Overexpression of McHB7 Transcription Factor from Mesembryanthemum crystallinum Improves Plant Salt Tolerance
Abstract
:1. Introduction
2. Results
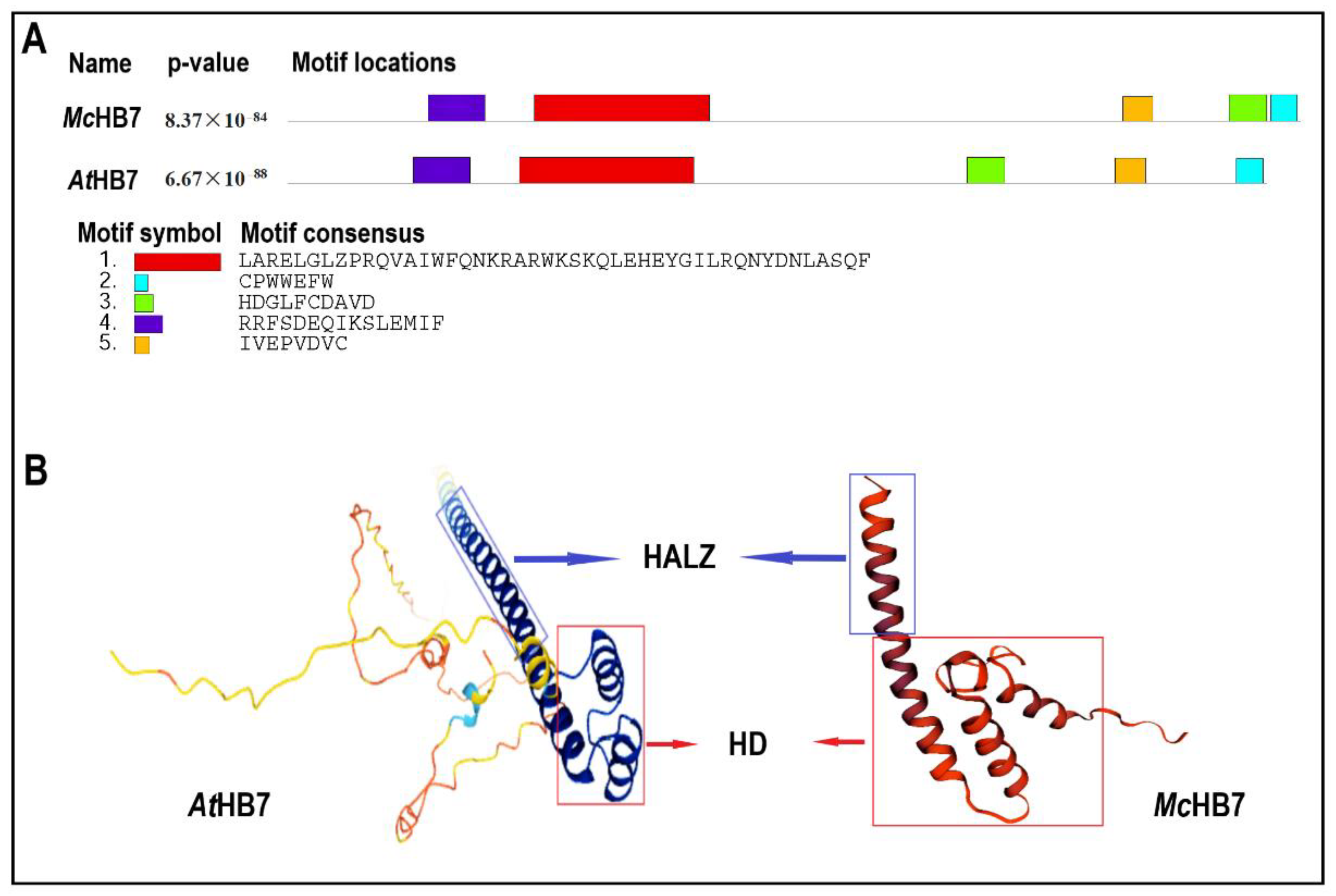
2.1. AtHB7 Is the Homolog of McHB7
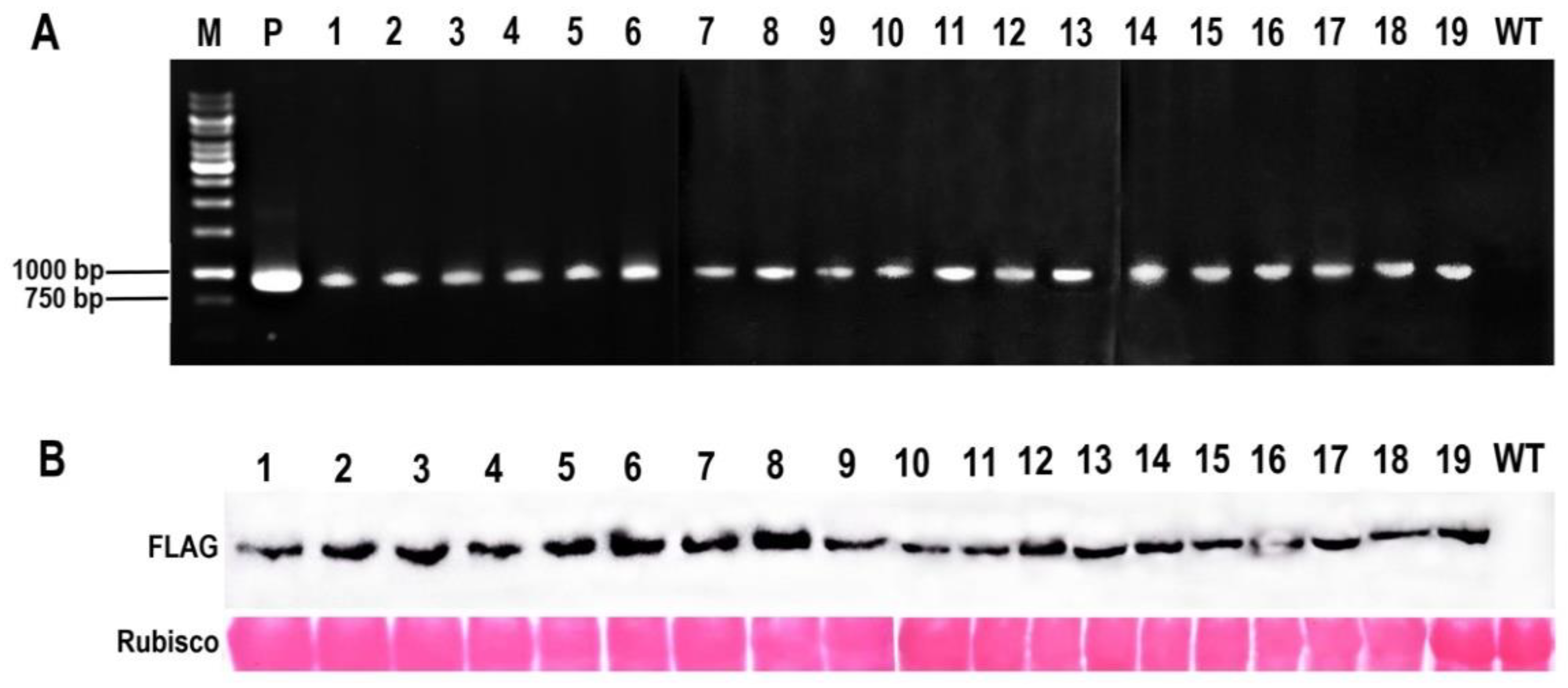
2.2. Creation of McHB7 Transgenic Arabidopsis
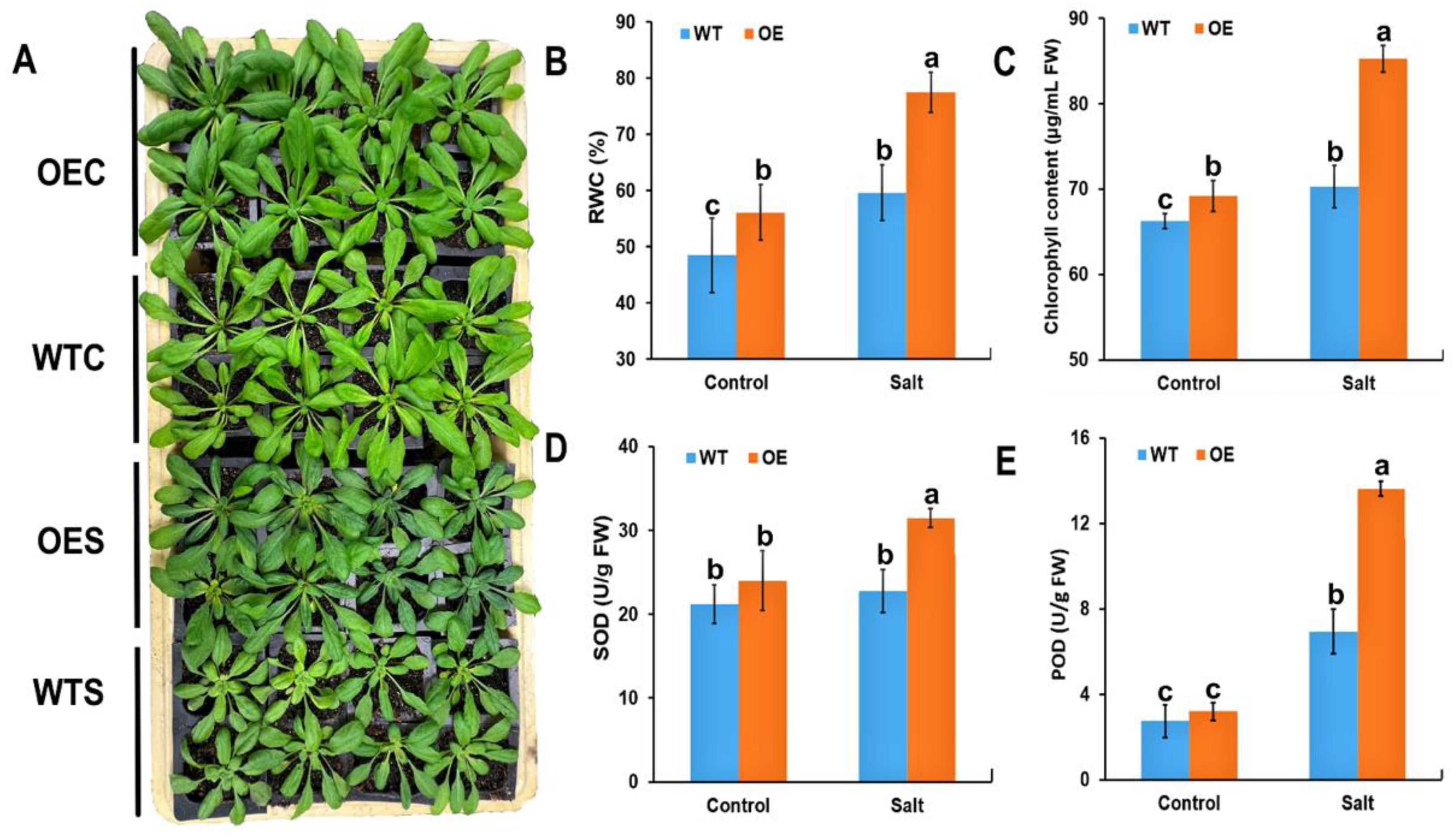
2.3. Physiological Characteristics of McHB7-OE Plants under Salt Stress
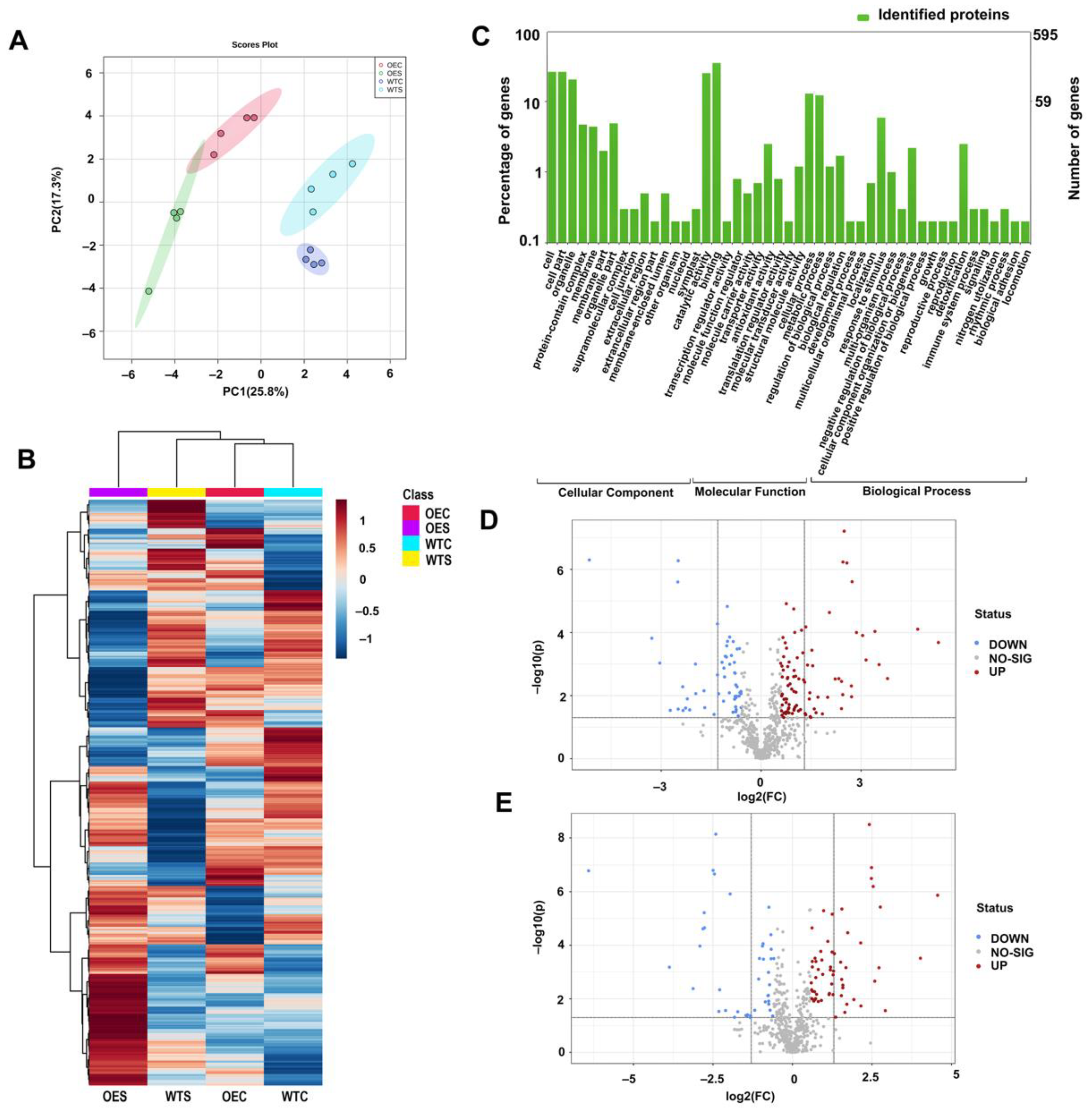
2.4. Protein Changes in McHB7-OE Plants under Salt Stress Conditions
2.5. Metabolite Changes in McHB7-OE Plants under Salt Stress Conditions
2.6. Integrated Physiological, Proteomics, and Metabolomics of the OE Plants under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material and Salt Treatment
4.2. McHB7 Sequence Analysis
4.3. Generation of McHB7 Transgenic Arabidopsis Lines
4.4. Identification of Transgenic Arabidopsis by PCR and Western Blot
4.5. Physiological Measurement of Transgenic Arabidopsis
4.6. Protein Extraction and Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
4.7. Metabolite Extraction and Metabolomics Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamers, J.; Meer, T.; Testerink, C. How Plants Sense and Respond to Stressful Environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julkowska, M.M.; Testerink, C. Tuning Plant Signaling and Growth to Survive Salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalifa, Y.; Perlson, E.; Gilad, A.; Konrad, Z.; Bar-Zvi, D. Over-Expression of the Water and Salt Stress-Regulated Asr1 Gene Confers an Increased Salt Tolerance. Plant Cell Environ. 2010, 27, 1459–1468. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C.F. The Regulatory Roles of Ethylene and Reactive Oxygen Species (ROS) in Plant Salt Stress Responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Yasar, F.; Ellialtioglu, S.; Yildiz, K. Effect of Salt Stress on Antioxidant Defense Systems, Lipid Peroxidation, and Chlorophyll Content in Green Bean. Russ. J. Plant Physiol. 2008, 55, 782. [Google Scholar] [CrossRef]
- Duan, L.; Dietrich, D.; Chong, H.N.; Chan, P.; Bhalerao, R.; Bennett, M.J.; Dinneny, J.R. Endodermal ABA Signaling Promotes Lateral Root Quiescence during Salt Stress in Arabidopsis Seedlings. Plant Cell 2013, 25, 324–341. [Google Scholar] [CrossRef] [Green Version]
- Hakeem, K.; Chandna, R.; Rehman, R.; Tahir, I.; Sabir, M.; Iqbal, M. Unravelling Salt Stress in Plants through Proteomics. In Salt Stress in Plants; Springer: New York, NY, USA, 2013; pp. 47–61. [Google Scholar]
- Cheong, Y.H.; Kim, K.-N.; Pandey, G.K.; Gupta, R.; Grant, J.J.; Luan, S. CBL1, a Calcium Sensor That Differentially Regulates Salt, Drought, and Cold Responses in Arabidopsis. Plant Cell 2003, 15, 1833–1845. [Google Scholar] [CrossRef] [Green Version]
- Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.; Hassan, F. How Salt Stress-Responsive Proteins Regulate Plant Adaptation to Saline Conditions. Plant Mol. Biol. 2021, 108, 175–224. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the Molecular Mechanisms Mediating Plant Salt-Stress Responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Belfield, E.J.; Cao, Y.; Smith, J.A.C.; Harberd, N.P. An Arabidopsis Soil-Salinity-Tolerance Mutation Confers Ethylene-Mediated Enhancement of Sodium/Potassium Homeostasis. Plant Cell 2013, 25, 3535–3552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt Tolerance in Rice: Physiological Responses and Molecular Mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Lv, D.W.; Zhu, G.R.; Zhu, D.; Bian, Y.W.; Liang, X.N.; Cheng, Z.W.; Deng, X.; Yan, Y.M. Proteomic and Phosphoproteomic Analysis Reveals the Response and Defense Mechanism in Leaves of Diploid Wheat T. Monococcum under Salt Stress and Recovery. J. Proteom. 2016, 143, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Song, L.; Huang, Z.; Yang, Y.; Wang, S.; Wang, Z.; Tong, J.; Gu, W.; Ma, H.; Xiao, L. Comparative Proteomic Analysis Reveals Molecular Mechanism of Seedling Roots of Different Salt Tolerant Soybean Genotypes in Responses to Salinity Stress. EuPA Open Proteom. 2014, 4, 40–57. [Google Scholar] [CrossRef] [Green Version]
- Nosek, M.; Kaczmarczyk, A.; Śliwa, M.; Jędrzejczyk, R.; Kornaś, A.; Supel, P.; Kaszycki, P.; Miszalski, Z. The Response of a Model C3/CAM Intermediate Semi-Halophyte Mesembryanthemum crystallinum L. to Elevated Cadmium Concentrations. J. Plant Physiol. 2019, 240, 153005. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Fan, C.; Wei, Y.; Zhong, C. Genome-Wide Analysis of MYB Transcription Factors and Their Responses to Salt Stress in Casuarina equisetifolia. BMC Plant Biol. 2021, 21, 328. [Google Scholar] [CrossRef]
- Yang, R.; Jie, L.; Zhong, L.; Wei, S.; Zhang, Y. ERF Transcription Factors Involved in Salt Response in Tomato. Plant Growth Regul. 2018, 84, 573–582. [Google Scholar] [CrossRef]
- Wang, S.; He, S.; Hui, D. Genome-Wide Analysis of the BZIP Transcription Factors in Populus in Response to Salt Stress. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2018, 38, 1–7. [Google Scholar]
- Li, S.; Wang, N.; Ji, D.; Zhang, W.; Xiang, F. A GmSIN1/GmNCED3s/GmRbohBs Feed-Forward Loop Acts as a Signal Amplifier That Regulates Root Growth in Soybean Exposed to Salt Stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, J.; Guo, M.; Aslam, M.; Cao, S. Genome-Wide Characterization and Expression Profiling of Eucalyptus Grandis HD-Zip Gene Family in Response to Salt and Temperature Stress. BMC Plant Biol. 2020, 20, 451. [Google Scholar] [CrossRef]
- Irish, T. The Arabidopsis Zinc Finger-Homeodomain Genes Encode Proteins with Unique Biochemical Properties That are Coordinately Expressed during Floral Development. Plant Physiol. 2006, 140, 1095–1108. [Google Scholar]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L. The True Story of the HD-Zip Family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Haider, I.; Kohlen, W.; Jiang, L.; Bouwmeester, H.; Meijer, A.H.; Schluepmann, H.; Liu, C.M.; Ouwerkerk, P.B.F. Function of the HD-Zip I Gene Oshox22 in ABA-Mediated Drought and Salt Tolerances in Rice. Plant Mol. Biol. 2012, 80, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, H.; Jia, X.; Gao, H.; Mao, K.; Ma, F. The HD-Zip I Transcription Factor MdHB7-like Confers Tolerance to Salinity in Transgenic Apple (Malus Domestica). Physiol. Plant. 2021, 172, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.; Diet, A.; Verdenaud, M.; Gruber, V.; Frugier, F.; Chan, R.; Crespi, M. Environmental Regulation of Lateral Root Emergence in Medicago Truncatula Requires the HD-Zip I Transcription Factor HB1. Plant Cell 2010, 22, 2171–2183. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Yoo, M.J.; Noble, J.D.; Kelley, T.M.; Chen, S. Molecular Changes in Mesembryanthemum crystallinum Guard Cells Underlying the C3 to CAM Transition. Plant Mol. Biol. 2019, 106, 653–667. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.; Zhu, D.; Dufresne, D.; Chen, S. Proteomics of Homeobox7 Enhanced Salt Tolerance in Mesembryanthemum crystallinum. Int. J. Mol. Sci. 2021, 22, 6390. [Google Scholar] [CrossRef]
- Viola, I.L.; Gonzalez, D.H. Chapter6-Structure and Evolution of Plant Homeobox Genes. Plant Transcr. Factor 2016, 101–112. [Google Scholar]
- Yu, R.; Tang, Y.; Liu, C.; Du, X.; Miao, C.; Shi, G. Comparative Transcriptomic Analysis Reveals the Roles of ROS Scavenging Genes in Response to Cadmium in Two Pak Choi Cultivars. Sci. Rep. 2017, 7, 9217. [Google Scholar] [CrossRef] [Green Version]
- Liwa-Cebula, M.; Kaszycki, P.; Kaczmarczyk, A.; Nosek, M.; Miszalski, Z. The Common Ice Plant (Mesembryanthemum crystallinum L.) Phytoremediation Potential for Cadmium and Chromate-Contaminated Soils. Plants 2020, 9, 1230. [Google Scholar] [CrossRef]
- Cushman, J.C.; Michalowski, C.B.; Bohnert, H.J. Developmental Control of Crassulacean Acid Metabolism Inducibility by Salt Stress in the Common Ice Plant. Plant Physiol. 1990, 94, 1137–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niewiadomska, E.; Bilger, W.; Gruca, M.; Mulisch, M.; Miszalski, Z.; Krupinska, K. CAM-Related Changes in Chloroplastic Metabolism of Mesembryanthemum crystallinum L. Planta 2011, 233, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, K.; Ziegler, H. Induction of Crassulacean Acid Metabolism in Mesembryanthemum crystallinum Increases Reproductive Success under Conditions of Drought and Salinity Stress. Oecologia 1992, 92, 475–479. [Google Scholar] [CrossRef]
- Winter, K.; Holtum, J.A.M. The Effects of Salinity, Crassulacean Acid Metabolism and Plant Age on the Carbon Isotope Composition of Mesembryanthemum crystallinum L., a Halophytic C3-CAM Species. Planta 2005, 222, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sahbeni, G. Soil Salinity Mapping Using Landsat 8 OLI Data and Regression Modeling in the Great Hungarian Plain. SN Appl. Sci. 2021, 3, 587. [Google Scholar] [CrossRef]
- Wei, M.; Liu, A.; Zhang, Y.; Zhou, Y.; Li, D.; Dossa, K.; Zhou, R.; Zhang, X.; You, J. Genome-Wide Characterization and Expression Analysis of the HD-Zip Gene Family in Response to Drought and Salinity Stresses in Sesame. BMC Genom. 2019, 20, 748. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bao, X.; Wang, S.; Liu, Y.; Yu, X. A Physic Nut Stress-Responsive HD-Zip Transcription Factor, JcHDZ07, Confers Enhanced Sensitivity to Salinity Stress in Transgenic Arabidopsis. Front. Plant Sci. 2019, 10, 942. [Google Scholar] [CrossRef] [Green Version]
- Sharif, R.; Raza, A.; Chen, P.; Li, Y.; El-Esawi, M.A. HD-ZIP Gene Family: Potential Roles in Improving Plant Growth and Regulating Stress-Responsive Mechanisms in Plants. Genes 2021, 12, 1256. [Google Scholar] [CrossRef]
- Li, S.; Chen, N.; Li, F.; Mei, F.; Mao, H. Characterization of Wheat Homeodomain-Leucine Zipper Family Genes and Functional Analysis of TaHDZ5-6A in Drought Tolerance in Transgenic Arabidopsis. BMC Plant Biol. 2020, 20, 50. [Google Scholar] [CrossRef] [Green Version]
- Sharif, R.; Xie, C.; Wang, J.; Cao, Z.; Zhang, H.; Chen, P.; Li, Y. Genome Wide Identification, Characterization and Expression Analysis of HD-ZIP Gene Family in Cucumis sativus L. under Biotic and Various Abiotic Stresses. Int. J. Biol. Macromol. 2020, 158, 502–520. [Google Scholar] [CrossRef]
- Kovalchuk, N.; Chew, W.; Sornaraj, P.; Borisjuk, N.; Yang, N.; Singh, R.; Bazanova, N.; Shavrukov, Y.; Guendel, A.; Munz, E.; et al. The Homeodomain Transcription Factor TaHDZipI-2 from Wheat Regulates Frost Tolerance, Flowering Time and Spike Development in Transgenic Barley. New Phytol. 2016, 211, 671–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Luang, S.; Harris, J.; Riboni, M.; Li, Y.; Bazanova, N.; Hrmova, M.; Haefele, S.; Kovalchuk, N.; Lopato, S. Overexpression of the Class I Homeodomain Transcription Factor TaHDZipI Increases Drought and Frost Tolerance in Transgenic Wheat. Plant Biotechnol. J. 2017, 16, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Hironaka, T.; Suzuki, T.; Nishikawa, K.; Agarie, S.; Ishiguro, S.; Higashiyama, T. RNA-Seq Analysis of the Response of the Halophyte, Mesembryanthemum crystallinum (Ice Plant) to High Salinity. PLoS ONE 2015, 10, e0118339. [Google Scholar]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H.; Wu, J.S. Physiological Response to Drought Stress in Camptotheca Acuminata Seedlings from Two Provenances. Front. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Wang, Y.L.; Cheng, L.S.; Zhou, L.L.; Xu, Q.T.; Liu, D.C.; Deng, X.Y.; Mei, F.Z.; Zhou, Z.Q. Mutual Regulation of ROS Accumulation and Cell Autophagy in Wheat Roots under Hypoxia Stress. Plant Physiol. Biochem. 2021, 158, 91–102. [Google Scholar] [CrossRef]
- Sahi, C.; Singh, A.; Blumwald, E.; Grover, A. Beyond Osmolytes and Transporters: Novel Plant Salt-stress Tolerance-related Genes from Transcriptional Profiling Data. Physiol. Plant. 2010, 127, 1–9. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, M.J.; Guo, Y.P. Regulation of Photosynthesis by Light Quality and Its Mechanism in Plants. Ying Yong Sheng Tai Xue Bao 2008, 19, 1619–1624. [Google Scholar]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of Salinity Stress on Chloroplast Structure and Function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Nosek, M.; Gawrońska, K.; Rozpądek, P.; Sujkowska-Rybkowska, M.; Miszalski, Z.; Kornaś, A. At the Edges of Photosynthetic Metabolic Plasticity-On the Rapidity and Extent of Changes Accompanying Salinity Stress-Induced CAM Photosynthesis Withdrawal. Int. J. Mol. Sci. 2021, 22, 8426. [Google Scholar] [CrossRef]
- Ré, D.A.; Capella, M.; Bonaventure, G.; Chan, R.L. Arabidopsis AtHB7 and AtHB12 Evolved Divergently to Fine Tune Processes Associated with Growth and Responses to Water Stress. BMC Plant Biol. 2014, 14, 150. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Zhang, L.; Cui, Y.N.; Wang, S.M.; Bao, A.K. Identification of Candidate Genes Related to Salt Tolerance of the Secretohalophyte Atriplex Canescens by Transcriptomic Analysis. BMC Plant Biol. 2019, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Ngara, R.; Ndimba, R.; Borch-Jensen, J.; Jensen, O.N.; Ndimba, B. Identification and Profiling of Salinity Stress-Responsive Protein in Sorghum Bicolor Seedlings. J. Proteom. 2012, 75, 4139–4150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory Metabolism: Glycolysis, the TCA Cycle and Mitochondrial Electron Transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Swart, C.; Alseekh, S.; Scossa, F.; Jiang, L.; Obata, T.; Graf, A.; Fernie, A.R. The Extra-Pathway Interactome of the TCA Cycle: Expected and Unexpected Metabolic Interactions. Plant Physiol. 2018, 177, 966–979. [Google Scholar] [CrossRef] [Green Version]
- Clough, S. Floral Dip: A Simplified Method for Transformation of Arabidopsis. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.; Tan, B.; Kelley, T.M.; Tian, J.; Chen, S. Physiological Changes in Mesembryanthemum crystallinum During the C3 to CAM Transition Induced by Salt Stress. Front. Plant Sci. 2020, 11, 283. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cheng, Z.; Yao, W.; Zhao, K.; Jiang, T. Functional Characterization of PsnNAC036 under Salinity and High Temperature Stresses. Int. J. Mol. Sci. 2021, 22, 2656. [Google Scholar] [CrossRef]
- Zhang, T.; Schneider, J.D.; Lin, C.; Geng, S.; Ma, T.; Lawrence, S.R.; Dufresne, C.P.; Harmon, A.C.; Chen, S. MPK4 Phosphorylation Dynamics and Interacting Proteins in Plant Immunity. J. Proteome Res. 2019, 18, 826–840. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tan, B.; Cheng, Z.; Zhu, D.; Jiang, T.; Chen, S. Overexpression of McHB7 Transcription Factor from Mesembryanthemum crystallinum Improves Plant Salt Tolerance. Int. J. Mol. Sci. 2022, 23, 7879. https://doi.org/10.3390/ijms23147879
Zhang X, Tan B, Cheng Z, Zhu D, Jiang T, Chen S. Overexpression of McHB7 Transcription Factor from Mesembryanthemum crystallinum Improves Plant Salt Tolerance. International Journal of Molecular Sciences. 2022; 23(14):7879. https://doi.org/10.3390/ijms23147879
Chicago/Turabian StyleZhang, Xuemei, Bowen Tan, Zihan Cheng, Dan Zhu, Tingbo Jiang, and Sixue Chen. 2022. "Overexpression of McHB7 Transcription Factor from Mesembryanthemum crystallinum Improves Plant Salt Tolerance" International Journal of Molecular Sciences 23, no. 14: 7879. https://doi.org/10.3390/ijms23147879
APA StyleZhang, X., Tan, B., Cheng, Z., Zhu, D., Jiang, T., & Chen, S. (2022). Overexpression of McHB7 Transcription Factor from Mesembryanthemum crystallinum Improves Plant Salt Tolerance. International Journal of Molecular Sciences, 23(14), 7879. https://doi.org/10.3390/ijms23147879







