 8 | 29.6 | 16 | 100 | 0 | 0 |
| NGCB | 18 | 66.7 | 0 | 0 | 5 | 35.7 |
| Unclassifiable | 1 | 3.7 | 0 | 0 | 9 | 64.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhl, B.; Prochazka, K.T.; Pansy, K.; Wenzl, K.; Strobl, J.; Baumgartner, C.; Szmyra, M.M.; Waha, J.E.; Wolf, A.; Tomazic, P.V.; et al. Distinct Chemokine Receptor Expression Profiles in De Novo DLBCL, Transformed Follicular Lymphoma, Richter’s Trans-Formed DLBCL and Germinal Center B-Cells. Int. J. Mol. Sci. 2022, 23, 7874. https://doi.org/10.3390/ijms23147874
Uhl B, Prochazka KT, Pansy K, Wenzl K, Strobl J, Baumgartner C, Szmyra MM, Waha JE, Wolf A, Tomazic PV, et al. Distinct Chemokine Receptor Expression Profiles in De Novo DLBCL, Transformed Follicular Lymphoma, Richter’s Trans-Formed DLBCL and Germinal Center B-Cells. International Journal of Molecular Sciences. 2022; 23(14):7874. https://doi.org/10.3390/ijms23147874
Chicago/Turabian StyleUhl, Barbara, Katharina T. Prochazka, Katrin Pansy, Kerstin Wenzl, Johanna Strobl, Claudia Baumgartner, Marta M. Szmyra, James E. Waha, Axel Wolf, Peter V. Tomazic, and et al. 2022. "Distinct Chemokine Receptor Expression Profiles in De Novo DLBCL, Transformed Follicular Lymphoma, Richter’s Trans-Formed DLBCL and Germinal Center B-Cells" International Journal of Molecular Sciences 23, no. 14: 7874. https://doi.org/10.3390/ijms23147874
APA StyleUhl, B., Prochazka, K. T., Pansy, K., Wenzl, K., Strobl, J., Baumgartner, C., Szmyra, M. M., Waha, J. E., Wolf, A., Tomazic, P. V., Steinbauer, E., Steinwender, M., Friedl, S., Weniger, M., Küppers, R., Pichler, M., Greinix, H. T., Stary, G., Ramsay, A. G., ... Deutsch, A. J. (2022). Distinct Chemokine Receptor Expression Profiles in De Novo DLBCL, Transformed Follicular Lymphoma, Richter’s Trans-Formed DLBCL and Germinal Center B-Cells. International Journal of Molecular Sciences, 23(14), 7874. https://doi.org/10.3390/ijms23147874






