Advances in the Structural Strategies of the Self-Assembly of Photoresponsive Supramolecular Systems
Abstract
:1. Introduction
2. Supramolecular Structures with Photoresponsive Units
3. Photoswitchable Control of Supramolecular Organic Systems
3.1. Metal–Organic Polyhedra (MOP)
3.2. Metal–Organic Framework (MOF)
3.3. Hydrogen-Bonded Organic Framework (HOF)
4. Azobenzene and Diethienyl Bearing Photoswitchable Catalysis
4.1. Azobenzene Photoswitchable Catalysts
4.1.1. Single Molecules
4.1.2. Molecular Assemblies
4.1.3. Other Systems
4.2. Dithienylethene Photoswitchable Catalysts
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, W.; Wang, J.; Parak, W.J.; Farokhzad, O.C.; Shi, J. Nanobuffering of PH-Responsive Polymers: A Known but Sometimes Overlooked Phenomenon and Its Biological Applications. ACS Nano 2019, 13, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Chen, D.; Zhan, T.G.; Zhang, K.-D. Ionic Liquid-Based Stimuli-Responsive Functional Materials. Adv. Funct. Mater. 2020, 30, 2005522. [Google Scholar] [CrossRef]
- Brighenti, R.; Cosma, M.P. Swelling Mechanism in Smart Polymers Responsive to Mechano-Chemical Stimuli. J. Mech. Phys. Solids 2020, 143, 104011. [Google Scholar] [CrossRef]
- Wani, O.M.; Schenning, A.P.H.J.; Priimagi, A. A Bifacial Colour-Tunable System: Via Combination of a Cholesteric Liquid Crystal Network and Hydrogel. J. Mater. Chem. C 2020, 8, 10191–10196. [Google Scholar] [CrossRef]
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical Stimuli-Responsive Vesicles in Drug Delivery: Beyond Liposomes and Polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Choi, H.J. Synthesis of Smart Poly(Diphenylamine)/Magnetic Particle Composites and Their Electric/Magnetic Stimuli-Response. Macromol. Res. 2018, 26, 667–670. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Chen, Y.R.; Wang, S.J.; Zhao, F.; Wang, X.G.; Yang, F.; Shi, J.J.; Ge, Z.G.; Ding, W.Y.; Yang, Y.C.; et al. Orchestrated Biomechanical, Structural, and Biochemical Stimuli for Engineering Anisotropic Meniscus. Sci. Transl. Med. 2019, 11, eaao0750. [Google Scholar] [CrossRef]
- Ru, Y.; Shi, Z.; Zhang, J.; Wang, J.; Chen, B.; Huang, R.; Liu, G.; Yu, T. Recent Progress of Photochromic Materials towards Photocontrollable Devices. Mater. Chem. Front. 2021, 5, 7737–7758. [Google Scholar] [CrossRef]
- Göstl, R.; Senf, A.; Hecht, S. Remote-Controlling Chemical Reactions by Light: Towards Chemistry with High Spatio-Temporal Resolution. Chem. Soc. Rev. 2014, 43, 1982–1996. [Google Scholar] [CrossRef]
- Lugger, S.J.D.; Houben, S.J.A.; Foelen, Y.; Debije, M.G.; Schenning, A.P.H.J.; Mulder, D.J. Hydrogen-Bonded Supramolecular Liquid Crystal Polymers: Smart Materials with Stimuli-Responsive, Self-Healing, and Recyclable Properties. Chem. Rev. 2022, 122, 4946–4975. [Google Scholar] [CrossRef]
- Pilz Da Cunha, M.; Van Thoor, E.A.J.; Debije, M.G.; Broer, D.J.; Schenning, A.P.H.J. Unravelling the Photothermal and Photomechanical Contributions to Actuation of Azobenzene-Doped Liquid Crystal Polymers in Air and Water. J. Mater. Chem. C 2019, 7, 13502–13509. [Google Scholar] [CrossRef] [Green Version]
- Gelebart, A.H.; Vantomme, G.; Meijer, E.W.; Broer, D.J. Mastering the Photothermal Effect in Liquid Crystal Networks: A General Approach for Self-Sustained Mechanical Oscillators. Adv. Mater. 2017, 29, 1606712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Zhang, H.; Fei, G.; Yu, B.; Tong, X.; Xia, H.; Zhao, Y. Liquid-Crystalline Dynamic Networks Doped with Gold Nanorods Showing Enhanced Photocontrol of Actuation. Adv. Mater. 2018, 30, e1706597. [Google Scholar] [CrossRef] [PubMed]
- Neilson, B.M.; Bielawski, C.W. Illuminating Photoswitchable Catalysis. ACS Catal. 2013, 3, 1874–1885. [Google Scholar] [CrossRef]
- Jiang, Y.; Heinke, L. Photoswitchable Metal-Organic Framework Thin Films: From Spectroscopy to Remote-Controllable Membrane Separation and Switchable Conduction. Langmuir 2021, 37, 2–15. [Google Scholar] [CrossRef]
- Adelizzi, B.; Gielen, V.; Le Saux, T.; Dedecker, P.; Jullien, L. Quantitative Model for Reversibly Photoswitchable Sensors. ACS Sensors 2021, 6, 1157–1165. [Google Scholar] [CrossRef]
- Cheng, H.B.; Cui, Y.; Wang, R.; Kwon, N.; Yoon, J. The Development of Light-Responsive, Organic Dye Based, Supramolecular Nanosystems for Enhanced Anticancer Therapy. Coord. Chem. Rev. 2019, 392, 237–254. [Google Scholar] [CrossRef]
- Shen, X.; Song, J.; Sevencan, C.; Leong, D.T.; Ariga, K. Bio-Interactive Nanoarchitectonics with Two-Dimensional Materials and Environments. Sci. Technol. Adv. Mater. 2022, 23, 199–224. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Zhao, L.; Duan, C. Photochemical Properties of Host-Guest Supramolecular Systems with Structurally Confined Metal-Organic Capsules. Acc. Chem. Res. 2019, 52, 100–109. [Google Scholar] [CrossRef]
- Jiao, Y.; Zuo, Y.; Yang, H.; Gao, X.; Duan, C. Photoresponse within Dye-Incorporated Metal-Organic Architectures. Coord. Chem. Rev. 2021, 430, 213648. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional Nanoparticles through π-Conjugated Polymer Self-Assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Zhu, Z.; Fan, J.B.; Tian, Y.; Meng, J.; Wang, S. Photo-Irresponsive Molecule-Amplified Cell Release on Photoresponsive Nanostructured Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 29681–29688. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Wang, Q.; Wang, X.; Yao, P.; Feng, W.; Yuan, Q.; Qi, X.; Chen, S.; Pu, W.; et al. Self-Illuminating Triggered Release of Therapeutics from Photocleavable Nanoprodrug for the Targeted Treatment of Breast Cancer. ACS Appl. Mater. Interfaces 2022, 14, 8766–8781. [Google Scholar] [CrossRef]
- Ikeda, T.; Iijima, T.; Sekiya, R.; Takahashi, O.; Haino, T. Cooperative Self-Assembly of Carbazole Derivatives Driven by Multiple Dipole-Dipole Interactions. J. Org. Chem. 2016, 81, 6832–6837. [Google Scholar] [CrossRef]
- Chen, C.H.; Wang, Y.; Michinobu, T.; Chang, S.W.; Chiu, Y.C.; Ke, C.Y.; Liou, G.S. Donor-Acceptor Effect of Carbazole-Based Conjugated Polymer Electrets on Photoresponsive Flash Organic Field-Effect Transistor Memories. ACS Appl. Mater. Interfaces 2020, 12, 6144–6150. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Hirao, T.; Ikeda, T.; Haino, T. Self-Assembling Behavior and Chiroptical Properties of Carbazole-Cored Phenyl Isoxazolyl Benzenes. J. Org. Chem. 2021, 86, 5499–5505. [Google Scholar] [CrossRef] [PubMed]
- Piwoński, H.; Michinobu, T.; Habuchi, S. Controlling Photophysical Properties of Ultrasmall Conjugated Polymer Nanoparticles through Polymer Chain Packing. Nat. Commun. 2017, 8, 15256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, A.; De Sarkar, S.; Das, A. Supramolecular Engineering and Self-Assembly Strategies in Photoredox Catalysis. ACS Catal. 2021, 11, 710–733. [Google Scholar] [CrossRef]
- Banerjee, T.; Podjaski, F.; Kröger, J.; Biswal, B.P.; Lotsch, B.V. Polymer Photocatalysts for Solar-to-Chemical Energy Conversion. Nat. Rev. Mater. 2021, 6, 168–190. [Google Scholar] [CrossRef]
- Sánchez-Fernández, J.A.; Cué-Sampedro, R. Biopolymers and Their Composites for Drug Delivery. In Green Biocomposites for Biomedical Engineering: Design, Properties, and Applications; Hoque, E., Sharif, A., Jawaid, M., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 363–387. [Google Scholar] [CrossRef]
- Walunj, M.B.; Dutta, S.; Srivatsan, S.G. Architectures of Nucleolipid Assemblies and Their Applications. In Molecular Architectonics and Nanoarchitectonics; Govindaraju, T., Ariga, K., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; pp. 307–334. [Google Scholar] [CrossRef]
- Begley, M.R.; Gianola, D.S.; Ray, T.R. Bridging Functional Nanocomposites to Robust Macroscale Devices. Science 2019, 364, eaav4299. [Google Scholar] [CrossRef]
- Alesanco, Y.; Viñuales, A.; Cabañero, G.; Rodriguez, J.; Tena-Zaera, R. Colorless to Neutral Color Electrochromic Devices Based on Asymmetric Viologens. ACS Appl. Mater. Interfaces 2016, 8, 29619–29627. [Google Scholar] [CrossRef] [PubMed]
- Mehlana, G.; Bourne, S.A. Unravelling Chromism in Metal-Organic Frameworks. CrystEngComm 2017, 19, 4238–4259. [Google Scholar] [CrossRef]
- Adams, T.J.; Brotherton, A.R.; Molai, J.A.; Parmar, N.; Palmer, J.R.; Sandor, K.A.; Walter, M.G. Obtaining Reversible, High Contrast Electrochromism, Electrofluorochromism, and Photochromism in an Aqueous Hydrogel Device Using Chromogenic Thiazolothiazoles. Adv. Funct. Mater. 2021, 31, 2103408. [Google Scholar] [CrossRef]
- Liu, J.J.; Fu, J.J.; He, C.X.; Liu, T.; Cheng, F.X. A Viologen-Derived Host-Guest MOF Material: Photochromism, Photoswitchable Luminescence, and Inkless and Erasable Printing. J. Solid State Chem. 2022, 306, 122812. [Google Scholar] [CrossRef]
- Castellanos, S.; Kapteijn, F.; Gascon, J. Photoswitchable Metal Organic Frameworks: Turn on the Lights and Close the Windows. CrystEngComm 2016, 18, 4006–4012. [Google Scholar] [CrossRef] [Green Version]
- Dattler, D.; Fuks, G.; Heiser, J.; Moulin, E.; Perrot, A.; Yao, X.; Giuseppone, N. Design of Collective Motions from Synthetic Molecular Switches, Rotors, and Motors. Chem. Rev. 2020, 120, 310–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.; McGonigal, P.R.; Schneebeli, S.T.; Li, H.; Vermeulen, N.A.; Ke, C.; Stoddart, J.F. An Artificial Molecular Pump. Nat. Nanotechnol. 2015, 10, 547–553. [Google Scholar] [CrossRef]
- Pezzato, C.; Nguyen, M.T.; Cheng, C.; Kim, D.J.; Otley, M.T.; Stoddart, J.F. An Efficient Artificial Molecular Pump. Tetrahedron 2017, 73, 4849–4857. [Google Scholar] [CrossRef]
- Feng, Y.; Ovalle, M.; Seale, J.S.W.; Lee, C.K.; Kim, D.J.; Astumian, R.D.; Stoddart, J.F. Molecular Pumps and Motors. J. Am. Chem. Soc. 2021, 143, 5569–5591. [Google Scholar] [CrossRef]
- Baroncini, M.; Silvi, S.; Credi, A. Photo- And Redox-Driven Artificial Molecular Motors. Chem. Rev. 2020, 120, 200–268. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, C.; Marullo, S.; Billeci, F.; D’Anna, F. Catalysis in Supramolecular Systems: The Case of Gel Phases. Eur. J. Org. Chem. 2021, 2021, 3148–3169. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J. Recent Advances in Stimuli-Responsive DNA-Based Hydrogels. ACS Appl. Bio Mater. 2022, 5, 1934–1953. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J. Coordination Copolymerization Mediated by Zn4O(CO 2R)6 Metal Clusters: A Balancing Act between Statistics and Geometry. J. Am. Chem. Soc. 2010, 132, 15005–15010. [Google Scholar] [CrossRef] [PubMed]
- Diercks, C.S.; Yaghi, O.M. The Atom, the Molecule, and the Covalent Organic Framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef]
- Lisensky, G.C.; Yaghi, O.M. Visualizing Pore Packing and Topology in MOFs. J. Chem. Educ. 2022, 99, 1998–2004. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, H.; Nam, D.; Lah, M.S.; Choe, W. The Rise of Metal-Organic Polyhedra. Chem. Soc. Rev. 2021, 50, 528–555. [Google Scholar] [CrossRef]
- Gosselin, A.J.; Rowland, C.A.; Bloch, E.D. Permanently Microporous Metal-Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014. [Google Scholar] [CrossRef]
- Jiang, Y.; Park, J.; Tan, P.; Feng, L.; Liu, X.Q.; Sun, L.B.; Zhou, H.C. Maximizing Photoresponsive Efficiency by Isolating Metal−organic Polyhedra into Confined Nanoscaled Spaces. J. Am. Chem. Soc. 2020, 141, 8221–8227. [Google Scholar] [CrossRef]
- Carné-Sánchez, A.; Craig, G.A.; Larpent, P.; Guillerm, V.; Urayama, K.; Maspoch, D.; Furukawa, S. A Coordinative Solubilizer Method to Fabricate Soft Porous Materials from Insoluble Metal–Organic Polyhedra. Angew. Chem. 2019, 131, 6413–6416. [Google Scholar] [CrossRef]
- Khobotov-Bakishev, A.; Hernández-López, L.; von Baeckmann, C.; Albalad, J.; Carné-Sánchez, A.; Maspoch, D. Metal–Organic Polyhedra as Building Blocks for Porous Extended Networks. Adv. Sci. 2022, 9, 2104753. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Zou, Y.Q.; Nitschke, J.R. Metal–Organic Cages for Molecular Separations. Nat. Rev. Chem. 2021, 5, 168–182. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Pan, M.; Su, C.Y. Metal-Organic Cages for Biomedical Applications. Isr. J. Chem. 2019, 59, 209–219. [Google Scholar] [CrossRef]
- Wezenberg, S.J. Light-Switchable Metal-Organic Cages. Chem. Lett. 2020, 49, 609–615. [Google Scholar] [CrossRef]
- Tarzia, A.; Jelfs, K.E. Unlocking the Computational Design of Metal–Organic Cages. Chem. Commun. 2022, 58, 3717–3730. [Google Scholar] [CrossRef]
- Sánchez-González, E.; Tsang, M.Y.; Troyano, J.; Craig, G.A.; Furukawa, S. Assembling Metal–Organic Cages as Porous Materials. Chem. Soc. Rev. 2022, 51, 4876–4889. [Google Scholar] [CrossRef]
- Jahović, I.; Zou, Y.Q.; Adorinni, S.; Nitschke, J.R.; Marchesan, S. Cages Meet Gels: Smart Materials with Dual Porosity. Matter 2021, 4, 2123–2140. [Google Scholar] [CrossRef]
- Hanopolskyi, A.I.; De, S.; Białek, M.J.; Diskin-Posner, Y.; Avram, L.; Feller, M.; Klajn, R. Reversible Switching of Arylazopyrazole within a Metal-Organic Cage. Beilstein J. Org. Chem. 2019, 15, 2398–2407. [Google Scholar] [CrossRef] [Green Version]
- Oldenhuis, N.J.; Qin, K.P.; Wang, S.; Ye, H.Z.; Alt, E.A.; Willard, A.P.; Van Voorhis, T.; Craig, S.L.; Johnson, J.A. Photoswitchable Sol–Gel Transitions and Catalysis Mediated by Polymer Networks with Coumarin-Decorated Cu24L24 Metal–Organic Cages as Junctions. Angew. Chem. Int. Ed. 2020, 59, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.G.; Kondo, M.; Nakamura, K.; Sasai, H.; Takizawa, S. Photoswitchable Chiral Cation-Binding Catalyst: Photocontrol of Catalytic Activity on Enantioselective Aminal Synthesis. Org. Lett. 2022, 24, 2670–2674. [Google Scholar] [CrossRef]
- Sheng, K.; Liu, Y.N.; Gupta, R.K.; Kurmoo, M.; Sun, D. Arylazopyrazole-Functionalized Photoswitchable Octanuclear Zn(II)-Silsesquioxane Nanocage. Sci. China Chem. 2021, 64, 419–425. [Google Scholar] [CrossRef]
- Clever, G.H.; Tashiro, S.; Shionoya, M. Light-Triggered Crystallization of a Molecular Host-Guest Complex. J. Am. Chem. Soc. 2010, 132, 9973–9975. [Google Scholar] [CrossRef] [PubMed]
- Stuckhardt, C.; Roke, D.; Danowski, W.; Otten, E.; Wezenberg, S.J.; Feringa, B.L. A Chiral Self-Sorting Photoresponsive Coordination Cage Based on Overcrowded Alkenes. Beilstein J. Org. Chem. 2019, 15, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zang, H.; Gai, F.; Feng, Z.; Li, M.; Duan, C. Photoswitchable Cu(Ii)/Cu(i) Catalyses Assisted by Enzyme-like Non-Covalent Interactions in Cu(Ii)-Melamine Coordination Polymers for Installing CO2/CS2and CF3groups in Heterocycles. New J. Chem. 2020, 44, 15131–15139. [Google Scholar] [CrossRef]
- Li, R.J.; Pezzato, C.; Berton, C.; Severin, K. Light-Induced Assembly and Disassembly of Polymers with PdnL2n-Type Network Junctions. Chem. Sci. 2021, 12, 4981–4984. [Google Scholar] [CrossRef]
- Dalton, D.M.; Ellis, S.R.; Nichols, E.M.; Mathies, R.A.; Dean Toste, F.; Bergman, R.G.; Raymond, K.N. Supramolecular Ga4L612- Cage Photosensitizes 1,3-Rearrangement of Encapsulated Guest via Photoinduced Electron Transfer. J. Am. Chem. Soc. 2015, 137, 10128–10131. [Google Scholar] [CrossRef]
- Park, J.; Sun, L.B.; Chen, Y.P.; Perry, Z.; Zhou, H.C. Azobenzene-Functionalized Metal-Organic Polyhedra for the Optically Responsive Capture and Release of Guest Molecules. Angew. Chem. Int. Ed. 2014, 53, 5842–5846. [Google Scholar] [CrossRef]
- Li, R.J.; Holstein, J.J.; Hiller, W.G.; Andréasson, J.; Clever, G.H. Mechanistic Interplay between Light Switching and Guest Binding in Photochromic [Pd2Dithienylethene4] Coordination Cages. J. Am. Chem. Soc. 2019, 141, 2097–2103. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Alt, E.A.; Wang, H.; Li, X.; Willard, A.P.; Johnson, J.A. Photoswitching Topology in Polymer Networks with Metal–Organic Cages as Crosslinks. Nature 2018, 560, 65–69. [Google Scholar] [CrossRef]
- Gong, W.; Chen, Z.; Dong, J.; Liu, Y.; Cui, Y. Chiral Metal-Organic Frameworks. Chem. Rev. 2022, 122, 9078–9144. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Feng, X.; Wang, S.; Wang, B. Recent Advances in AIEgen-Based Luminescent Metal-Organic Frameworks and Covalent Organic Frameworks. Mater. Chem. Front. 2017, 1, 2474–2486. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular Materials Based on AIE Luminogens (AIEgens): Construction and Applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lee, M.M.S.; Zhang, Z.; Sung, H.H.Y.; Williams, I.D.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, D.; Tang, B.Z. Facile Synthesis of AIEgens with Wide Color Tunability for Cellular Imaging and Therapy. Chem. Sci. 2019, 10, 3494–3501. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Wang, L.; Chen, X.; Tang, H.; Cao, D.; Zhang, G.; Chen, W. Pyridinium-Substituted Tetraphenylethylene Salt-Based Photosensitizers by Varying Counter Anions: A Highly Efficient Photodynamic Therapy for Cancer Cell Ablation and Bacterial Inactivation. J. Mater. Chem. B 2020, 8, 5234–5244. [Google Scholar] [CrossRef]
- Pandey, N.K.; Xiong, W.; Wang, L.; Chen, W.; Bui, B.; Yang, J.; Amador, E.; Chen, M.; Xing, C.; Athavale, A.A.; et al. Aggregation-Induced Emission Luminogens for Highly Effective Microwave Dynamic Therapy. Bioact. Mater. 2022, 7, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Wang, Y.; Compaan, A.; Ren, T.; Dong, W.J. Increasing the Power Output of a CdTe Solar Cell via Luminescent down Shifting Molecules with Intramolecular Charge Transfer and Aggregation-Induced Emission Characteristics. Energy Environ. Sci. 2013, 6, 2907–2911. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Xiong, W.; Ran, X.; Tang, H.; Cao, D. Design and Synthesis of an AIEgen with Multiple Functions: Solvatochromism, Chromism, Lipid Droplet Imaging. Dye. Pigments 2020, 181, 108537. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Y.; Liu, J.; Du, J.; Yu, Y.; Wang, S.; Liang, Z.; Yu, J. Multifunctional Porous Tröger’s Base Polymers with Tetraphenylethene Units: CO2 Adsorption, Luminescence and Sensing Properties. Polym. Chem. 2017, 8, 4842–4848. [Google Scholar] [CrossRef]
- Peng, Y.; Li, L.; Zhu, C.; Chen, B.; Zhao, M.; Zhang, Z.; Lai, Z.; Zhang, X.; Tan, C.; Han, Y.; et al. Intramolecular Hydrogen Bonding-Based Topology Regulation of Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 13162–13169. [Google Scholar] [CrossRef]
- Sun, N.; Jin, Y.; Wang, H.; Yu, B.; Wang, R.; Wu, H.; Zhou, W.; Jiang, J. Photoresponsive Covalent Organic Frameworks with Diarylethene Switch for Tunable Singlet Oxygen Generation. Chem. Mater. 2022, 34, 1956–1964. [Google Scholar] [CrossRef]
- Xia, Y.; Duan, H.; Hao, A.; Xing, P. Photoresponsive Supramolecular Chiral Composites Based on Hydrogen-Bonded Coassembly. J. Phys. Chem. C 2021, 125, 28108–28114. [Google Scholar] [CrossRef]
- Goto, T.; Okazaki, Y.; Ueki, M.; Kuwahara, Y.; Takafuji, M.; Oda, R.; Ihara, H. Induction of Strong and Tunable Circularly Polarized Luminescence of Nonchiral, Nonmetal, Low-Molecular-Weight Fluorophores Using Chiral Nanotemplates. Angew. Chem. Int. Ed. 2017, 56, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M.; Poppe, S.; Tschierske, C. Photoresponsive Halogen Bonded Polycatenar Liquid Crystals. J. Mol. Liq. 2019, 277, 233–240. [Google Scholar] [CrossRef]
- Szell, P.M.J.; Zablotny, S.; Bryce, D.L. Halogen Bonding as a Supramolecular Dynamics Catalyst. Nat. Commun. 2019, 10, 916. [Google Scholar] [CrossRef] [Green Version]
- Bashir, D.J.; Manzoor, S.; Khan, I.A.; Bashir, M.; Agarwal, N.B.; Rastogi, S.; Arora, I.; Samim, M. Nanonization of Magnoflorine-Encapsulated Novel Chitosan-Collagen Nanocapsules for Neurodegenerative Diseases: In Vitro Evaluation. ACS Omega 2022, 7, 6472–6480. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Shrestha, L.K. Supramolecular Nanoarchitectonics for Functional Materials. APL Mater. 2019, 7, 120903. [Google Scholar] [CrossRef]
- Momeni, M.R.; Zhang, Z.; Dell’Angelo, D.; Shakib, F.A. Tuning Electronic Properties of Conductive 2D Layered Metal-Organic Frameworks via Host-Guest Interactions: Dioxygen as an Electroactive Chemical Stimuli. APL Mater. 2021, 9, 051109. [Google Scholar] [CrossRef]
- Park, J.; Suh, B.L.; Kim, J. Computational Design of a Photoresponsive Metal-Organic Framework for Post Combustion Carbon Capture. J. Phys. Chem. C 2020, 124, 13162–13167. [Google Scholar] [CrossRef]
- Sánchez-Fernández, J.A.; Cué-Sampedro, R.; Flores-Hernandez, D.R. Metal-Organic Frameworks-Polymer Composites for Practical Applications: Compatibility, Processability, and Biotribology Studies. In Biotribology: Emerging Technologies and Applications; Rao, T.V.V.L.N., Kasolang, S.B., Guoxin, X., Katiyar, J.K., Rani, A.M.A., Eds.; CRC Press: Boca Raton, NJ, USA, 2022; pp. 123–145. [Google Scholar]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy Storage: The Future Enabled by Nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Guo, K.; Zeng, S.L.; Xu, L.; Xing, C.Y.; Zhang, S.; Li, B.J. Cross-Stacking Aligned Non-Woven Fabrics with Automatic Self-Healing Properties for Electromagnetic Interference Shielding. Carbon 2020, 162, 445–454. [Google Scholar] [CrossRef]
- Ji, B.; Fan, S.; Ma, X.; Hu, K.; Wang, L.; Luan, C.; Deng, J.; Cheng, L.; Zhang, L. Electromagnetic Shielding Behavior of Heat-Treated Ti3C2TX MXene Accompanied by Structural and Phase Changes. Carbon 2020, 165, 150–162. [Google Scholar] [CrossRef]
- Aïssa, B.; Sinopoli, A.; Ali, A.; Zakaria, Y.; Zekri, A.; Helal, M.; Nedil, M.; Rosei, F.; Mansour, S.; Mahmoud, K.A. Nanoelectromagnetic of a Highly Conductive 2D Transition Metal Carbide (MXene)/Graphene Nanoplatelets Composite in the EHF M-Band Frequency. Carbon 2021, 173, 528–539. [Google Scholar] [CrossRef]
- Zhang, W.X.; Kholkin, A.; Rocha, J.; Xu, W.J.; Romanyuk, K.; Martinho, J.M.G.; Zeng, Y.; Zhang, X.W.; Ushakov, A.; Shur, V.; et al. Photoresponsive Organic-Inorganic Hybrid Ferroelectric Designed at the Molecular Level. J. Am. Chem. Soc. 2020, 142, 16990–16998. [Google Scholar] [CrossRef]
- Yang, T.; Cai, F.; Zhang, X.; Huang, Y. Nitrogen and Sulfur Codoped Graphene Quantum Dots as a New Fluorescent Probe for Au3+ Ions in Aqueous Media. RSC Adv. 2015, 5, 107340–107347. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Buchholz, M.; Füllgrabe, N.; Grosjean, S.; Bebensee, F.; Bräse, S.; Wöll, C.; Heinke, L. Cis-to-Trans Isomerization of Azobenzene Investigated by Using Thin Films of Metal-Organic Frameworks. Phys. Chem. Chem. Phys. 2015, 17, 22721–22725. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Peng, M.; Ma, Y.; Guo, R.; Lin, W. Rational Design of a Lipid-Droplet-Polarity Based Fluorescent Probe for Potential Cancer Diagnosis. Chem. Commun. 2018, 54, 12093–12096. [Google Scholar] [CrossRef]
- Wu, K.; Wu, X.; Zhang, Y.; Chen, S.; Qiao, Z.; Wei, D.; Sun, J.; Fan, H. Semiconvertible Hyaluronic Hydrogel Enabled Red-Light-Responsive Reversible Mechanics, Adhesion, and Self-Healing. Biomacromolecules 2022, 23, 1030–1040. [Google Scholar] [CrossRef]
- Zhang, H.; Hui, J.; Chen, H.; Chen, J.; Xu, W.; Shuai, Z.; Zhu, D.; Guo, X. Synergistic Photomodulation of Capacitive Coupling and Charge Separation Toward Functional Organic Field-Effect Transistors with High Responsivity. Adv. Electron. Mater. 2015, 1, 1500159. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Hisaki, I.; Xin, C.; Takahashi, K.; Nakamura, T. Designing Hydrogen-Bonded Organic Frameworks (HOFs) with Permanent Porosity. Angew. Chem. 2019, 131, 11278–11288. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional Porous Hydrogen-Bonded Organic Framework Materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Wang, H.L.; Zou, H.H.; Liang, F.P. Metal Hydrogen-Bonded Organic Frameworks: Structure and Performance. Dalt. Trans. 2020, 49, 10708–10723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Samanta, J.; Ke, C. Assembling Guests as Cyclic Tetramers in a Porous Hydrogen-Bonded Organic Framework. Cryst. Growth Des. 2022, 22, 3421–3427. [Google Scholar] [CrossRef]
- Ke, Z.; Chen, K.; Li, Z.; Huang, J.; Yao, Z.; Dai, W.; Wang, X.; Liu, C.; Xiang, S.; Zhang, Z. Dual-Functional Hydrogen-Bonded Organic Frameworks for Aniline and Ultraviolet Sensitive Detection. Chin. Chem. Lett. 2021, 32, 3109–3112. [Google Scholar] [CrossRef]
- Boer, S.A.; Morshedi, M.; Tarzia, A.; Doonan, C.J.; White, N.G. Molecular Tectonics: A Node-and-Linker Building Block Approach to a Family of Hydrogen-Bonded Frameworks. Chem. A Eur. J. 2019, 25, 10006–10012. [Google Scholar] [CrossRef]
- Nicks, J.; Boer, S.A.; White, N.G.; Foster, J.A. Monolayer Nanosheets Formed by Liquid Exfoliation of Charge-Assisted Hydrogen-Bonded Frameworks. Chem. Sci. 2021, 12, 3322–3327. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, S.; Tao, W.; Guo, J.; Xie, S.; Ding, Y.; Xu, G.; Chen, C.; Sun, X.; Zhang, Z.; et al. Multiple yet Switchable Hydrogen-Bonded Organic Frameworks with White-Light Emission. Nat. Commun. 2022, 13, 1882. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, J.; Zhang, Z.; Yang, Y.; Yang, Q.; Ren, Q.; Bao, Z. Crystal Structure Transformation in Hydrogen-Bonded Organic Frameworks via Ion Exchange. Chem. Asian J. 2021, 16, 3978–3984. [Google Scholar] [CrossRef]
- Bao, Z.; Xie, D.; Chang, G.; Wu, H.; Li, L.; Zhou, W.; Wang, H.; Zhang, Z.; Xing, H.; Yang, Q.; et al. Fine Tuning and Specific Binding Sites with a Porous Hydrogen-Bonded Metal-Complex Framework for Gas Selective Separations. J. Am. Chem. Soc. 2018, 140, 4596–4603. [Google Scholar] [CrossRef] [Green Version]
- Nugent, P.S.; Rhodus, V.L.; Pham, T.; Forrest, K.; Wojtas, L.; Space, B.; Zaworotko, M.J. A Robust Molecular Porous Material with High CO2 Uptake and Selectivity. J. Am. Chem. Soc. 2013, 135, 10950–10953. [Google Scholar] [CrossRef]
- Han, Y.F.; Yuan, Y.X.; Wang, H.B. Porous Hydrogen-Bonded Organic Frameworks. Molecules 2017, 22, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Wang, J.W.; Zhang, J.H.; Lai, S.; Zhong, D.C. Hydrogen-Bonded Organic Frameworks: Design, Structures and Potential Applications. CrystEngComm 2018, 20, 5884–5898. [Google Scholar] [CrossRef]
- Su, J.; Yuan, S.; Cheng, Y.X.; Yang, Z.M.; Zuo, J.L. Coordination-Bond-Directed Synthesis of Hydrogen-Bonded Organic Frameworks from Metal-Organic Frameworks as Templates. Chem. Sci. 2021, 12, 14254–14259. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Gutiérrez, M.; Tanaka, S.; Gomez, E.; Tohnai, N.; Yasuda, N.; Matubayasi, N.; Douhal, A.; Hisaki, I. Construction of Isostructural Hydrogen-Bonded Organic Frameworks: Limitations and Possibilities of Pore Expansion. Chem. Sci. 2021, 12, 9607–9618. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Jiang, H.B.; Jiang, K.B.; Hu, D.L.; Cai, L.Z.; Wang, M.S.; Guo, G.C. Photochromic Semiconductive Hydrogen-Bonded Organic Framework (HOF) with Broadband Absorption. ACS Appl. Mater. Interfaces 2022, 14, 11619–11625. [Google Scholar] [CrossRef]
- Tao, K.; Makam, P.; Aizen, R.; Gazit, E. Self-Assembling Peptide Semiconductors. Science 2017, 358, eaam9756. [Google Scholar] [CrossRef] [Green Version]
- Kaler, S.; McKeown, P.; Ward, B.D.; Jones, M.D. Aluminium(III) and Zinc(II) Complexes of Azobenzene-Containing Ligands for Ring-Opening Polymerisation of ϵ-Caprolactone and: Rac-Lactide. Inorg. Chem. Front. 2021, 8, 711–719. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Xia, F.; Dai, Y. Azobenzene-Based Photoswitchable Catalysts: State of the Art and Perspectives. J. Catal. 2022, 409, 33–40. [Google Scholar] [CrossRef]
- Dorel, R.; Feringa, B.L. Photoswitchable Catalysis Based on the Isomerisation of Double Bonds. Chem. Commun. 2019, 55, 6477–6486. [Google Scholar] [CrossRef] [Green Version]
- Vassalini, I.; Alessandri, I. Switchable Stimuli-Responsive Heterogeneous Catalysis. Catalysts 2018, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Stoll, R.S.; Peters, M.V.; Kuhn, A.; Heiles, S.; Goddard, R.; Bühl, M.; Thiele, C.M.; Hecht, S. Photoswitchable Catalysts: Correlating Structure and Conformational Dynamics with Reactivity by a Combined Experimental and Computational Approach. J. Am. Chem. Soc. 2009, 131, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Imahori, T.; Yamaguchi, R.; Kurihara, S. Azobenzene-Tethered Bis(Trityl Alcohol) as a Photoswitchable Cooperative Acid Catalyst for Morita-Baylis-Hillman Reactions. Chem. A Eur. J. 2012, 18, 10802–10807. [Google Scholar] [CrossRef] [PubMed]
- Samanta, M.; Krishna, V.S.R.; Bandyopadhyay, S. A Photoresponsive Glycosidase Mimic. Chem. Commun. 2014, 50, 10577–10579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnetta, L.; Bermudez, M.; Riefolo, F.; Matera, C.; Claro, E.; Messerer, R.; Littmann, T.; Wolber, G.; Holzgrabe, U.; Decker, M. Fluorination of Photoswitchable Muscarinic Agonists Tunes Receptor Pharmacology and Photochromic Properties. J. Med. Chem. 2019, 62, 3009–3020. [Google Scholar] [CrossRef]
- Cabré, G.; Garrido-Charles, A.; Moreno, M.; Bosch, M.; Porta-de-la-Riva, M.; Krieg, M.; Gascón-Moya, M.; Camarero, N.; Gelabert, R.; Lluch, J.M.; et al. Rationally Designed Azobenzene Photoswitches for Efficient Two-Photon Neuronal Excitation. Nat. Commun. 2019, 10, 907. [Google Scholar] [CrossRef]
- Dudek, M.; Pokładek, Z.; Deiana, M.; Matczyszyn, K. Molecular Design and Structural Characterization of Photoresponsive Azobenzene-Based Polyamide Units. Dye. Pigments 2020, 180, 108501. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Zhao, Z.; Barrett, S.L.; Shelton, S.D.; Zafferani, M.; Anderson, H.E.; Blumenthal, M.O.; Jones, L.R.; Wang, L.; Li, X.; et al. Photoresponsive Azo-Combretastatin A-4 Analogues. Eur. J. Med. Chem. 2018, 143, 1–7. [Google Scholar] [CrossRef]
- Trads, J.B.; Hüll, K.; Matsuura, B.S.; Laprell, L.; Fehrentz, T.; Görldt, N.; Kozek, K.A.; Weaver, C.D.; Klöcker, N.; Barber, D.M.; et al. Sign Inversion in Photopharmacology: Incorporation of Cyclic Azobenzenes in Photoswitchable Potassium Channel Blockers and Openers. Angew. Chem. Int. Ed. 2019, 58, 15421–15428. [Google Scholar] [CrossRef]
- Gao, F.; Bi, Z.; Wang, S.; Zhao, Z.; Dong, Y.; Li, X. An Amphiphilic Azobenzene Derivative as a Crosslinker in the Construction of Smart Supramacromolecular Hydrogels. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 647, 129088. [Google Scholar] [CrossRef]
- Homma, K.; Chang, A.C.; Yamamoto, S.; Tamate, R.; Ueki, T.; Nakanishi, J. Design of Azobenzene-Bearing Hydrogel with Photoswitchable Mechanics Driven by Photo-Induced Phase Transition for in Vitro Disease Modeling. Acta Biomater. 2021, 132, 103–113. [Google Scholar] [CrossRef]
- Pessoni, L.; Siniscalco, D.; Boussonnière, A.; Castanet, A.-S.; Billon, L.; Delorme, N. Photo-Reversible Solid to Liquid Transition of Azobenzene Containing Polymers: Impact of the Chemical Structure and Chain Length. Eur. Polym. J. 2022, 174, 111297. [Google Scholar] [CrossRef]
- Telleria, A.; Van Leeuwen, P.W.N.M.; Freixa, Z. Azobenzene-Based Ruthenium(Ii) Catalysts for Light-Controlled Hydrogen Generation. Dalt. Trans. 2017, 46, 3569–3578. [Google Scholar] [CrossRef]
- Saha, M.; Hossain, M.S.; Bandyopadhyay, S. A Photoregulated Racemase Mimic. Angew. Chem. Int. Ed. 2021, 60, 5220–5224. [Google Scholar] [CrossRef] [PubMed]
- Niedek, D.; Erb, F.R.; Topp, C.; Seitz, A.; Wende, C.; Eckhardt, A.K.; Kind, J.; Herold, D.; Thiele, C.M.; Schreiner, P.R. In Situ Switching of Site-Selectivity with Light in the Acetylation of Sugars with Azopeptide Catalysts. J. Org. Chem. 2020, 85, 1835–1846. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Wang, Y.; Xia, F.; Dai, Y. Cofactor-Free Organic Nanozyme with Assembly-Induced Catalysis and Light-Regulated Activity. Chem. Eng. J. 2021, 426, 130855. [Google Scholar] [CrossRef]
- Zhao, Y.; Lei, B.; Wang, M.; Wu, S.; Qi, W.; Su, R.; He, Z. A Supramolecular Approach to Construct a Hydrolase Mimic with Photo-Switchable Catalytic. J. Mater. Chem. B 2018, 6, 2444–2449. [Google Scholar] [CrossRef]
- Szewczyk, M.; Sobczak, G.; Sashuk, V. Photoswitchable Catalysis by a Small Swinging Molecule Confined on the Surface of a Colloidal Particle. ACS Catal. 2018, 8, 2810–2814. [Google Scholar] [CrossRef]
- Ueno, A.; Takahashi, K.; Osa, T. Photoregulation of Catalytic Activity of β-Cyclodextrin by an Azo Inhibitor. J. Chem. Soc. Chem. Commun. 1980, 837–838. [Google Scholar] [CrossRef]
- Kondo, M.; Nakamura, K.; Krishnan, C.G.; Takizawa, S.; Abe, T.; Sasai, H. Photoswitchable Chiral Phase Transfer Catalyst. ACS Catal. 2021, 11, 1863–1867. [Google Scholar] [CrossRef]
- Honnigfort, C.; Topp, L.; García Rey, N.; Heuer, A.; Braunschweig, B. Dynamic Wetting of Photoresponsive Arylazopyrazole Monolayers Is Controlled by the Molecular Kinetics of the Monolayer. J. Am. Chem. Soc. 2022, 144, 4026–4038. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Shodai, A.; Yasui, H.; Sakurai, H.; Hirota, S. Photocontrol of Spatial Orientation and DNA Cleavage Activity of Copper(II)-Bound Dipeptides Linked by an Azobenzene Derivative. Inorg. Chem. 2008, 47, 5045–5047. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.L.; Yao, W.T.; Liu, Z.Q.; Zheng, A.M.; Li, J.Q.; Feng, X.F.; Ma, L.F.; Yan, C.S.; Luo, M.B.; Luo, F. Photoswitching Storage of Guest Molecules in Metal-Organic Framework for Photoswitchable Catalysis: Exceptional Product, Ultrahigh Photocontrol, and Photomodulated Size Selectivity. J. Mater. Chem. A 2017, 5, 7961–7967. [Google Scholar] [CrossRef]
- Nagai, Y.; Ishiba, K.; Yamamoto, R.; Yamada, T.; Morikawa, M.; Kimizuka, N. Light-Triggered, Non-Centrosymmetric Self-Assembly of Aqueous Arylazopyrazoles at the Air–Water Interface and Switching of Second-Harmonic Generation. Angew. Chem. Int. Ed. 2021, 60, 6333–6338. [Google Scholar] [CrossRef]
- Cheng, H.B.; Zhang, S.; Bai, E.; Cao, X.; Wang, J.; Qi, J.; Liu, J.; Zhao, J.; Zhang, L.; Yoon, J. Future-Oriented Advanced Diarylethene Photoswitches: From Molecular Design to Spontaneous Assembly Systems. Adv. Mater. 2022, 34, 2108289. [Google Scholar] [CrossRef]
- Majee, D.; Presolski, S. Dithienylethene-Based Photoswitchable Catalysts: State of the Art and Future Perspectives. ACS Catal. 2021, 11, 2244–2252. [Google Scholar] [CrossRef]
- Sud, D.; Norsten, T.B.; Branda, N.R. Photoswitching of Stereoselectivity in Catalysis Using a Copper Dithienylethene Complex. Angew. Chem. Int. Ed. 2005, 44, 2019–2021. [Google Scholar] [CrossRef]
- Vomasta, D.; Högner, C.; Branda, N.R.; König, B. Regulation of Human Carbonic Anhydrase I (HCAI) Activity by Using a Photochromic Inhibitor. Angew. Chem. Int. Ed. 2008, 47, 7644–7647. [Google Scholar] [CrossRef]
- Eisenreich, F.; Kathan, M.; Dallmann, A.; Ihrig, S.P.; Schwaar, T.; Schmidt, B.M.; Hecht, S. A Photoswitchable Catalyst System for Remote-Controlled (Co)Polymerization in Situ. Nat. Catal. 2018, 1, 516–522. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, C.Y.; Jang, W.D.; Jaung, J.Y. Effect of Donor-π-Acceptor Structure on Photochromism of Dithienylethene-Based Dyes. Dye. Pigments 2020, 177, 108315. [Google Scholar] [CrossRef]
- Afonin, S.; Babii, O.; Reuter, A.; Middel, V.; Takamiya, M.; Strähle, U.; Komarov, I.V.; Ulrich, A.S. Light-Controllable Dithienylethene-Modified Cyclic Peptides: Photoswitching the in Vivo Toxicity in Zebrafish Embryos. Beilstein J. Org. Chem. 2020, 16, 39–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, R.; Frasco, M.F.; Sales, M.G.F. Photonics in Nature and Bioinspired Designs: Sustainable Approaches for a Colourful World. Nanoscale Adv. 2020, 2, 5106–5129. [Google Scholar] [CrossRef]
- Pei, K.; Wu, J.; Zhao, M.; Feng, X.; Li, Y.; Ma, Y.; Li, H.; Zhai, T. Polarized Emission of Lanthanide Metal–Organic Framework (Ln-MOF) Crystals for High-Capacity Photonic Barcodes. Adv. Opt. Mater. 2022, 10, 2102143. [Google Scholar] [CrossRef]
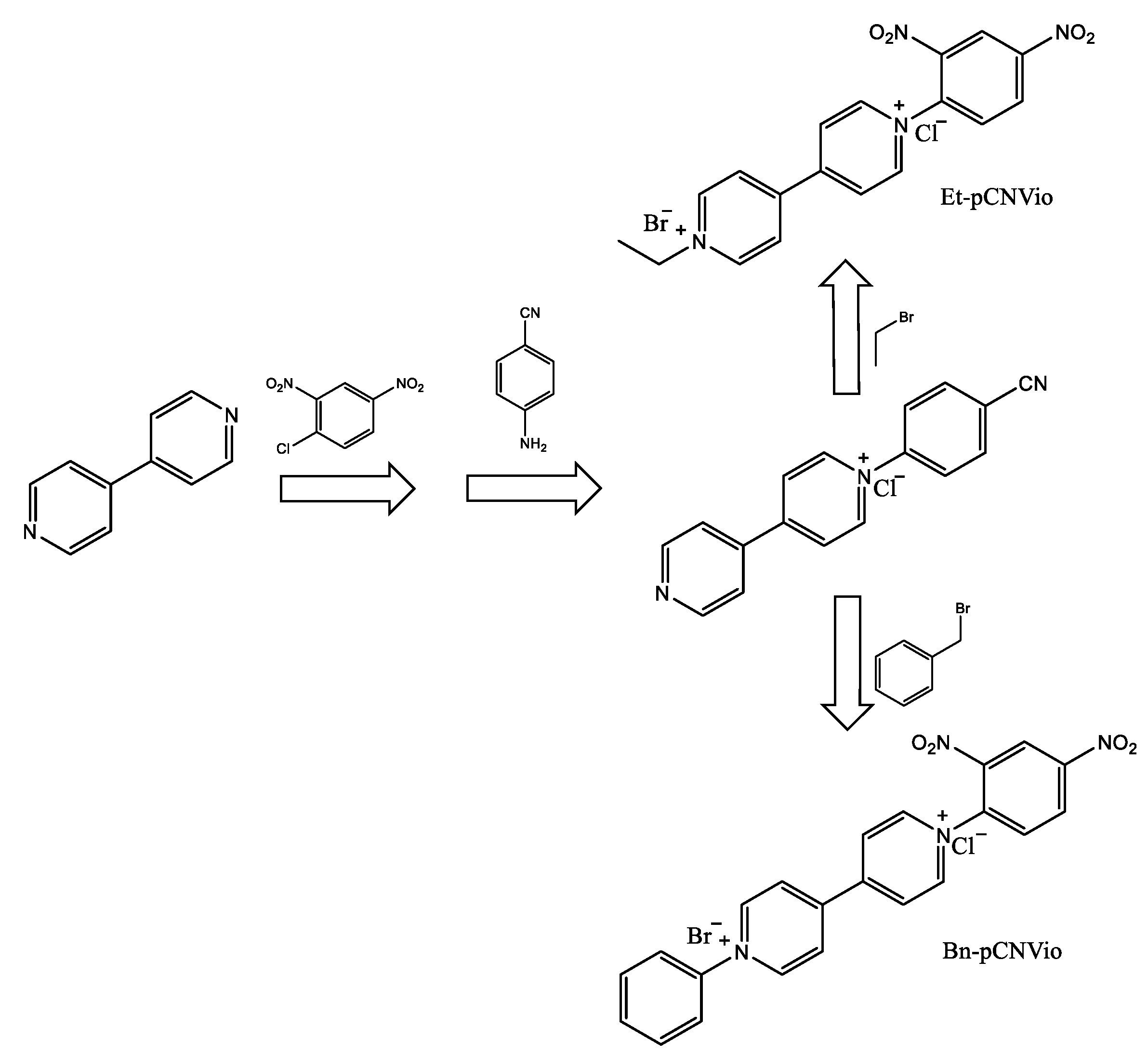
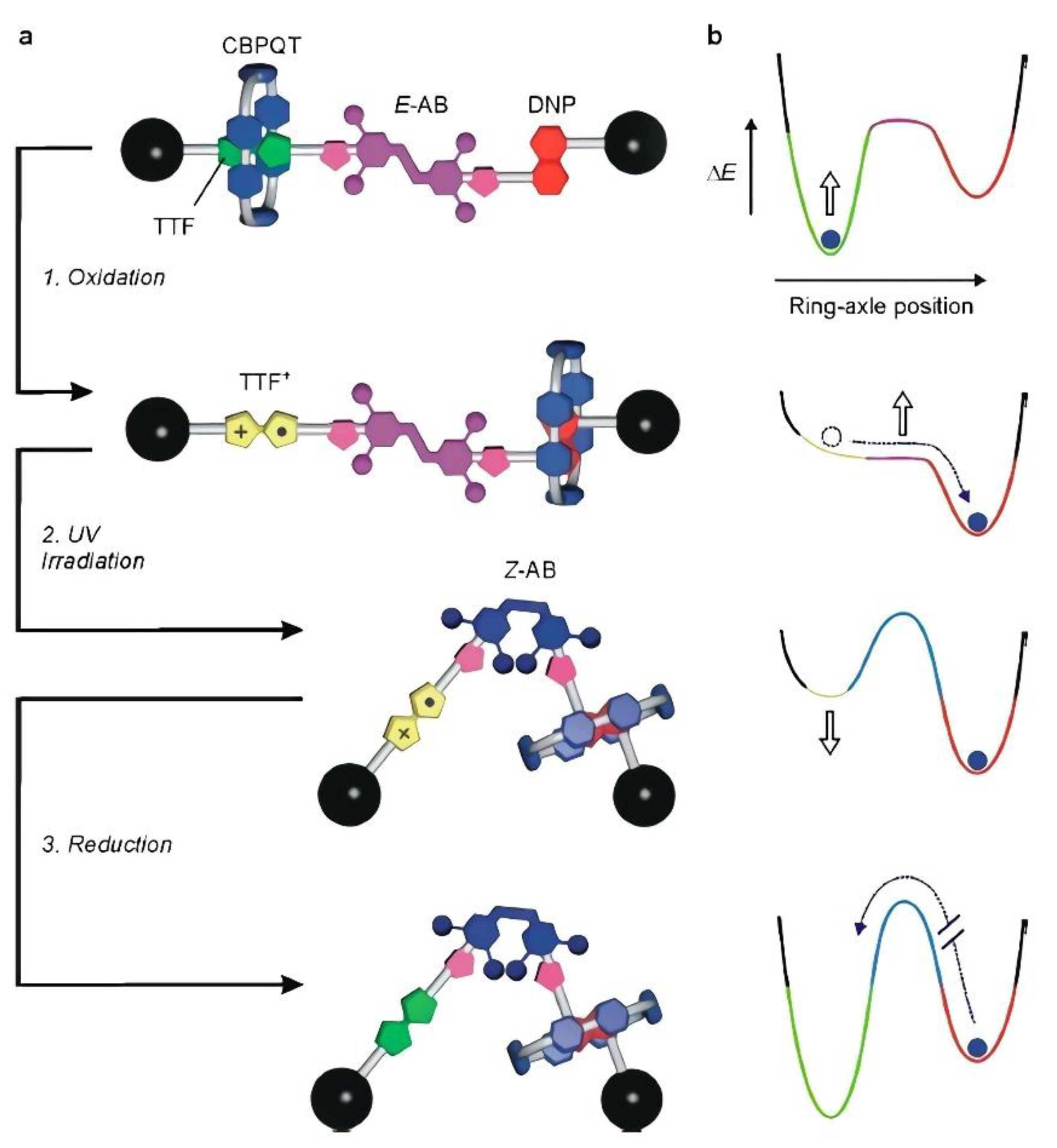
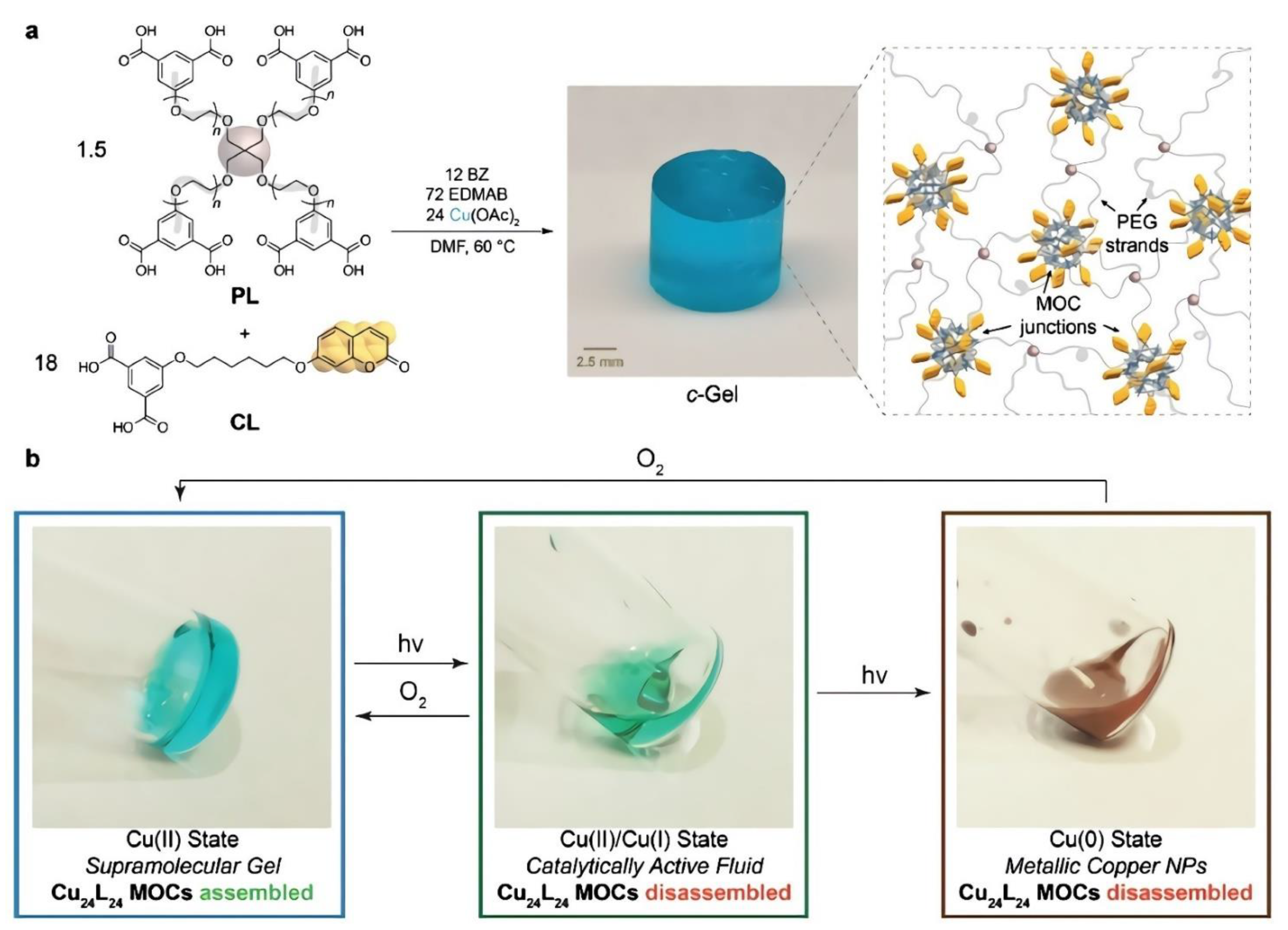
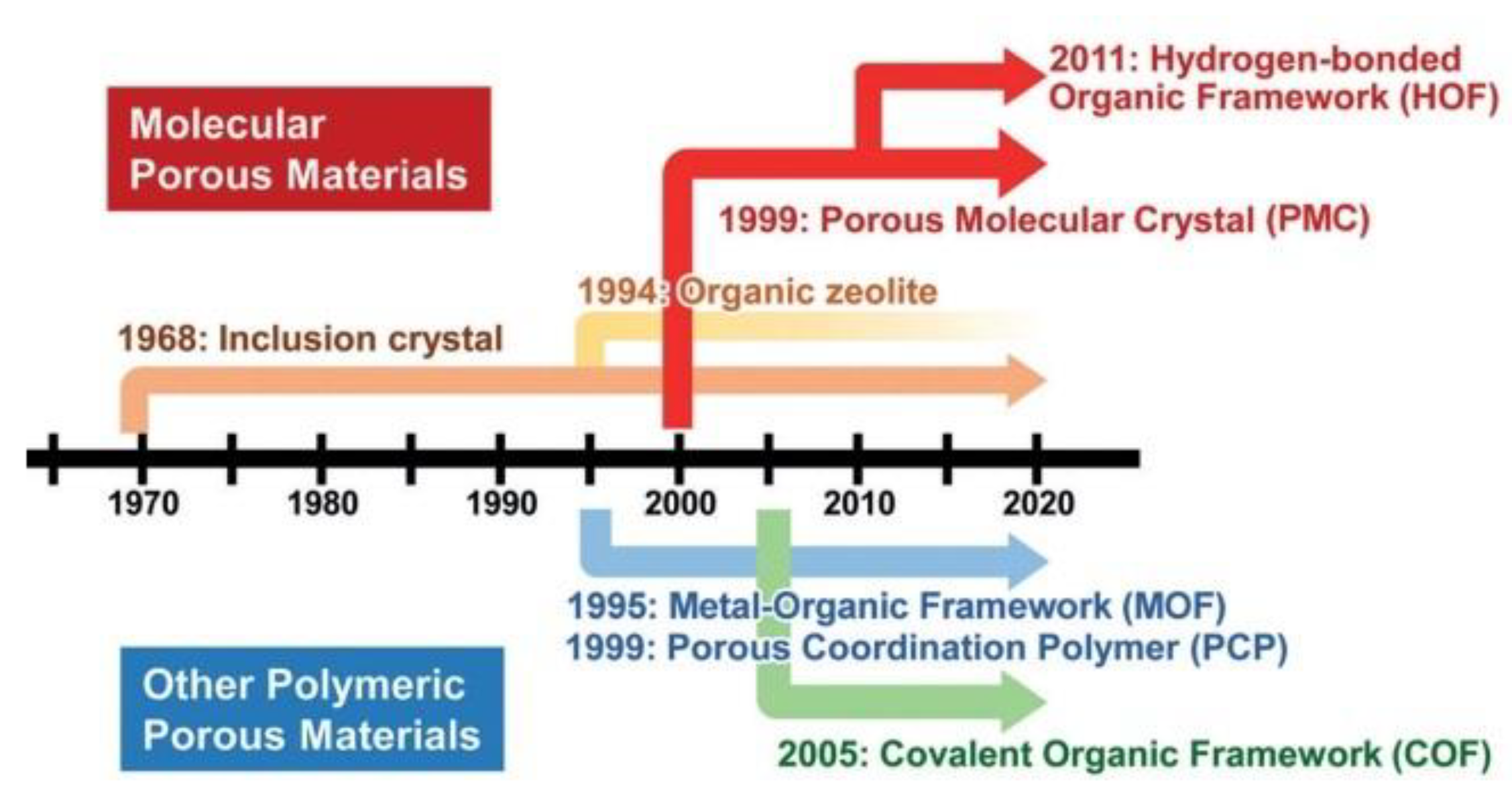

| Ref. | Metal Ion | Organic Ligand | Ligand Nature | Cage Structure | Geometry | Guest | Cage Containing Polymer | Irradiating Light λ (Reversible) | Light Exposure Time (min) | Reusability (Cycles) | Mechanism | Application |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [61] | - | ABOEG | Chiral acyclic oligoethylene glycol | - | - | K+/Cs+ | - | 365/448 nm | 30/30 | - | E/Z isomerization of the azobenzene unit | Synthesis of optically active amines |
| [60] | Cu(II) | m-BDC | Coumarin based | Cu24L24 | Cuboctahedra | - | PEG | 368 nm | 480 | 4, 12 states | Transition between Cu(II),Cu(I), and Cu(0) states | Catalysis of azide-alkyne cycloaddition (CuAAC) reactions/Sol–gel transition/Color switches |
| [64] | Pd(II) | Z1 | Bidentate bispyridyl | Pd2L4 | Square planar | TA | 312/365 nm | - | - | Photochromic ligand isomer interconversion | Tuning guest binding affinity and selectivity properties of supramolecular coordination complexes | |
| [65] | Cu(II) | H6TDPAT | Melamine based | - | Cuboctahedron/Truncated tetrahedron/Truncated octahedron | CO2/CS2 | - | >400 nm | 720 | 5 | Cu(II)/Cu(I) transition | Incorporate CO2/CS2 and CF3 groups into value-added heterocycles |
| [62] | Zn(II) | SD | Tetrameric silsesquioxane and pyrazole functionalized with azobenzene | Zn8L | Octanuclear space craft-like | - | 365/450 nm | 10/2 | - | trans/cis ligand isomerization | Light sensitive molecular pump | |
| [66] | Pd(II) | - | Tetratopic N-donor | Pd2L4 | Hexanuclear | - | PEG | 456 nm | 60 | 4 | Reversible cleavage of the metal–ligand bond | Sol–gel transitioning |
| [67] | Ga | DAB | Polyaromatic bridge | Ga4L6 | - | CA | 400 nm | - | photoinduced electron transfer (PET): Ga4L6 12− cage absorbs photons and transfers an electron to the guest ion | Transfer energy to encapsulated guest molecules | ||
| [63] | Pd(II) | - | Bent bis-monodentate pyridyl | Pd2L4 | - | cis-4,4′-AB | PEG | 365 nm/white light | - | 4 | cis/trans isomerization of guest | Expelling the guest out of the cage/Spatially controlled lithographic deposition |
| [59] | Pd | IA | - | - | - | AAP | - | 365/520 nm | 5 | 10 | E to Z isomerization of arylazopyrazole | Solubilization of E isomer of arylazopyrazole in water |
| [68] | Cu(II) | DPD | Azobenzene containing unit | Cu12L24 | Cuboctahedra | MB | - | 365/blue light | 30 | 5 | trans/cis isomerization of ligand | Expel the guest from the cavity |
| [69] | Pd(II) | DTE | - | Pd2L4 | - | R/S CSA | 313 nm | - | Interconversion between ligand open and photoisomeric closed forms | Expel the guest from the cavity |
| Azo-Based Photoswitch | Substituent Groups | Substituent Configuration | Substituent Nature | (nm) trans | (nm) trans | Tethered Ligand | Ligand Configuration | Conformational Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Tetra fluorinated azobenzene | Fluorine (4) | ortho | EWG | 447 | 320 | Iperoxo | para | Improve binding affinity to muscarinic receptor | [128] |
| MAG azobenzene | Fluorine (1) | ortho | EWG | 473 | 365 | Maleimide Glutamate | para | Control over activation of glutamate receptors | [129] |
| Azobenzene based polyamide | -NHCO-(2) | para | EDG | 425 | 323 | - | - | Decrease in the thermal half-life of the cis isomer | [130] |
| Azo-combretastatin | Methoxy (3) Ethoxy (1) | meta (2) para (1) meta (1) | EWG | - | <400 | Ethoxy | para | Improve binding affinity to Tubulin through colchicine pocket | [131] |
| Diazocine | Acetamide derivative | - | - | - | <400 | - | - | Inversion of conventional cis active state to a trans active state | [132] |
| APA | - | - | - | 450 | 335 | PEG | para | Control over polymer phase transition | [133] |
| Azo-AA monomer | - | - | - | >436 | >365 | 4-phenylazophenyl acrylate-DMF | para | Up-shift in the phase transition temperature | [134] |
| Azobenzene based monomer | CH3 | para | EDG | 450 | 350 | HOC6H12O | para | Solid to liquid transition of the azopolymer | [135] |
| Azobenzene-Schiff base | - | - | EWG | 365 | 500 | Al(III)-Cl substituted Salicylaldehyde derivative Schiff base | para- | Ring opening polymerization ε-caprolactone | [120] |
| Tri-azo substituted phosphine | Phosphorus | para | EDG | 345 | - | Ruthenium complexes | - | H generation by hydrolytic decomposition of AB | [136] |
| PLP–photoswitch–imidazole triad | PLP | meta | EWG | 431 | 319 | Imidazole | meta | Conversion of L-amino acids toD-isomers | [137] |
| Azopeptide catalysts | Pmh | meta (2) | - | 448 | - | - | - | Acetylation ofsugars | [138] |
| Azo-GFGH | Cyclodextrin | - | - | - | 324 | GFGH | - | Hydrolysis of 4-nitrophenylacetate | [139] |
| Azo-GFGH | Histidine residue | - | - | - | 325 | GFGH | - | Hydrolysis of p-NPA | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaria-Garcia, V.J.; Flores-Hernandez, D.R.; Contreras-Torres, F.F.; Cué-Sampedro, R.; Sánchez-Fernández, J.A. Advances in the Structural Strategies of the Self-Assembly of Photoresponsive Supramolecular Systems. Int. J. Mol. Sci. 2022, 23, 7998. https://doi.org/10.3390/ijms23147998
Santamaria-Garcia VJ, Flores-Hernandez DR, Contreras-Torres FF, Cué-Sampedro R, Sánchez-Fernández JA. Advances in the Structural Strategies of the Self-Assembly of Photoresponsive Supramolecular Systems. International Journal of Molecular Sciences. 2022; 23(14):7998. https://doi.org/10.3390/ijms23147998
Chicago/Turabian StyleSantamaria-Garcia, Vivian J., Domingo R. Flores-Hernandez, Flavio F. Contreras-Torres, Rodrigo Cué-Sampedro, and José Antonio Sánchez-Fernández. 2022. "Advances in the Structural Strategies of the Self-Assembly of Photoresponsive Supramolecular Systems" International Journal of Molecular Sciences 23, no. 14: 7998. https://doi.org/10.3390/ijms23147998
APA StyleSantamaria-Garcia, V. J., Flores-Hernandez, D. R., Contreras-Torres, F. F., Cué-Sampedro, R., & Sánchez-Fernández, J. A. (2022). Advances in the Structural Strategies of the Self-Assembly of Photoresponsive Supramolecular Systems. International Journal of Molecular Sciences, 23(14), 7998. https://doi.org/10.3390/ijms23147998








