Early Gβγ-GRK2 Inhibition Ameliorates Osteoarthritis Development by Simultaneous Anti-Inflammatory and Chondroprotective Effects
Abstract
1. Introduction
2. Results
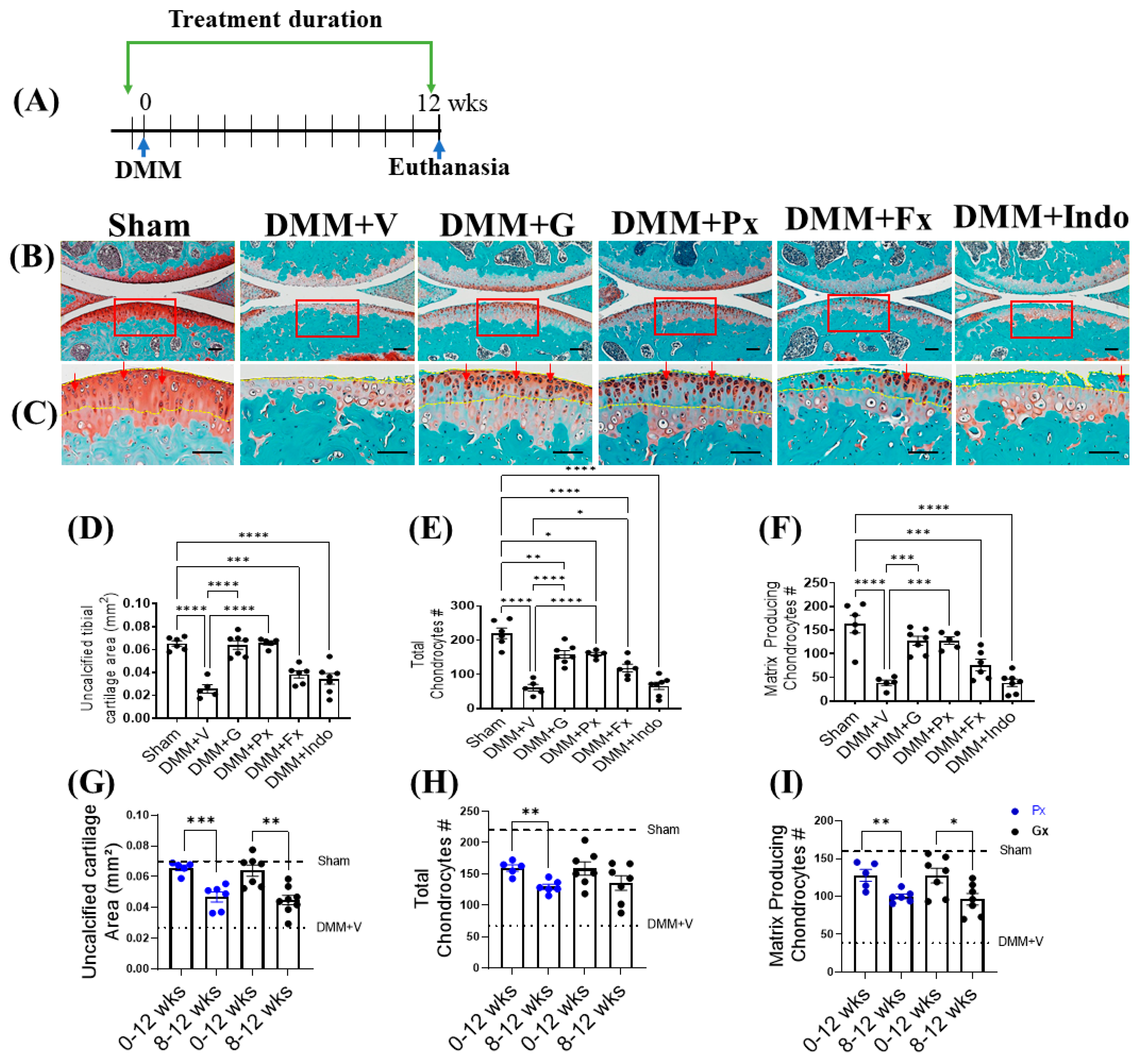
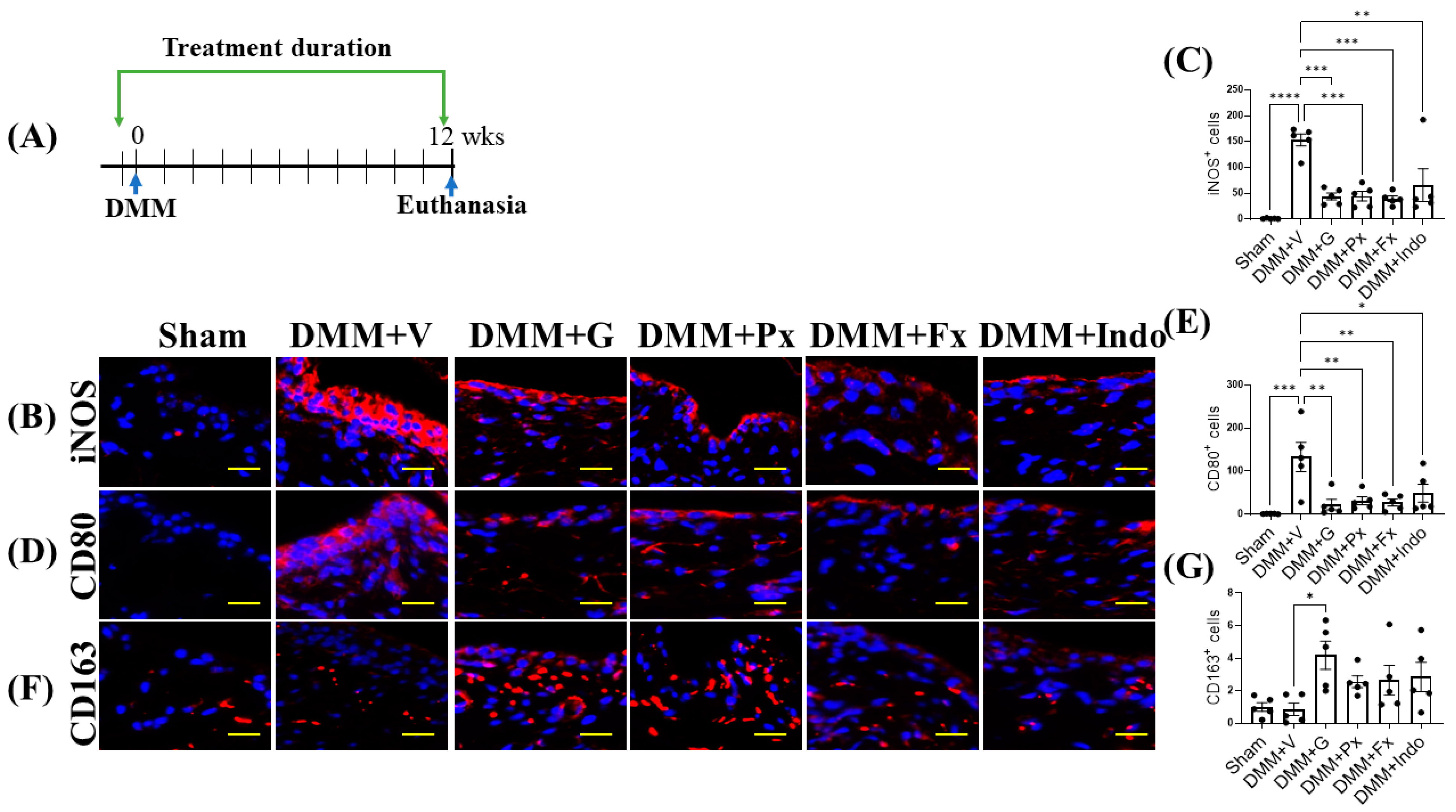
2.1. Early Continuous Gβγ-GRK2 Inhibition Attenuates OA Development in Late-Stage DMM, with Higher Therapeutic Efficacy Than Delayed Treatment
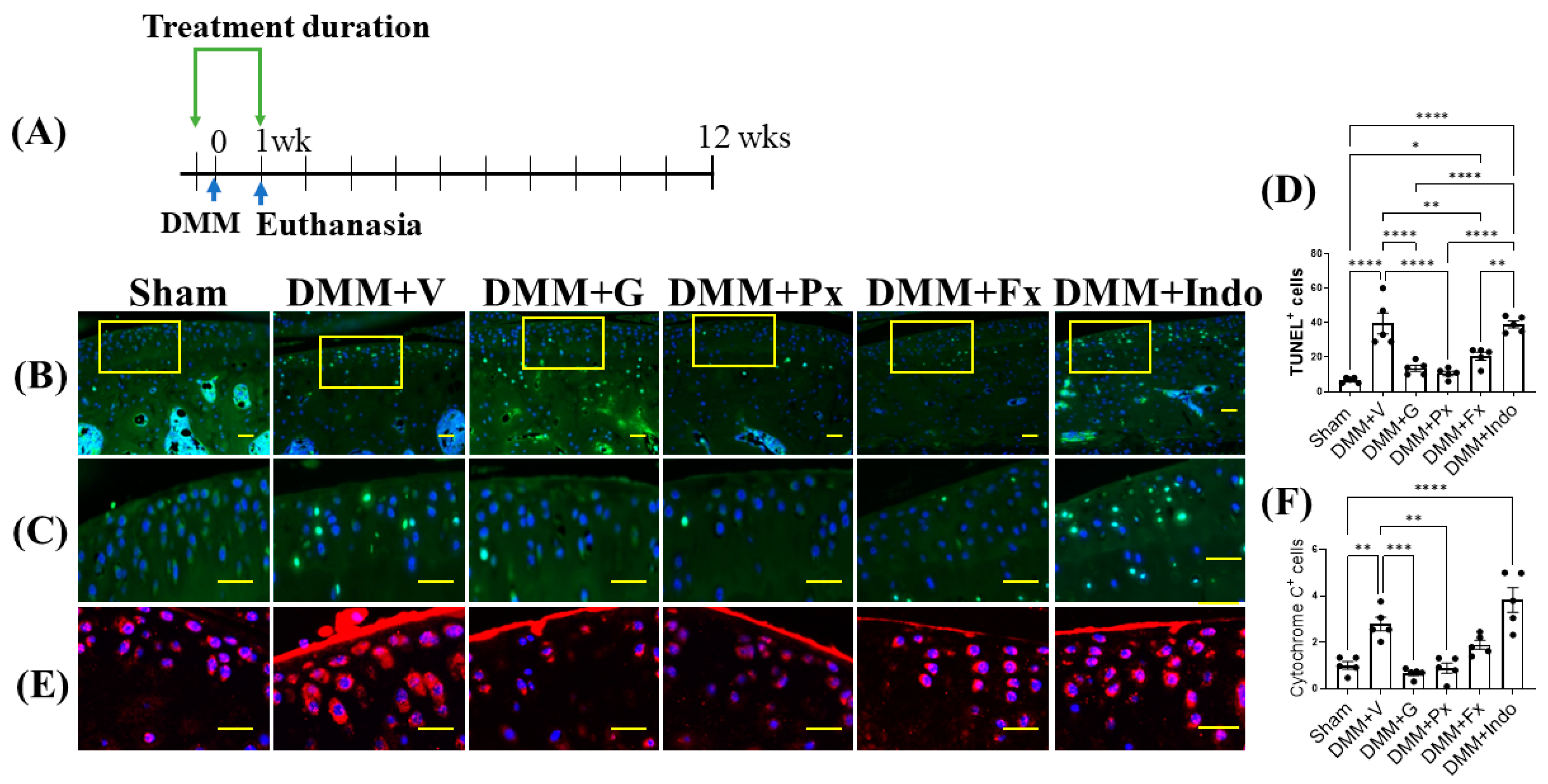
2.2. Early Gβγ-GRK2 Inhibition Attenuates Chondrocyte Apoptosis and Cytochrome C in the Acute Inflammatory Phase of DMM
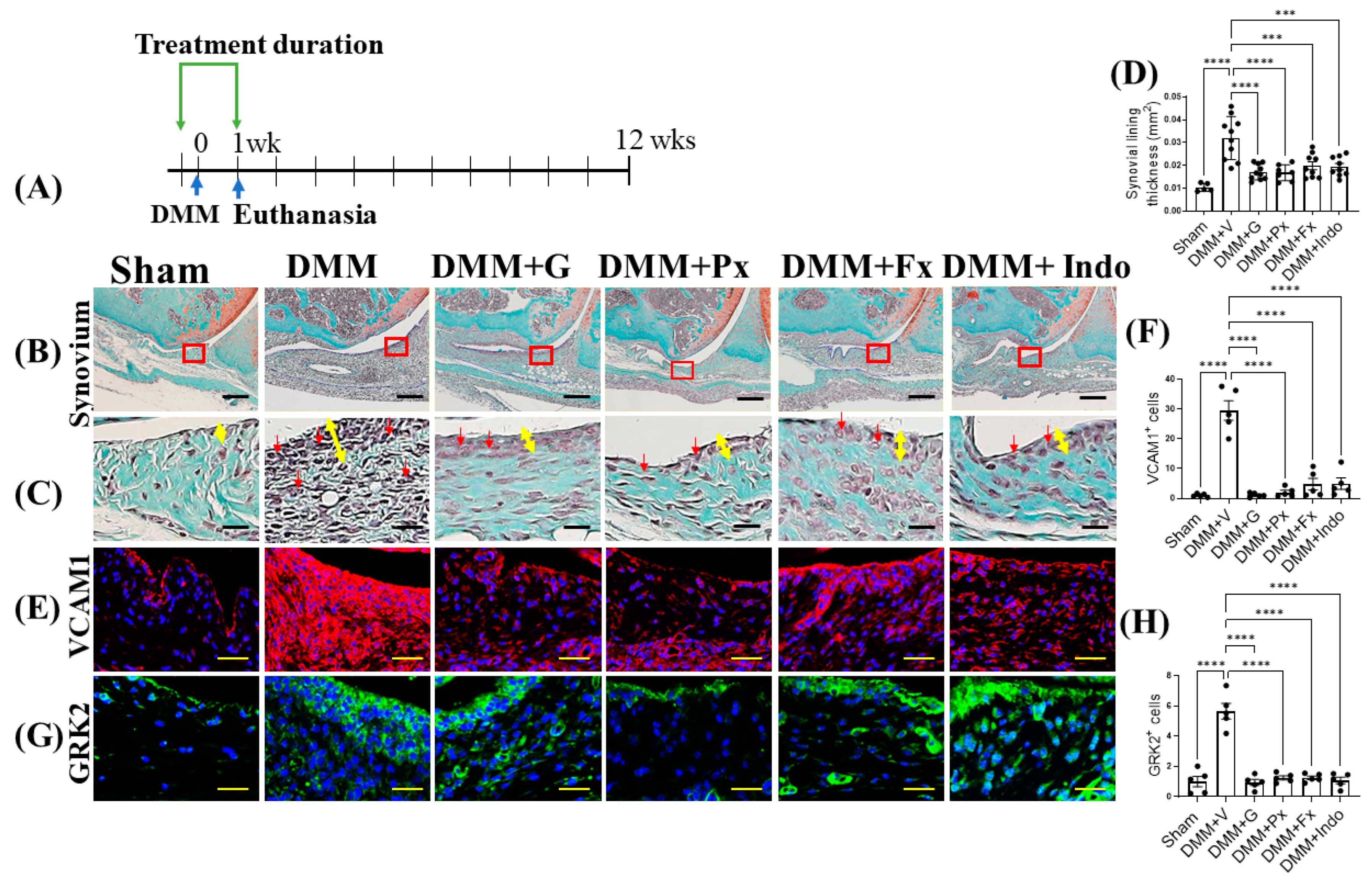
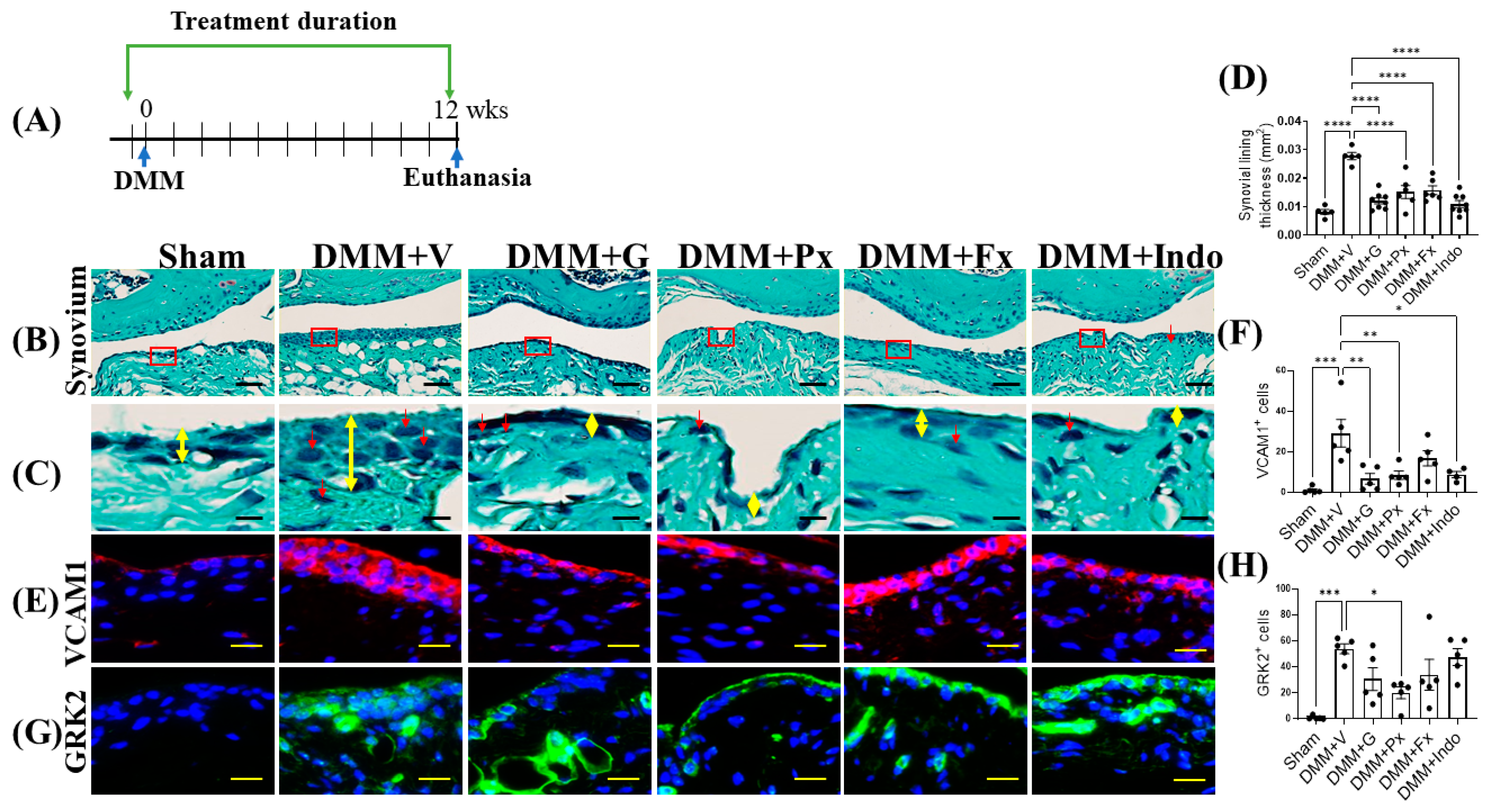
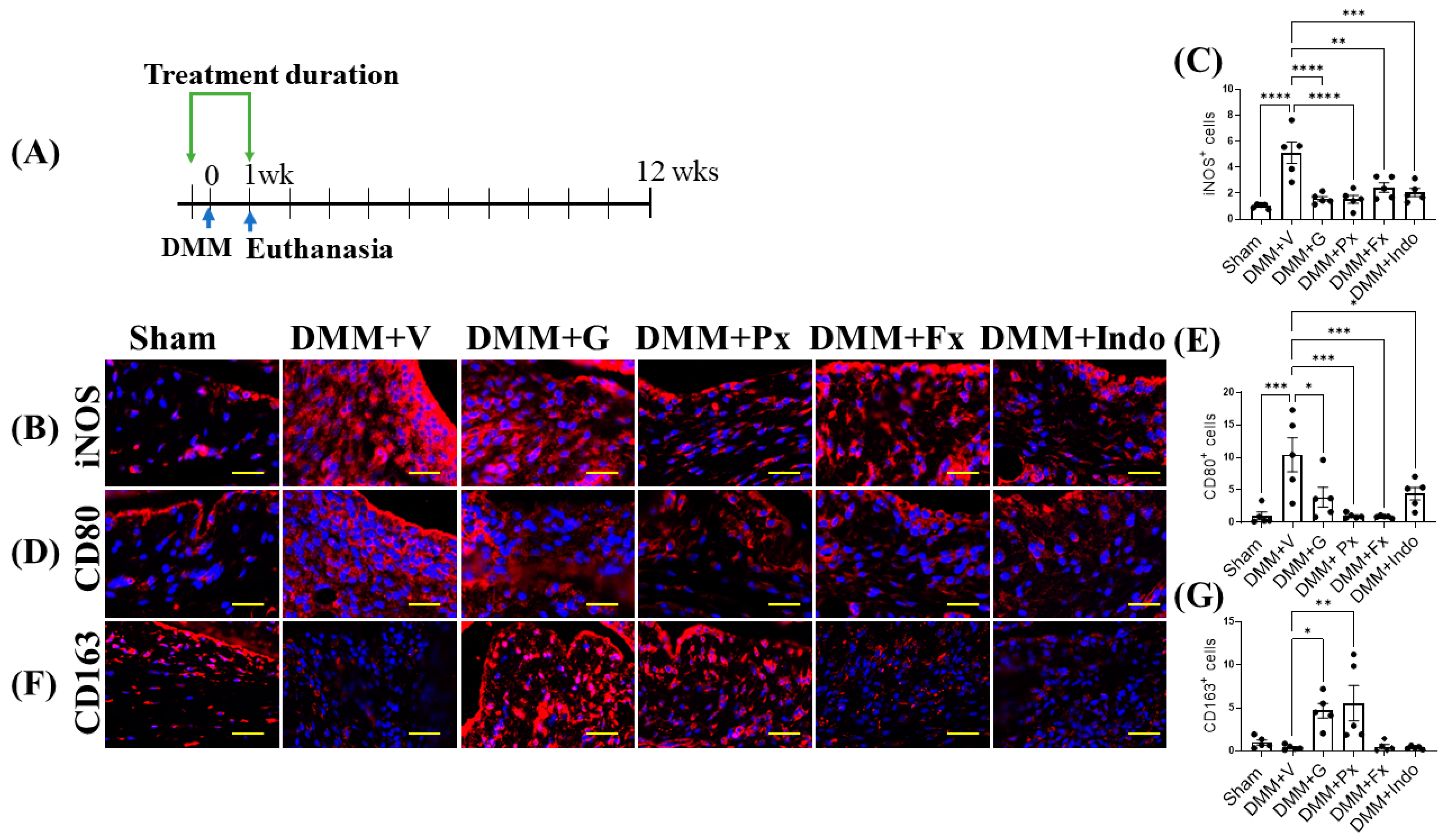
2.3. Elevated Synovial GRK2 Expression in the Early Inflammatory Phase following DMM Is Reduced by Gβγ-GRK2 Inhibition
2.4. Gβγ-GRK2 Inhibition Promotes M2 over M1 Macrophage Phenotype in Early and Late DMM
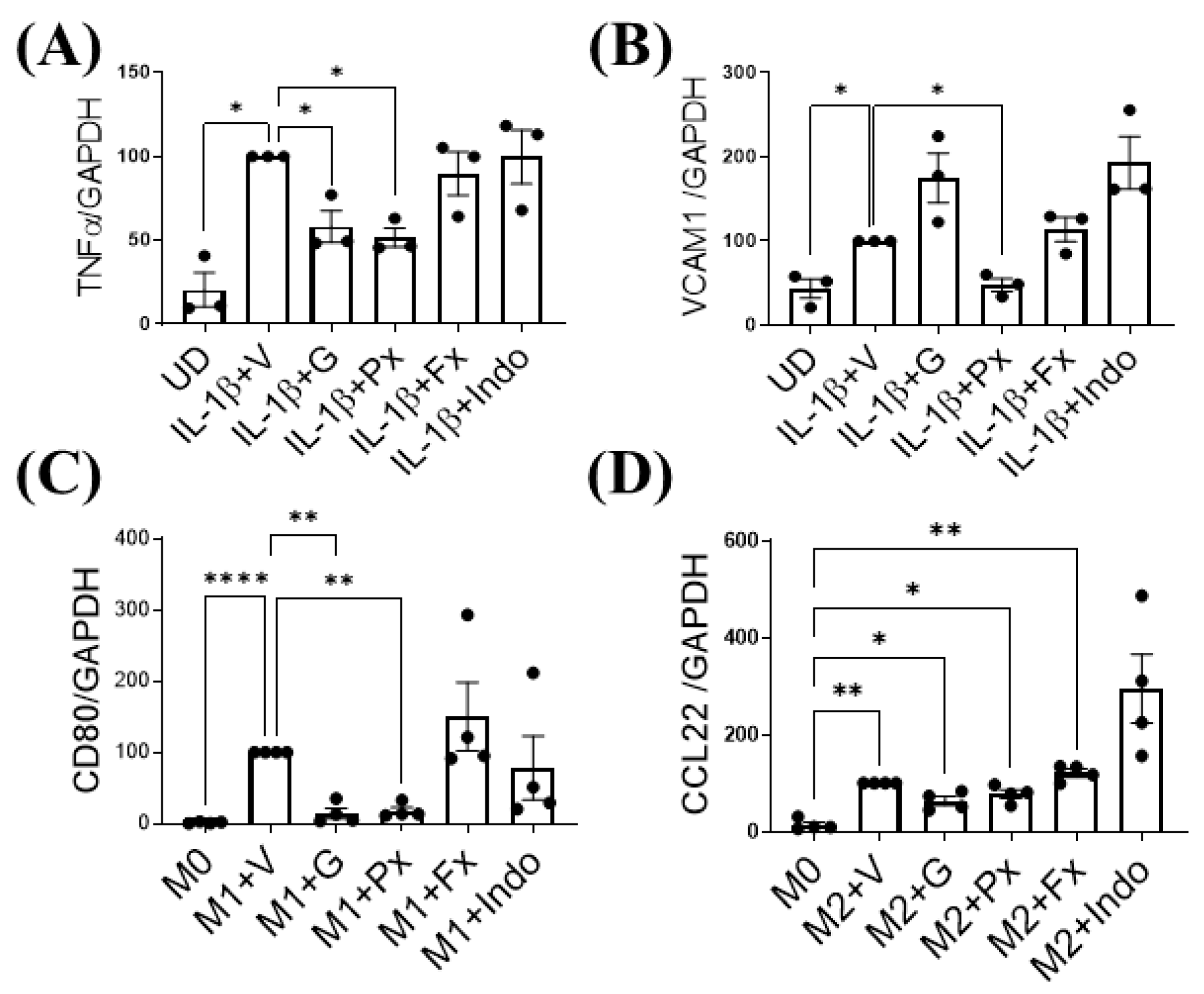
2.5. Gβγ-GRK2 Inhibition Ameliorates Human Synoviocytes and M1 Macrophage Inflammatory Differentiation In Vitro
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Study Design
4.3. Animals
4.4. DMM Surgery
4.5. Experimental Groups
4.6. Histomorphometry (Safranin-O/Fast Green) Coupled with Histomorphometry Using the Osteomeasure® System
4.7. Immunofluorescence (IF) Staining
4.8. Cell Culture and Treatment
- (i)
- Human THP1 cells were differentiated into resting (MΦ) macrophages (by treating with 100 nM phorbol 12-myristate 13-acetate (PMA) for 72 h). Next, these MΦ macrophages were differentiated into M1 macrophages (by treating with 100 ng/mL lipopolysaccharides (LPS) and 20 ng/mL IFN γ for 48 h) or M2 macrophages (by treating with 100 ng/mL interleukin (IL)-4 for 48 h) [48]. At the time of M1 and M2 macrophage differentiation, we treated cells with phosphate-buffered saline (PBS) (vehicle), 10 µM paroxetine (GRK2 inhibitor), 10 µM gallein (Gβγ inhibitor), 10 µM fluoxetine (SSRI control), or 50 µM indomethacin (anti-inflammatory control) for 48 h.
- (ii)
- SW982 synoviocytes were differentiated into the inflammatory phenotype (FLS) by treating cultures with 5 ng/mL IL-1β for 24 h [49]. At the time of synoviocyte differentiation, we treated with vehicle, paroxetine (10 µM), gallein (10 µM), fluoxetine (10 µM), or indomethacin (50 µM) for 24 h.
4.9. RNA Purification and Real Time-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.10. TUNEL Staining
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. eClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Atukorala, I.; Kwoh, C.K.; Guermazi, A.; Roemer, F.W.; Boudreau, R.M.; Hannon, M.J.; Hunter, D.J. Synovitis in knee osteoarthritis: A precursor of disease? Ann. Rheum. Dis. 2016, 75, 390–395. [Google Scholar] [CrossRef]
- Smith, M.D.; Barg, E.; Weedon, H.; Papengelis, V.; Smeets, T.; Tak, P.P.; Kraan, M.; Coleman, M.; Ahern, M.J. Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints. Ann. Rheum. Dis. 2003, 62, 303–307. [Google Scholar] [CrossRef]
- Wu, T.J.; Chang, S.L.; Lin, C.Y.; Lai, C.Y.; He, X.Y.; Tsai, C.H.; Ko, C.Y.; Fong, Y.C.; Su, C.M.; Tang, C.H. IL-17 Facilitates VCAM-1 Production and Monocyte Adhesion in Osteoarthritis Synovial Fibroblasts by Suppressing miR-5701 Synthesis. Int. J. Mol. Sci. 2022, 23, 6804. [Google Scholar] [CrossRef]
- Lee, H.R.; Yoo, S.J.; Kim, J.; Park, C.K.; Kang, S.W. Reduction of Oxidative Stress in Peripheral Blood Mononuclear Cells Attenuates the Inflammatory Response of Fibroblast-like Synoviocytes in Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 12411. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- Fahy, N.; de Vries-van Melle, M.L.; Lehmann, J.; Wei, W.; Grotenhuis, N.; Farrell, E.; van der Kraan, P.M.; Murphy, J.M.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthr. Cartil. 2014, 22, 1167–1175. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Bajpai, A.; Meng, S.; Arumugam, S.; Sreedhar, R.; Giridharan, V.V.; Guha, A.; Bhimaraj, A.; Youker, K.A.; Palaniyandi, S.S.; et al. Small molecule disruption of G protein betagamma subunit signaling reprograms human macrophage phenotype and prevents autoimmune myocarditis in rats. PLoS ONE 2018, 13, e0200697. [Google Scholar]
- Eijkelkamp, N.; Heijnen, C.J.; Willemen, H.L.; Deumens, R.; Joosten, E.A.; Kleibeuker, W.; den Hartog, I.J.; van Velthoven, C.T.; Nijboer, C.; Nassar, M.A.; et al. GRK2: A novel cell-specific regulator of severity and duration of inflammatory pain. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 2138–2149. [Google Scholar] [CrossRef]
- Willemen, H.L.; Huo, X.J.; Mao-Ying, Q.L.; Zijlstra, J.; Heijnen, C.J.; Kavelaars, A. MicroRNA-124 as a novel treatment for persistent hyperalgesia. J. Neuroinflamm. 2012, 9, 143. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Zhao, Y.; Li, S.; Zhao, T.; Tai, Y.; Zhang, B.; Wang, X.; Wang, C.; Chen, J.; et al. GRK2 Mediated Abnormal Transduction of PGE2-EP4-cAMP-CREB Signaling Induces the Imbalance of Macrophages Polarization in Collagen-Induced Arthritis Mice. Cells 2019, 8, 1596. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.M.; Seneviratne, A.M.; Smrcka, A.V. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol. Pharmacol. 2008, 73, 410–418. [Google Scholar] [CrossRef]
- Kamal, F.A.; Travers, J.G.; Schafer, A.E.; Ma, Q.; Devarajan, P.; Blaxall, B.C. G Protein-Coupled Receptor-G-Protein betagamma-Subunit Signaling Mediates Renal Dysfunction and Fibrosis in Heart Failure. J. Am. Soc. Nephrol. JASN 2017, 28, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.L.; Karuppagounder, V.; Pinamont, W.J.; Yoshioka, N.K.; Ahmad, A.; Schott, E.M.; Le Bleu, H.K.; Zuscik, M.J.; Elbarbary, R.A.; Kamal, F. Paroxetine-mediated GRK2 inhibition is a disease-modifying treatment for osteoarthritis. Sci. Transl. Med. 2021, 13, eaau8491. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.M.; Homan, K.T.; Chen, J.; Wu, E.K.; Hinkle, P.M.; Huang, Z.M.; Chuprun, J.K.; Song, J.; Gao, E.; Cheung, J.Y.; et al. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem. Biol. 2012, 7, 1830–1839. [Google Scholar] [CrossRef]
- Schumacher, S.M.; Gao, E.; Zhu, W.; Chen, X.; Chuprun, J.K.; Feldman, A.M.; Tesmer, J.J.; Koch, W.J. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci. Transl. Med. 2015, 7, 277ra31. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Wu, L.; Zhang, M.; Hu, S.; Wang, R.; Han, Y.; Wu, Y.; Zhang, L.; Wang, X.; et al. Paroxetine alleviates T lymphocyte activation and infiltration to joints of collagen-induced arthritis. Sci. Rep. 2017, 7, 45364. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Wang, Y.; Cui, D.; Luo, T.; Zhang, Y.; Ma, Y.; Wei, W. Development of Inflammatory Immune Response-Related Drugs Based on G Protein-Coupled Receptor Kinase 2. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 729–745. [Google Scholar] [CrossRef]
- Kamal, F.A.; Mickelsen, D.M.; Wegman, K.M.; Travers, J.G.; Moalem, J.; Hammes, S.R.; Smrcka, A.V.; Blaxall, B.C. Simultaneous adrenal and cardiac g-protein-coupled receptor-gbetagamma inhibition halts heart failure progression. J. Am. Coll. Cardiol. 2014, 63, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Yassin, N.Z.; El-Shenawy, S.M.; Abdel-Rahman, R.F.; Yakoot, M.; Hassan, M.; Helmy, S. Effect of a topical copper indomethacin gel on inflammatory parameters in a rat model of osteoarthritis. Drug Des. Dev. Ther. 2015, 9, 1491–1498. [Google Scholar]
- Krasnokutsky, S.; Belitskaya-Levy, I.; Bencardino, J.; Samuels, J.; Attur, M.; Regatte, R.; Rosenthal, P.; Greenberg, J.; Schweitzer, M.; Abramson, S.B.; et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011, 63, 2983–2991. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Felson, D.T.; Niu, J.; Nevitt, M.C.; Crema, M.D.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Zhang, Y. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: The MOST study. Ann. Rheum. Dis. 2011, 70, 1804–1809. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ball, H.C.; Wase, S.J.; Novak, K.; Haqqi, T.M. Lysosomal dysfunction in osteoarthritis and aged cartilage triggers apoptosis in chondrocytes through BAX mediated release of Cytochrome c. Osteoarthr. Cartil. 2021, 29, 100–112. [Google Scholar] [CrossRef]
- Aguer, C.; Harper, M.E. Skeletal muscle mitochondrial energetics in obesity and type 2 diabetes mellitus: Endocrine aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Ni, S.; Yang, Z.C.; Liu, R.P. Oxidative Stress Induces Chondrocyte Apoptosis through Caspase-Dependent and Caspase-Independent Mitochondrial Pathways and the Antioxidant Mechanism of Angelica Sinensis Polysaccharide. Oxidative Med. Cell. Longev. 2020, 2020, 3240820. [Google Scholar] [CrossRef]
- Blanco, F.J.; Valdes, A.M.; Rego-Perez, I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018, 14, 327–340. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef]
- Jemmerson, R.; LaPlante, B.; Treeful, A. Release of intact, monomeric cytochrome c from apoptotic and necrotic cells. Cell Death Differ. 2002, 9, 538–548. [Google Scholar] [CrossRef]
- Nahon, E.; Israelson, A.; Abu-Hamad, S.; Varda, S.B. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett. 2005, 579, 5105–5110. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Matsura, T.; Kai, M.; Matsui, H.; Kawasaki, H.; Yamada, K. Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells. Proc. Soc. Exp. Biol. Med. 2000, 224, 102–108. [Google Scholar] [CrossRef]
- de Groot, D.J.; Timmer, T.; Spierings, D.C.; Le, T.K.; de Jong, S.; de Vries, E.G. Indomethacin-induced activation of the death receptor-mediated apoptosis pathway circumvents acquired doxorubicin resistance in SCLC cells. Br. J. Cancer 2005, 92, 1459–1466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, C.; Li, Y.; Zhang, Y.; Wang, Y.; Cui, D.; Luo, T.; Zhang, Y.; Liu, Q.; Li, H.; Wang, C.; et al. Targeted inhibition of GRK2 kinase domain by CP-25 to reverse fibroblast-like synoviocytes dysfunction and improve collagen-induced arthritis in rats. Acta Pharm. Sin. B 2021, 11, 1835–1852. [Google Scholar] [CrossRef] [PubMed]
- Sacre, S.; Medghalchi, M.; Gregory, B.; Brennan, F.; Williams, R. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010, 62, 683–693. [Google Scholar] [CrossRef]
- Kao, C.Y.; He, Z.; Zannas, A.S.; Hahn, O.; Kuhne, C.; Reichel, J.M.; Binder, E.B.; Wotjak, C.T.; Khaitovich, P.; Turck, C.W. Fluoxetine treatment prevents the inflammatory response in a mouse model of posttraumatic stress disorder. J. Psychiatr. Res. 2016, 76, 74–83. [Google Scholar] [CrossRef]
- Diaz-Torne, C.; Schumacher, H.R.; Yu, X.; Gomez-Vaquero, C.; Dai, L.; Chen, L.X.; Clayburne, G.; Einhorn, E.; Sachdeva, R.M.; Singh, J.A.; et al. Absence of histologic evidence of synovitis in patients with Gulf War veterans’ illness with joint pain. Arthritis Rheum. 2007, 57, 1316–1323. [Google Scholar] [CrossRef][Green Version]
- Pessler, F.; Chen, L.X.; Dai, L.; Gomez-Vaquero, C.; Diaz-Torne, C.; Paessler, M.E.; Scanzello, C.; Cakir, N.; Einhorn, E.; Schumacher, H.R. A histomorphometric analysis of synovial biopsies from individuals with Gulf War Veterans’ Illness and joint pain compared to normal and osteoarthritis synovium. Clin. Rheumatol. 2008, 27, 1127–1134. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef]
- Blom, A.B.; van Lent, P.L.; Holthuysen, A.E.; van der Kraan, P.M.; Roth, J.; van Rooijen, N.; van den Berg, W.B. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2004, 12, 627–635. [Google Scholar] [CrossRef]
- Wu, C.L.; McNeill, J.; Goon, K.; Little, D.; Kimmerling, K.; Huebner, J.; Kraus, V.; Guilak, F. Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice. Arthritis Rheumatol. 2017, 69, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Yoon, Y.N.; Son, D.; Jung, D.; Gu, G.J.; Seok, S.H. Consistent inhibition of cyclooxygenase drives macrophages towards the inflammatory phenotype. PLoS ONE 2015, 10, e0118203. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Yoon, Y.N.; Son, D.I.; Seok, S.H. Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS ONE 2013, 8, e63451. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Takeda, K.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Reduction of knee joint load suppresses cartilage degeneration, osteophyte formation, and synovitis in early-stage osteoarthritis using a post-traumatic rat model. PLoS ONE 2021, 16, e0254383. [Google Scholar] [CrossRef] [PubMed]
- Culley, K.L.; Dragomir, C.L.; Chang, J.; Wondimu, E.B.; Coico, J.; Plumb, D.A.; Otero, M.; Goldring, M.B. Mouse models of osteoarthritis: Surgical model of posttraumatic osteoarthritis induced by destabilization of the medial meniscus. Methods Mol. Biol. 2015, 1226, 143–173. [Google Scholar] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef]
- Pinamont, W.J.; Yoshioka, N.K.; Young, G.M.; Karuppagounder, V.; Carlson, E.L.; Ahmad, A.; Elbarbary, R.; Kamal, F. Standardized Histomorphometric Evaluation of Osteoarthritis in a Surgical Mouse Model. JoVE 2020, 159, e60991. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, T.; Wang, X.; Wei, X.; Chen, Y.; Guo, L.; Zhang, J.; Wang, C. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and its Signaling Pathways. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 631–641. [Google Scholar] [CrossRef]
- Castejon, M.L.; Rosillo, M.A.; Montoya, T.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C. Oleuropein down-regulated IL-1beta-induced inflammation and oxidative stress in human synovial fibroblast cell line SW982. Food Funct. 2017, 8, 1890–1898. [Google Scholar] [CrossRef]
- Le Bleu, H.K.; Kamal, F.A.; Kelly, M.; Ketz, J.P.; Zuscik, M.J.; Elbarbary, R.A. Extraction of high-quality RNA from human articular cartilage. Anal. Biochem. 2017, 518, 134–138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppagounder, V.; Pinamont, W.; Yoshioka, N.; Elbarbary, R.; Kamal, F. Early Gβγ-GRK2 Inhibition Ameliorates Osteoarthritis Development by Simultaneous Anti-Inflammatory and Chondroprotective Effects. Int. J. Mol. Sci. 2022, 23, 7933. https://doi.org/10.3390/ijms23147933
Karuppagounder V, Pinamont W, Yoshioka N, Elbarbary R, Kamal F. Early Gβγ-GRK2 Inhibition Ameliorates Osteoarthritis Development by Simultaneous Anti-Inflammatory and Chondroprotective Effects. International Journal of Molecular Sciences. 2022; 23(14):7933. https://doi.org/10.3390/ijms23147933
Chicago/Turabian StyleKaruppagounder, Vengadeshprabhu, William Pinamont, Natalie Yoshioka, Reyad Elbarbary, and Fadia Kamal. 2022. "Early Gβγ-GRK2 Inhibition Ameliorates Osteoarthritis Development by Simultaneous Anti-Inflammatory and Chondroprotective Effects" International Journal of Molecular Sciences 23, no. 14: 7933. https://doi.org/10.3390/ijms23147933
APA StyleKaruppagounder, V., Pinamont, W., Yoshioka, N., Elbarbary, R., & Kamal, F. (2022). Early Gβγ-GRK2 Inhibition Ameliorates Osteoarthritis Development by Simultaneous Anti-Inflammatory and Chondroprotective Effects. International Journal of Molecular Sciences, 23(14), 7933. https://doi.org/10.3390/ijms23147933






