Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line
Abstract
:1. Introduction
2. Results
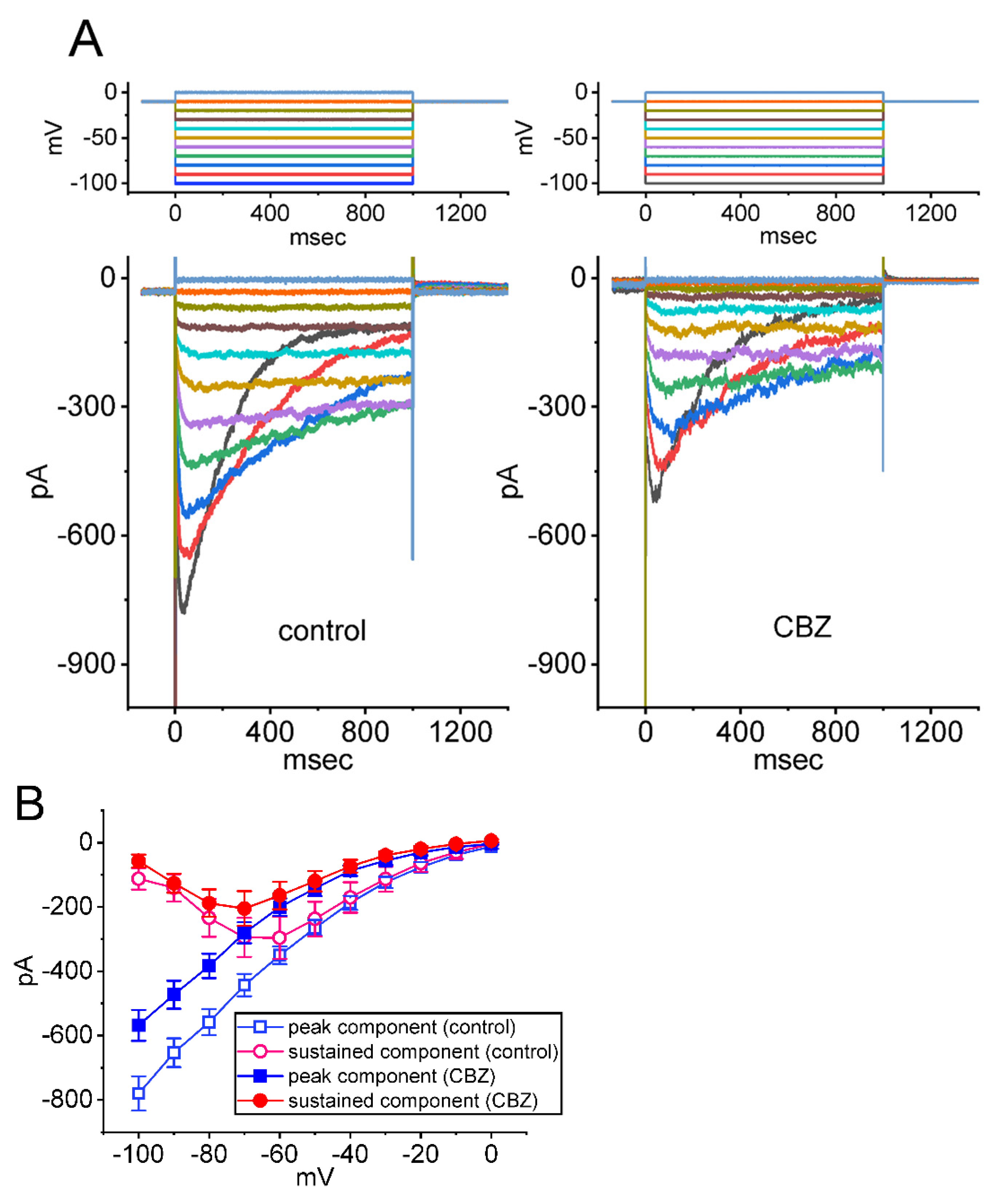
2.1. Modification by the CBZ Presence of Voltage-Gated Na+ Current (INa) Measured in Neuro-2a Cells
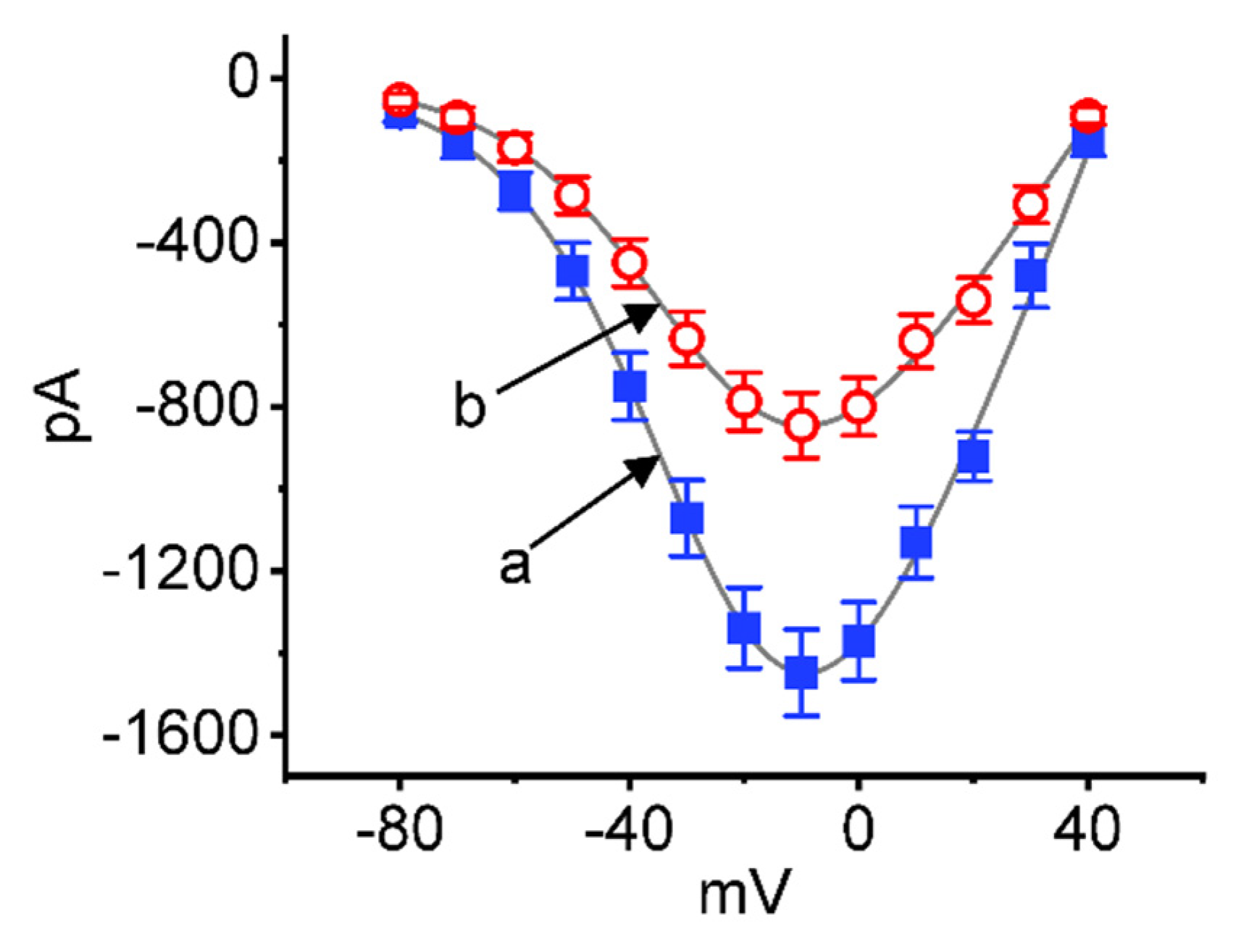
2.2. Effect of CBZ on the Quasi-Steady-State Current–Voltage (I–V) Relationship of INa(T)
2.3. Effect of CBZ on the Window Component of INa (INa(W)) Recorded from Neuro-2a Cells
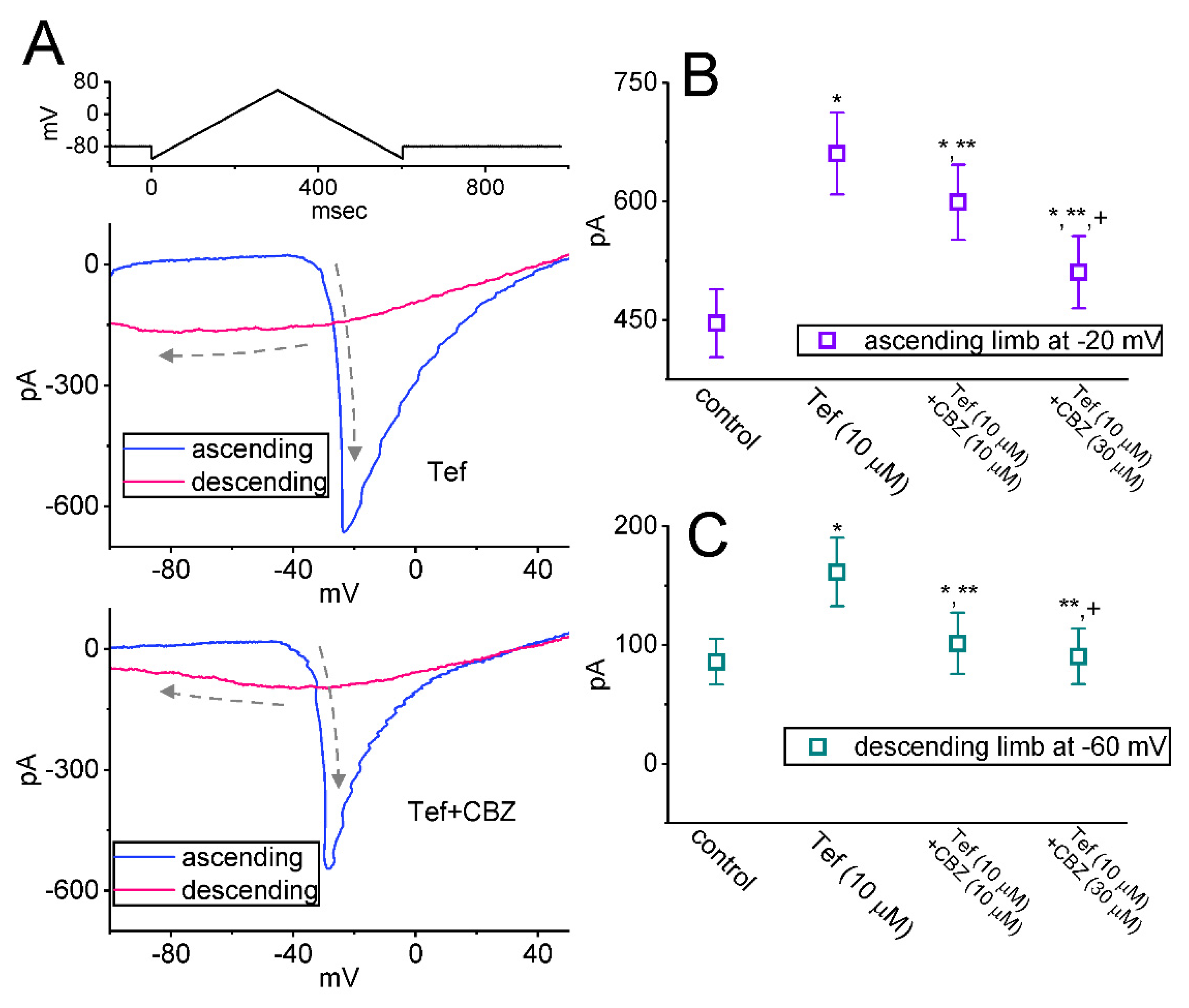
2.4. Effect of CBZ on the Hysteretic Behavior of Persistent Na+ Current (INa(P)) Triggered by Upright Isosceles-Triangular Vramp
2.5. Effect of CBZ on the Recovery from INa(T) Inactivation Evoked during Varying Interpulse Intervals
2.6. CBZ-Induced Increase in Cumulative Inhibition of INa(T) Inactivation in Neuro-2a Cells
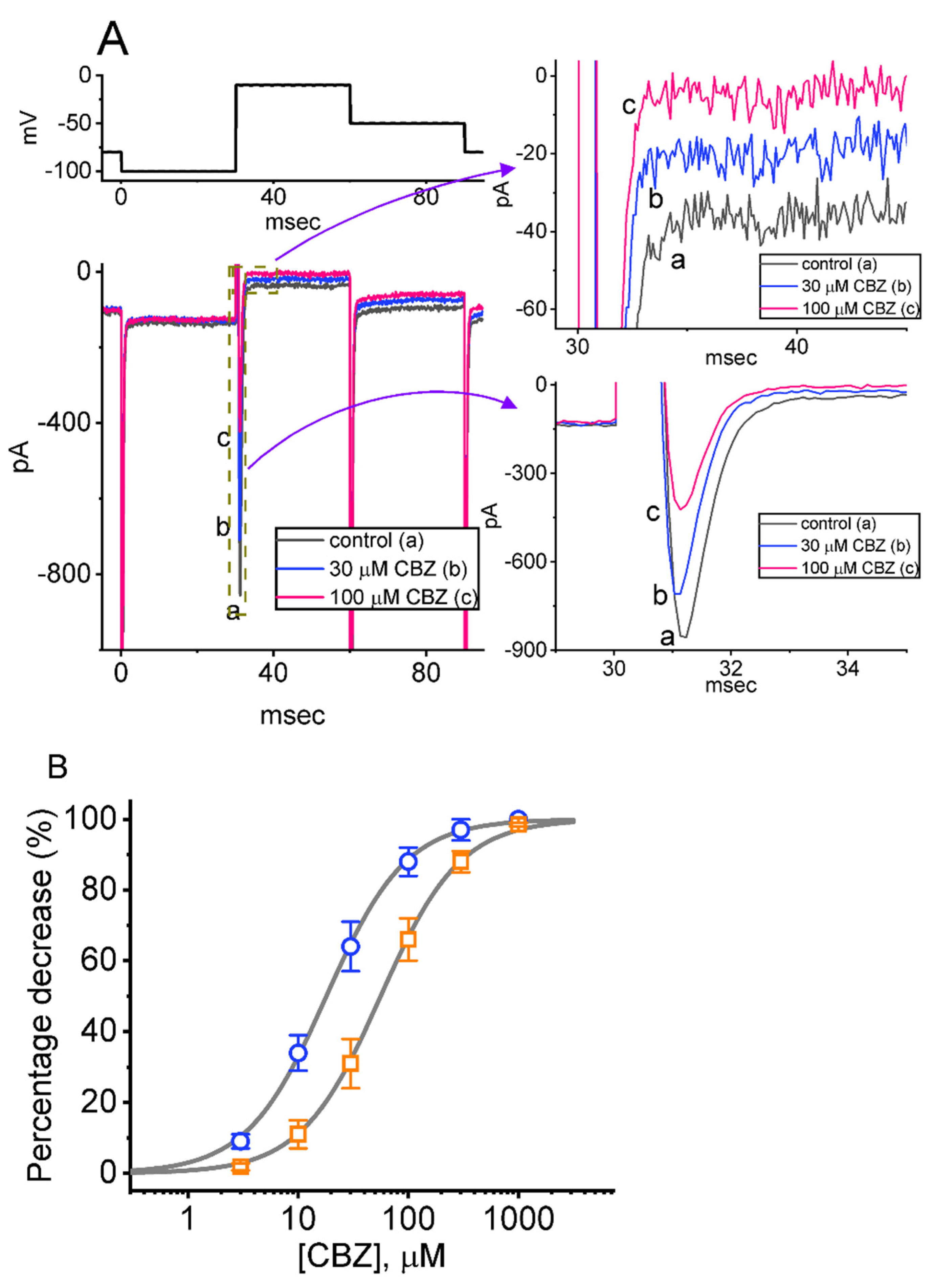
2.7. Inhibition of Erg-Mediated K+ Current (IK(erg)) Caused by CBZ
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs, Reagents and Solutions Used in This Work
4.2. Cell Preparations
4.3. Patch-Clamp Recordings: Electrophysiological Measurements
4.4. Data Analyses
4.5. Curve-Fitting Approximations and Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBZ | carbamazepine (Tegretol®, 5H-dibenzo[b,f]azepine-5-carboxamide, benzo[b][1]benzazepine-11-carboxamide) |
| erg | ether-à-go-go-related gene |
| Hys(V) | voltage-dependent hysteresis |
| I-V | current versus voltage |
| IC50 | the concentration required from half-maximal inhibition |
| IK(erg) | erg-mediated K+ current |
| INa | voltage-gated Na+ current |
| INa(L) | late (sustained) component of INa |
| INa(P) | persistent INa |
| INa(T) | transient (peak) component of INa |
| INa(W) | window INa |
| KATP channel | ATP-sensitive K+ channel |
| NaV (SCN) channel | voltage-gated Na+ channel |
| SEM | standard error of mean |
| τinact(S) | slow component in the inactivation time constant |
| Tef | tefluthrin |
| TTX | tetrodotoxin |
| Vramp | ramp voltage |
References
- Brodie, M.J. Sodium Channel Blockers in the Treatment of Epilepsy. CNS Drugs 2017, 31, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Araya, E.I.; Claudino, R.F.; Piovesan, E.J.; Chichorro, J.G. Trigeminal Neuralgia: Basic and Clinical Aspects. Curr. Neuropharmacol. 2020, 18, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L.; Zakrzewska, J.M.; Heinskou, T.B.; Hodaie, M.; Leal, P.R.L.; Nurmikko, T.; Obermann, M.; Cruccu, G.; Maarbjerg, S. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020, 19, 784–796. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef]
- Bridwell, R.E.; Brown, S.; Clerkin, S.; Birdsong, S.; Long, B. Neurologic toxicity of carbamazepine in treatment of trigeminal neuralgia. Am. J. Emerg. Med. 2022, 55, 231.e3–231.e5. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Helfer, B.; Dold, M.; Kissling, W.; McGrath, J. Carbamazepine for schizophrenia. Cochrane Database Syst. Rev. 2014, 2014, Cd001258. [Google Scholar] [CrossRef] [Green Version]
- Jitpimolmard, N.; Matthews, E.; Fialho, D. Treatment Updates for Neuromuscular Channelopathies. Curr. Treat. Options Neurol. 2020, 22, 34. [Google Scholar] [CrossRef]
- Grunze, A.; Amann, B.L.; Grunze, H. Efficacy of Carbamazepine and Its Derivatives in the Treatment of Bipolar Disorder. Medicina 2021, 57, 433. [Google Scholar] [CrossRef]
- Fuseya, Y.; Ishikawa, N.; Sasaki, R.; Yamashita, H. Teaching Video NeuroImage: Carbamazepine Improves Gait Initiation in Autosomal Recessive Myotonia Congenita. Neurology 2022, 98, e328. [Google Scholar] [CrossRef]
- Vasconcelos-Ferreira, A.; Carmo-Silva, S.; Codêsso, J.M.; Silva, P.; Martinez, A.R.M.; França, M.C., Jr.; Nóbrega, C.; Pereira de Almeida, L. The autophagy-enhancing drug carbamazepine improves neuropathology and motor impairment in mouse models of Machado-Joseph disease. Neuropathol. Appl. Neurobiol. 2022, 48, e12763. [Google Scholar] [CrossRef]
- Vieweg, W.V.; Godleski, L.S. Carbamazepine and hyponatremia. Am. J. Psychiatry 1988, 145, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Yassa, R.; Iskandar, H.; Nastase, C.; Camille, Y. Carbamazepine and hyponatremia in patients with affective disorder. Am. J. Psychiatry 1988, 145, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Gandelman, M.S. Review of carbamazepine-induced hyponatremia. Prog. Neuropsychopharmacol. Biol. Psychiatry 1994, 18, 211–233. [Google Scholar] [CrossRef]
- Van Amelsvoort, T.; Bakshi, R.; Devaux, C.B.; Schwabe, S. Hyponatremia associated with carbamazepine and oxcarbazepine therapy: A review. Epilepsia 1994, 35, 181–188. [Google Scholar] [CrossRef]
- Kelly, B.D.; Hillery, J. Hyponatremia during carbamazepine therapy in patients with intellectual disability. J. Intellect. Disabil. Res. 2001, 45, 152–156. [Google Scholar] [CrossRef]
- Dong, X.; Leppik, I.E.; White, J.; Rarick, J. Hyponatremia from oxcarbazepine and carbamazepine. Neurology 2005, 65, 1976–1978. [Google Scholar] [CrossRef]
- Tateno, A.; Sawada, K.; Takahashi, I.; Hujiwara, Y. Carbamazepine-induced transient auditory pitch-perception deficit. Pediatr. Neurol. 2006, 35, 131–134. [Google Scholar] [CrossRef]
- Holtschmidt-Täschner, B.; Soyka, M. Hyponatremia-induced seizure during carbamazepine treatment. World J. Biol. Psychiatry 2007, 8, 51–53. [Google Scholar] [CrossRef]
- Berghuis, B.; de Haan, G.J.; van den Broek, M.P.; Sander, J.W.; Lindhout, D.; Koeleman, B.P. Epidemiology, pathophysiology and putative genetic basis of carbamazepine- and oxcarbazepine-induced hyponatremia. Eur. J. Neurol. 2016, 23, 1393–1399. [Google Scholar] [CrossRef] [Green Version]
- Berghuis, B.; van der Palen, J.; de Haan, G.J.; Lindhout, D.; Koeleman, B.P.C.; Sander, J.W. Carbamazepine- and oxcarbazepine-induced hyponatremia in people with epilepsy. Epilepsia 2017, 58, 1227–1233. [Google Scholar] [CrossRef]
- Berghuis, B.; Hulst, J.; Sonsma, A.; McCormack, M.; de Haan, G.J.; Sander, J.W.; Lindhout, D.; Koeleman, B.P.C. Symptomatology of carbamazepine- and oxcarbazepine-induced hyponatremia in people with epilepsy. Epilepsia 2021, 62, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Intravooth, T.; Staack, A.M.; Juerges, K.; Stockinger, J.; Steinhoff, B.J. Antiepileptic drugs-induced hyponatremia: Review and analysis of 560 hospitalized patients. Epilepsy Res. 2018, 143, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Palacios Argueta, P.J.; Sánchez Rosenberg, G.F.; Pineda, A. Walking hyponatremia syndrome of inappropriate antidiuretic hormone secretion secondary to carbamazepine use: A case report. J. Med. Case Rep. 2018, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Kaeley, N.; Kabi, A.; Bhatia, R.; Mohanty, A. Carbamazepine-induced hyponatremia—A wakeup call. J. Family Med. Prim. Care 2019, 8, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Altun, Y.; Yasar, E. Effects of valproate, carbamazepine and levetiracetam on Tp-e interval, Tp-e/QT and Tp-e/QTc ratio. Ideggyogy Sz 2020, 73, 121–127. [Google Scholar] [CrossRef]
- Asoğlu, R.; Özdemir, M.; Aladağ, N.; Asoğlu, E. Evaluation of Cardiac Repolarization Indices in Epilepsy Patients Treated with Carbamazepine and Valproic Acid. Medicina 2020, 56, 20. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Jo, C.H.; Kim, G.H. The Role of Vasopressin V2 Receptor in Drug-Induced Hyponatremia. Front. Physiol. 2021, 12, 797039. [Google Scholar] [CrossRef]
- Okayasu, H.; Shinozaki, T.; Takano, Y.; Sugawara, N.; Fujii, K.; Yasui-Furukori, N.; Ozeki, Y.; Shimoda, K. Effects of Antipsychotics on Arrhythmogenic Parameters in Schizophrenia Patients: Beyond Corrected QT Interval. Neuropsychiatr. Dis. Treat. 2021, 17, 239–249. [Google Scholar] [CrossRef]
- Chen, P.C.; Olson, E.M.; Zhou, Q.; Kryukova, Y.; Sampson, H.M.; Thomas, D.Y.; Shyng, S.L. Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism. J. Biol. Chem. 2013, 288, 20942–20954. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Lin, Y.C.; Yang, S.B.; Chen, P.C. Carbamazepine promotes surface expression of mutant Kir6.2-A28V ATP-sensitive potassium channels by modulating Golgi retention and autophagy. J. Biol. Chem. 2022, 298, 101904. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, F.; Feng, Y.; Wang, H. Carbamazepine Restores Neuronal Signaling, Protein Synthesis, and Cognitive Function in a Mouse Model of Fragile X Syndrome. Int. J. Mol. Sci. 2020, 21, 9327. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.C.; Werkman, T.R.; Wadman, W.J. Kinetic changes and modulation by carbamazepine on voltage-gated sodium channels in rat CA1 neurons after epilepsy. Acta Pharmacol. Sin. 2006, 27, 1537–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theile, J.W.; Cummins, T.R. Inhibition of Navβ4 peptide-mediated resurgent sodium currents in Nav1.7 channels by carbamazepine, riluzole, and anandamide. Mol. Pharmacol. 2011, 80, 724–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.; Bean, B.P. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol. Pharmacol. 2014, 85, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Petty, S.J.; Milligan, C.J.; Todaro, M.; Richards, K.L.; Kularathna, P.K.; Pagel, C.N.; French, C.R.; Hill-Yardin, E.L.; O’Brien, T.J.; Wark, J.D.; et al. The antiepileptic medications carbamazepine and phenytoin inhibit native sodium currents in murine osteoblasts. Epilepsia 2016, 57, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Wu, S.N.; Chen, B.S.; Hsu, T.I.; Peng, H.; Wu, Y.H.; Lo, Y.C. Analytical studies of rapidly inactivating and noninactivating sodium currents in differentiated NG108-15 neuronal cells. J. Theor. Biol. 2009, 259, 828–836. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Characterization of Direct Perturbations on Voltage-Gated Sodium Current by Esaxerenone, a Nonsteroidal Mineralocorticoid Receptor Blocker. Biomedicines 2021, 9, 549. [Google Scholar] [CrossRef]
- Chen, B.S.; Lo, Y.C.; Peng, H.; Hsu, T.I.; Wu, S.N. Effects of ranolazine, a novel anti-anginal drug, on ion currents and membrane potential in pituitary tumor GH(3) cells and NG108-15 neuronal cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.N.; So, E.C.; Liao, Y.K.; Huang, Y.M. Reversal by ranolazine of doxorubicin-induced prolongation in the inactivation of late sodium current in rat dorsal root ganglion neurons. Pain Med. 2015, 16, 1032–1034. [Google Scholar] [CrossRef] [Green Version]
- Chuang, T.H.; Cho, H.Y.; Wu, S.N. The Evidence for Sparsentan-Mediated Inhibition of I(Na) and I(K(erg)): Possibly Unlinked to Its Antagonism of Angiotensin II or Endothelin Type a Receptor. Biomedicines 2021, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Chen, P.C.; Chuang, T.H.; Yu, M.C.; Wu, S.N. Activation of Voltage-Gated Na(+) Current by GV-58, a Known Activator of Ca(V) Channels. Biomedicines 2022, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Wu, Y.H.; Chen, B.S.; Lo, Y.C.; Liu, Y.C. Underlying mechanism of actions of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology 2009, 258, 70–77. [Google Scholar] [CrossRef]
- Tan, J.; Soderlund, D.M. Actions of Tefluthrin on Rat Na(v)1.7 Voltage-Gated Sodium Channels Expressed in Xenopus Oocytes. Pestic. Biochem. Physiol. 2011, 101, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of I(Na) activation and inactivation as well as on resurgent and persistent I(Na) in a pituitary cell line (GH(3)). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.Y.; Laezza, F.; Gerber, B.R.; Xiao, M.; Yamada, K.A.; Hartmann, H.; Craig, A.M.; Nerbonne, J.M.; Ornitz, D.M. Fibroblast growth factor 14 is an intracellular modulator of voltage-gated sodium channels. J. Physiol. 2005, 569, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Martiszus, B.J.; Tsintsadze, T.; Chang, W.; Smith, S.M. Enhanced excitability of cortical neurons in low-divalent solutions is primarily mediated by altered voltage-dependence of voltage-gated sodium channels. eLife 2021, 10, e67914. [Google Scholar] [CrossRef]
- Wu, C.L.; Chuang, C.W.; Cho, H.Y.; Chuang, T.H.; Wu, S.N. The Evidence for Effective Inhibition of I(Na) Produced by Mirogabalin ((1R,5S,6S)-6-(aminomethyl)-3-ethyl-bicyclo [3.2.0] hept-3-ene-6-acetic acid), a Known Blocker of Ca(V) Channels. Int. J. Mol. Sci. 2022, 23, 3845. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Effectiveness of Columbianadin, a Bioactive Coumarin Derivative, in Perturbing Transient and Persistent I(Na). Int. J. Mol. Sci. 2021, 22, 621. [Google Scholar] [CrossRef]
- Morris, C.E.; Boucher, P.A.; Joós, B. Left-shifted nav channels in injured bilayer: Primary targets for neuroprotective nav antagonists? Front. Pharmacol. 2012, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Tryba, A.K.; Ramirez, J.M. Background sodium current stabilizes bursting in respiratory pacemaker neurons. J. Neurobiol. 2004, 60, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Ben-Mabrouk, F.; Tryba, A.K. Background sodium current underlying respiratory rhythm regularity. Eur. J. Neurosci. 2008, 28, 2423–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.N.; Lo, Y.C.; Shen, A.Y.; Chen, B.S. Contribution of non-inactivating Na+ current induced by oxidizing agents to the firing behavior of neuronal action potentials: Experimental and theoretical studies from NG108-15 neuronal cells. Chin. J. Physiol. 2011, 54, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Khaliq, Z.M.; Bean, B.P. Pacemaking in dopaminergic ventral tegmental area neurons: Depolarizing drive from background and voltage-dependent sodium conductances. J. Neurosci. 2010, 30, 7401–7413. [Google Scholar] [CrossRef]
- Guérineau, N.C.; Monteil, A.; Lory, P. Sodium background currents in endocrine/neuroendocrine cells: Towards unraveling channel identity and contribution in hormone secretion. Front. Neuroendocrinol. 2021, 63, 100947. [Google Scholar] [CrossRef] [PubMed]
- Taddese, A.; Bean, B.P. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 2002, 33, 587–600. [Google Scholar] [CrossRef] [Green Version]
- Tsai, D.; Morley, J.W.; Suaning, G.J.; Lovell, N.H. Frequency-dependent reduction of voltage-gated sodium current modulates retinal ganglion cell response rate to electrical stimulation. J. Neural. Eng. 2011, 8, 066007. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.A.; Salari, A.; Lin, J.L.; Cowan, L.M.; Penington, N.J.; Milescu, M.; Milescu, L.S. Sodium channels implement a molecular leaky integrator that detects action potentials and regulates neuronal firing. eLife 2020, 9, e54940. [Google Scholar] [CrossRef]
- Schwarz, J.R.; Bauer, C.K. Functions of erg K+ channels in excitable cells. J. Cell Mol. Med. 2004, 8, 22–30. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Activation of voltage-gated sodium current and inhibition of erg-mediated potassium current caused by telmisartan, an antagonist of angiotensin II type-1 receptor, in HL-1 atrial cardiomyocytes. Clin. Exp. Pharmacol. Physiol. 2018, 45, 797–807. [Google Scholar] [CrossRef]
- Chen, L.; Cho, H.Y.; Chuang, T.H.; Ke, T.L.; Wu, S.N. The Effectiveness of Isoplumbagin and Plumbagin in Regulating Amplitude, Gating Kinetics, and Voltage-Dependent Hysteresis of erg-mediated K(+) Currents. Biomedicines 2022, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Ruan, J.S.; Wu, S.N. Evidence of Decreased Activity in Intermediate-Conductance Calcium-Activated Potassium Channels During Retinoic Acid-Induced Differentiation in Motor Neuron-Like NSC-34 Cells. Cell Physiol. Biochem. 2018, 48, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Bovee, T.F.; Kamelia, L.; Rietjens, I.M.; Hendriksen, P.J. Exploration of new functional endpoints in neuro-2a cells for the detection of the marine biotoxins saxitoxin, palytoxin and tetrodotoxin. Toxicol. In Vitro 2015, 30 Pt 1, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.N.; Tang, H.L.; Lin, G.W.; Chen, Y.H.; Lu, P.; Li, H.J.; Gao, M.M.; Zhao, Q.H.; Yi, Y.H.; Liao, W.P.; et al. Epigenetic Downregulation of Scn3a Expression by Valproate: A Possible Role in Its Anticonvulsant Activity. Mol. Neurobiol. 2017, 54, 2831–2842. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Chiba, Y.; Nakazaki, A.; Ishikawa, Y.; Nakane, Y.; Cho, Y.; Yotsu-Yamashita, M.; Nishikawa, T.; Wakamori, M.; Konoki, K. Inhibition of veratridine-induced delayed inactivation of the voltage-sensitive sodium channel by synthetic analogs of crambescin B. Bioorg. Med. Chem. Lett. 2017, 27, 1247–1251. [Google Scholar] [CrossRef]
- Khan, F.; Saify, Z.S.; Jamali, K.S.; Naz, S.; Hassan, S.; Siddiqui, S. Vitex negundo induces an anticonvulsant effect by inhibiting voltage gated sodium channels in murine Neuro 2A cell line. Pak. J. Pharm. Sci. 2018, 31 (Suppl. S1), 297–303. [Google Scholar]
- Chuang, T.H.; Cho, H.Y.; Wu, S.N. Effective Accentuation of Voltage-Gated Sodium Current Caused by Apocynin (4′-Hydroxy-3′-methoxyacetophenone), a Known NADPH-Oxidase Inhibitor. Biomedicines 2021, 9, 1146. [Google Scholar] [CrossRef]
- Villalba-Galea, C.A.; Chiem, A.T. Hysteretic Behavior in Voltage-Gated Channels. Front. Pharmacol. 2020, 11, 579596. [Google Scholar] [CrossRef]
- Popović, J.; Mikov, M.; Jakovljevic, V. Pharmacokinetics of carbamazepine derived from a new tablet formulation. Eur. J. Drug Metab. Pharmacokinet. 1995, 20, 297–300. [Google Scholar] [CrossRef]
- Hill, A.C.; Rower, J.E.; White, H.S. The pharmacokinetics of oral carbamazepine in rats dosed using an automated drug delivery system. Epilepsia 2019, 60, 1829–1837. [Google Scholar] [CrossRef]
- Van Calker, D.; Steber, R.; Klotz, K.N.; Greil, W. Carbamazepine distinguishes between adenosine receptors that mediate different second messenger responses. Eur. J. Pharmacol. 1991, 206, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Sula, A.; Hollingworth, D.; Ng, L.C.T.; Larmore, M.; DeCaen, P.G.; Wallace, B.A. A tamoxifen receptor within a voltage-gated sodium channel. Mol. Cell 2021, 81, 1160–1169.e5. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, M.; Curia, G.; Biagini, G.; Ragsdale, D.S.; Avoli, M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010, 9, 413–424. [Google Scholar] [CrossRef]
- Tibbs, G.R.; Posson, D.J.; Goldstein, P.A. Voltage-Gated Ion Channels in the PNS: Novel Therapies for Neuropathic Pain? Trends Pharmacol. Sci. 2016, 37, 522–542. [Google Scholar] [CrossRef]
- Im, J.K.; Son, H.S.; Kang, Y.M.; Zoh, K.D. Carbamazepine degradation by photolysis and titanium dioxide photocatalysis. Water Environ. Res. 2012, 84, 554–561. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, S.; Wu, Y.; Sheng, D.; Bu, L.; Zhou, S. Insights into the wavelength-dependent photolysis of chlorite: Elimination of carbamazepine and formation of chlorate. Chemosphere 2022, 288, 132505. [Google Scholar] [CrossRef]
- Olmsted, J.B.; Carlson, K.; Klebe, R.; Ruddle, F.; Rosenbaum, J. Isolation of microtubule protein from cultured mouse neuroblastoma cells. Proc. Natl. Acad. Sci. USA 1970, 65, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Methods 2010, 186, 60–67. [Google Scholar] [CrossRef]
- Markgraf, R.; Leipold, E.; Schirmeyer, J.; Paolini-Bertrand, M.; Hartley, O.; Heinemann, S.H. Mechanism and molecular basis for the sodium channel subtype specificity of µ-conopeptide CnIIIC. Br. J. Pharmacol. 2012, 167, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Chen, Y.C.; Chang, Y.C.; Wang, L.W.; Lin, Y.C.; Chiang, Y.L.; Ho, C.J.; Huang, C.C. Human umbilical vein endothelial cells protect against hypoxic-ischemic damage in neonatal brain via stromal cell-derived factor 1/C-X-C chemokine receptor type 4. Stroke 2013, 44, 1402–1409. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-M.; Cho, H.-Y.; Chiang, C.-W.; Chuang, T.-H.; Wu, S.-N.; Tu, Y.-F. Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line. Int. J. Mol. Sci. 2022, 23, 7892. https://doi.org/10.3390/ijms23147892
Wu P-M, Cho H-Y, Chiang C-W, Chuang T-H, Wu S-N, Tu Y-F. Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line. International Journal of Molecular Sciences. 2022; 23(14):7892. https://doi.org/10.3390/ijms23147892
Chicago/Turabian StyleWu, Po-Ming, Hsin-Yen Cho, Chi-Wu Chiang, Tzu-Hsien Chuang, Sheng-Nan Wu, and Yi-Fang Tu. 2022. "Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line" International Journal of Molecular Sciences 23, no. 14: 7892. https://doi.org/10.3390/ijms23147892
APA StyleWu, P.-M., Cho, H.-Y., Chiang, C.-W., Chuang, T.-H., Wu, S.-N., & Tu, Y.-F. (2022). Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line. International Journal of Molecular Sciences, 23(14), 7892. https://doi.org/10.3390/ijms23147892





