Human Chorionic Villous Differentiation and Placental Development
Abstract
1. Placental Function
2. Villous Differentiation and Placental Formation
2.1. From Villi Differentiation to Early Placental Formation
2.2. Formation of the Trophoblast
2.3. Placental Blood Vessel Development
3. Placental Formation and Hypoxia
4. Formation of the Decidua
4.1. Natural Killer (NK) Cells
4.1.1. Decidual Natural Killer (dNK) Cells
4.1.2. Role of dNK Cells
4.2. Decidual Macrophages
4.2.1. M1 and M2 Macrophages
4.2.2. Phenotypes of Decidual Macrophages
4.3. Regulatory T (Treg) Cells
Role of Treg Cells
5. Genes Involved in Villous Differentiation
6. Diseases Involving Placental Dysfunction
6.1. Fetal Growth Restriction (FGR)
6.2. Hypertensive Disorders of Pregnancy (HDP)
6.3. Gestational Diabetes Mellitus (GDM)
7. Trophoblast Research Tool
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, J.L.; Carter, A.M.; Chamley, L.W. Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation? Placenta 2012, 33, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Cuman, C.; Menkhorst, E.; Winship, A.; Van Sinderen, M.; Osianlis, T.; Rombauts, L.J.; Dimitriadis, E. Fetal-maternal communication: The role of Notch signalling in embryo implantation. Reproduction 2014, 147, R75–R86. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Q.; Gardner, L.; Turco, M.; Zhao, N.; Murray, M.J.; Coleman, N.; Rossant, J.; Hemberger, M.; Moffett, A. What Is Trophoblast? A Combination of Criteria Define Human First-Trimester Trophoblast. Stem Cell Rep. 2016, 6, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Degner, K.; Magness, R.R.; Shah, D.M. Establishment of the Human Uteroplacental Circulation: A Historical Perspective. Reprod. Sci. 2017, 24, 753–761. [Google Scholar] [CrossRef]
- Saghian, R.; Bogle, G.; James, J.L.; Clark, A.R. Establishment of maternal blood supply to the placenta: Insights into plugging, unplugging and trophoblast behaviour from an agent-based model. Interface Focus 2019, 9, 20190019. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.; Burton, G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am. J. Obstet. Gynecol. 2001, 184, 998–1003. [Google Scholar] [CrossRef]
- Rodesch, F.; Simon, P.; Donner, C.; Jauniaux, E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 1992, 80, 283–285. [Google Scholar]
- Burton, G.J.; Jauniaux, E.; Charnock-Jones, D.S. The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 2010, 54, 303–312. [Google Scholar] [CrossRef]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and Placental Development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef]
- Velicky, P.; Knöfler, M.; Pollheimer, J. Function and control of human invasive trophoblast subtypes: Intrinsic vs. maternal control. Cell Adhes. Migr. 2016, 10, 154–162. [Google Scholar] [CrossRef]
- Varney, M.L.; Olsen, K.J.; Mosley, R.L.; Singh, R.K. Paracrine regulation of vascular endothelial growth factor—A expression during macrophage-melanoma cell interaction: Role of monocyte chemotactic protein-1 and macrophage colony-stimulating factor. J. Interferon Cytokine Res. 2005, 25, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Heikkila, P.; Suojanen, J.; Pirila, E.; Vaananen, A.; Koivunen, E.; Sorsa, T.; Salo, T. Human tongue carcinoma growth is inhibited by selective antigelatinolytic peptides. Int. J. Cancer 2006, 118, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.A.; Lysiak, J.J.; Graham, C.H.; Hearn, S.; Han, V.K.; Lala, P.K. Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta 1995, 16, 413–433. [Google Scholar] [CrossRef]
- Genbacev, O.; Joslin, R.; Damsky, C.H.; Polliotti, B.M.; Fisher, S.J. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Investig. 1996, 97, 540–550. [Google Scholar] [CrossRef]
- Genbacev, O.; Zhou, Y.; Ludlow, J.W.; Fisher, S.J. Regulation of human placental development by oxygen tension. Science 1997, 277, 1669–1672. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the survival of early pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef]
- Douglas, R.M.; Haddad, G.G. Genetic models in applied physiology: Invited review: Effect of oxygen deprivation on cell cycle activity: A profile of delay and arrest. J. Appl. Physiol. 2003, 94, 2068–2083, discussion 2084. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, Q.Y.; Jiang, Y.Z.; Zheng, J. Role of oxygen in fetoplacental endothelial responses: Hypoxia, physiological normoxia, or hyperoxia? Am. J. Physiol. Cell Physiol. 2020, 318, C943–C953. [Google Scholar] [CrossRef]
- Watson, A.L.; Skepper, J.N.; Jauniaux, E.; Burton, G.J. Susceptibility of human placental syncytiotrophoblastic mitochondria to oxygen-mediated damage in relation to gestational age. J. Clin. Endocrinol. Metab. 1998, 83, 1697–1705. [Google Scholar] [CrossRef]
- Watson, A.L.; Palmer, M.E.; Jauniaux, E.; Burton, G.J. Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta 1997, 18, 295–299. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Sivridis, E.; Turley, H.; Talks, K.; Pezzella, F.; Gatter, K.C.; Harris, A.L. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br. J. Cancer 2001, 85, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Bergeron, D.L.; Simon, M.C. Hypoxia-inducible factor and the development of stem cells of the cardiovascular system. Stem Cells 2001, 19, 279–286. [Google Scholar] [CrossRef]
- Yu, N.; Wu, J.L.; Xiao, J.; Fan, L.; Chen, S.H.; Li, W. HIF-1alpha regulates angiogenesis via Notch1/STAT3/ETBR pathway in trophoblastic cells. Cell Cycle 2019, 18, 3502–3512. [Google Scholar] [CrossRef]
- Caniggia, I.; Mostachfi, H.; Winter, J.; Gassmann, M.; Lye, S.J.; Kuliszewski, M.; Post, M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J. Clin. Investig. 2000, 105, 577–587. [Google Scholar] [CrossRef]
- Nishi, H.; Nakada, T.; Hokamura, M.; Osakabe, Y.; Itokazu, O.; Huang, L.E.; Isaka, K. Hypoxia-inducible factor-1 transactivates transforming growth factor-beta3 in trophoblast. Endocrinology 2004, 145, 4113–4118. [Google Scholar] [CrossRef]
- Adelman, D.M.; Gertsenstein, M.; Nagy, A.; Simon, M.C.; Maltepe, E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000, 14, 3191–3203. [Google Scholar] [CrossRef]
- Gnarra, J.R.; Ward, J.M.; Porter, F.D.; Wagner, J.R.; Devor, D.E.; Grinberg, A.; Emmert-Buck, M.R.; Westphal, H.; Klausner, R.D.; Linehan, W.M. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc. Natl. Acad. Sci. USA 1997, 94, 9102–9107. [Google Scholar] [CrossRef]
- Genbacev, O.; Krtolica, A.; Kaelin, W.; Fisher, S.J. Human cytotrophoblast expression of the von Hippel-Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev. Biol. 2001, 233, 526–536. [Google Scholar] [CrossRef]
- Wakeland, A.K.; Soncin, F.; Moretto-Zita, M.; Chang, C.W.; Horii, M.; Pizzo, D.; Nelson, K.K.; Laurent, L.C.; Parast, M.M. Hypoxia Directs Human Extravillous Trophoblast Differentiation in a Hypoxia-Inducible Factor-Dependent Manner. Am. J. Pathol. 2017, 187, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jiang, Y.; Cao, Q.; Hou, Y.; Wang, C. Role of integrin switch and transforming growth factor Beta 3 in hypoxia-induced invasion inhibition of human extravillous trophoblast cells. Biol. Reprod. 2012, 87, 47. [Google Scholar] [CrossRef] [PubMed]
- Caniggia, I.; Grisaru-Gravnosky, S.; Kuliszewsky, M.; Post, M.; Lye, S.J. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J. Clin. Investig. 1999, 103, 1641–1650. [Google Scholar] [CrossRef]
- Farrell, A.; Alahari, S.; Ermini, L.; Tagliaferro, A.; Litvack, M.; Post, M.; Caniggia, I. Faulty oxygen sensing disrupts angiomotin function in trophoblast cell migration and predisposes to preeclampsia. JCI Insight 2019, 4, e127009. [Google Scholar] [CrossRef]
- Yinon, Y.; Nevo, O.; Xu, J.; Many, A.; Rolfo, A.; Todros, T.; Post, M.; Caniggia, I. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: Hypoxic regulation via transforming growth factor-beta 3. Am. J. Pathol. 2008, 172, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.H.; Yong, H.E.J.; Chollangi, T.; Brennecke, S.P.; Fisher, S.J.; Wallace, E.M.; Ebeling, P.R.; Murthi, P. Altered downstream target gene expression of the placental Vitamin D receptor in human idiopathic fetal growth restriction. Cell Cycle 2018, 17, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Pringle, K.G.; Kind, K.L.; Thompson, J.G.; Roberts, C.T. Complex interactions between hypoxia inducible factors, insulin-like growth factor-II and oxygen in early murine trophoblasts. Placenta 2007, 28, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, K.; Li, T.; Chen, J.; Xie, D.; Chang, X.; Yao, J.; Wu, J.; Zhou, Q.; Jia, Y.; et al. Hypoxia-induced TET1 facilitates trophoblast cell migration and invasion through HIF1alpha signaling pathway. Sci. Rep. 2017, 7, 8077. [Google Scholar] [CrossRef]
- Tedde, G.; Piras, A.T. Mitotic index of the Langhans’ cells in the normal human placenta from the early stages of pregnancy to the term. Cells Tissues Organs 1978, 100, 114–119. [Google Scholar] [CrossRef]
- Kilburn, B.A.; Wang, J.; Duniec-Dmuchowski, Z.M.; Leach, R.E.; Romero, R.; Armant, D.R. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol. Reprod. 2000, 62, 739–747. [Google Scholar] [CrossRef]
- Jiang, B.; Kamat, A.; Mendelson, C.R. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: Potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol. Endocrinol. 2000, 14, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Delforce, S.J.; Wang, Y.; Van-Aalst, M.E.; Corbisier de Meaultsart, C.; Morris, B.J.; Broughton-Pipkin, F.; Roberts, C.T.; Lumbers, E.R.; Pringle, K.G. Effect of oxygen on the expression of renin-angiotensin system components in a human trophoblast cell line. Placenta 2016, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Perlman, B.E.; Merriam, A.A.; Lemenze, A.; Zhao, Q.; Begum, S.; Nair, M.; Wu, T.; Wapner, R.J.; Kitajewski, J.K.; Shawber, C.J.; et al. Implications for preeclampsia: Hypoxia-induced Notch promotes trophoblast migration. Reproduction 2021, 161, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Colson, A.; Depoix, C.L.; Baldin, P.; Hubinont, C.; Sonveaux, P.; Debieve, F. Hypoxia-inducible factor 2 alpha impairs human cytotrophoblast syncytialization: New insights into placental dysfunction and fetal growth restriction. FASEB J. 2020, 34, 15222–15235. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, L.; Hirota, Y.; Saito-Fujita, T.; Takeda, N.; Tanaka, T.; Hiraoka, T.; Akaeda, S.; Fujita, H.; Shimizu-Hirota, R.; Igaue, S.; et al. HIF2alpha in the uterine stroma permits embryo invasion and luminal epithelium detachment. J. Clin. Investig. 2018, 128, 3186–3197. [Google Scholar] [CrossRef]
- Horii, M.; Li, Y.; Wakeland, A.K.; Pizzo, D.P.; Nelson, K.K.; Sabatini, K.; Laurent, L.C.; Liu, Y.; Parast, M.M. Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc. Natl. Acad. Sci. USA 2016, 113, E3882–E3891. [Google Scholar] [CrossRef]
- Maltepe, E.; Krampitz, G.W.; Okazaki, K.M.; Red-Horse, K.; Mak, W.; Simon, M.C.; Fisher, S.J. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development 2005, 132, 3393–3403. [Google Scholar] [CrossRef]
- Albers, R.E.; Kaufman, M.R.; Natale, B.V.; Keoni, C.; Kulkarni-Datar, K.; Min, S.; Williams, C.R.; Natale, D.R.C.; Brown, T.L. Trophoblast-Specific Expression of Hif-1alpha Results in Preeclampsia-Like Symptoms and Fetal Growth Restriction. Sci. Rep. 2019, 9, 2742. [Google Scholar] [CrossRef]
- Rowe, J.H.; Ertelt, J.M.; Xin, L.; Way, S.S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012, 490, 102–106. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Dunk, C.E.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 2009, 174, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Searle, R.F.; Robson, S.C.; Innes, B.A.; Bulmer, J.N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Rieger, L.; Segerer, S.; Bernar, T.; Kapp, M.; Majic, M.; Morr, A.K.; Dietl, J.; Kammerer, U. Specific subsets of immune cells in human decidua differ between normal pregnancy and preeclampsia--a prospective observational study. Reprod. Biol. Endocrinol. 2009, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 2019, 8, e52004. [Google Scholar] [CrossRef]
- Keskin, D.B.; Allan, D.S.; Rybalov, B.; Andzelm, M.M.; Stern, J.N.; Kopcow, H.D.; Koopman, L.A.; Strominger, J.L. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc. Natl. Acad. Sci. USA 2007, 104, 3378–3383. [Google Scholar] [CrossRef]
- Vacca, P.; Vitale, C.; Montaldo, E.; Conte, R.; Cantoni, C.; Fulcheri, E.; Darretta, V.; Moretta, L.; Mingari, M.C. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 2402–2407. [Google Scholar] [CrossRef]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Y.H.; Piao, H.L.; Zhou, W.J.; Zhang, D.; Fu, Q.; Wang, S.C.; Li, D.J.; Du, M.R. CD56(bright)CD25+ NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. Cell Mol. Immunol. 2015, 12, 77–86. [Google Scholar] [CrossRef][Green Version]
- Lu, H.; Jin, L.P.; Huang, H.L.; Ha, S.Y.; Yang, H.L.; Chang, R.Q.; Li, D.J.; Li, M.Q. Trophoblast-derived CXCL12 promotes CD56(bright) CD82(−) CD29(+) NK cell enrichment in the decidua. Am. J. Reprod. Immunol. 2020, 83, e13203–e13215. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Robson, S.C.; Bulmer, J.N. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta 2010, 31, S87–S92. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Colucci, F. Uterine NK cells: Active regulators at the maternal-fetal interface. J. Clin. Investig. 2014, 124, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.H.; Dunk, C.E.; Lye, S.J.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Extravillous Trophoblast and Endothelial Cell Crosstalk Mediates Leukocyte Infiltration to the Early Remodeling Decidual Spiral Arteriole Wall. J. Immunol. 2017, 198, 4115–4128. [Google Scholar] [CrossRef]
- Zhang, J.; Adams, M.A.; Croy, B.A. Alterations in maternal and fetal heart functions accompany failed spiral arterial remodeling in pregnant mice. Am. J. Obstet. Gynecol. 2011, 205, 485.e1–485.e16. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Medeiros, L.T.; Peracoli, J.C.; Bannwart-Castro, C.F.; Romao, M.; Weel, I.C.; Golim, M.A.; de Oliveira, L.G.; Kurokawa, C.S.; Medeiros Borges, V.T.; Peracoli, M.T. Monocytes from pregnant women with pre-eclampsia are polarized to a M1 phenotype. Am. J. Reprod. Immunol. 2014, 72, 5–13. [Google Scholar] [CrossRef]
- Chabtini, L.; Mfarrej, B.; Mounayar, M.; Zhu, B.; Batal, I.; Dakle, P.J.; Smith, B.D.; Boenisch, O.; Najafian, N.; Akiba, H.; et al. TIM-3 regulates innate immune cells to induce fetomaternal tolerance. J. Immunol. 2013, 190, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two unique human decidual macrophage populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Scherjon, S.A.; van der Mast, B.J.; Haasnoot, G.W.; Voort-Maarschalk, M.V.V.; Roelen, D.L.; van Rood, J.J.; Claas, F.H. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J. Reprod. Immunol. 2009, 82, 148–157. [Google Scholar] [CrossRef]
- Lissauer, D.; Piper, K.; Goodyear, O.; Kilby, M.D.; Moss, P.A. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 2012, 189, 1072–1080. [Google Scholar] [CrossRef]
- Jasper, M.J.; Tremellen, K.P.; Robertson, S.A. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol. Hum. Reprod. 2006, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Sasaki, Y.; Itoh, M.; Nakashima, A.; Ishii, N.; Sugamura, K.; Saito, S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010, 85, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kahn, D.A.; Baltimore, D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc. Natl. Acad. Sci. USA 2010, 107, 9299–9304. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, J.Y.; Hur, S.E.; Kim, C.J.; Na, B.J.; Lee, M.; Gilman-Sachs, A.; Kwak-Kim, J. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011, 26, 2964–2971. [Google Scholar] [CrossRef]
- Wang, W.J.; Hao, C.F.; Qu, Q.L.; Wang, X.; Qiu, L.H.; Lin, Q.D. The deregulation of regulatory T cells on interleukin-17-producing T helper cells in patients with unexplained early recurrent miscarriage. Hum. Reprod. 2010, 25, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, J.; Xu, H.L.; Xu, B.; Tong, X.H.; Kwak-Kim, J.; Liu, Y.S. IL-7/IL-7R signaling pathway might play a role in recurrent pregnancy losses by increasing inflammatory Th17 cells and decreasing Treg cells. Am. J. Reprod. Immunol. 2016, 76, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Liu, F.J.; Xin, L.; Hao, C.F.; Bao, H.C.; Qu, Q.L.; Liu, X.M. Adoptive transfer of pregnancy-induced CD4+CD25+ regulatory T cells reverses the increase in abortion rate caused by interleukin 17 in the CBA/JxBALB/c mouse model. Hum. Reprod. 2014, 29, 946–952. [Google Scholar] [CrossRef]
- Schwede, S.; Alfer, J.; von Rango, U. Differences in regulatory T-cell and dendritic cell pattern in decidual tissue of placenta accreta/increta cases. Placenta 2014, 35, 378–385. [Google Scholar] [CrossRef]
- Krendl, C.; Shaposhnikov, D.; Rishko, V.; Ori, C.; Ziegenhain, C.; Sass, S.; Simon, L.; Muller, N.S.; Straub, T.; Brooks, K.E.; et al. GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc. Natl. Acad. Sci. USA 2017, 114, E9579–E9588. [Google Scholar] [CrossRef] [PubMed]
- Home, P.; Kumar, R.P.; Ganguly, A.; Saha, B.; Milano-Foster, J.; Bhattacharya, B.; Ray, S.; Gunewardena, S.; Paul, A.; Camper, S.A.; et al. Genetic redundancy of GATA factors in the extraembryonic trophoblast lineage ensures the progression of preimplantation and postimplantation mammalian development. Development 2017, 144, 876–888. [Google Scholar] [PubMed]
- Li, Y.; Moretto-Zita, M.; Leon-Garcia, S.; Parast, M.M. p63 inhibits extravillous trophoblast migration and maintains cells in a cytotrophoblast stem cell-like state. Am. J. Pathol. 2014, 184, 3332–3343. [Google Scholar] [CrossRef]
- Lin, K.C.; Park, H.W.; Guan, K.L. Regulation of the Hippo Pathway Transcription Factor TEAD. Trends Biochem. Sci. 2017, 42, 862–872. [Google Scholar] [CrossRef]
- Haider, S.; Meinhardt, G.; Saleh, L.; Fiala, C.; Pollheimer, J.; Knofler, M. Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc. Natl. Acad. Sci. USA 2016, 113, E7710–E7719. [Google Scholar] [CrossRef]
- Saha, B.; Ganguly, A.; Home, P.; Bhattacharya, B.; Ray, S.; Ghosh, A.; Rumi, M.A.K.; Marsh, C.; French, V.A.; Gunewardena, S.; et al. TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss. Proc. Natl. Acad. Sci. USA 2020, 117, 17864–17875. [Google Scholar] [CrossRef]
- Pobbati, A.V.; Chan, S.W.; Lee, I.; Song, H.; Hong, W. Structural and functional similarity between the Vgll1-TEAD and the YAP-TEAD complexes. Structure 2012, 20, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Soncin, F.; Khater, M.; To, C.; Pizzo, D.; Farah, O.; Wakeland, A.; Arul Nambi Rajan, K.; Nelson, K.K.; Chang, C.W.; Moretto-Zita, M.; et al. Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development 2018, 145, dev156273. [Google Scholar] [CrossRef] [PubMed]
- Krebsbach, P.H.; Villa-Diaz, L.G. The Role of Integrin alpha6 (CD49f) in Stem Cells: More than a Conserved Biomarker. Stem Cells Dev. 2017, 26, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.J.; Chakraborty, D.; Mason, C.W.; Rumi, M.A.; Vivian, J.L.; Soares, M.J. OVO-like 1 regulates progenitor cell fate in human trophoblast development. Proc. Natl. Acad. Sci. USA 2015, 112, E6175–E6184. [Google Scholar] [CrossRef] [PubMed]
- Baczyk, D.; Drewlo, S.; Proctor, L.; Dunk, C.; Lye, S.; Kingdom, J. Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differ. 2009, 16, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Chen, H. GATA3 inhibits GCM1 activity and trophoblast cell invasion. Sci. Rep. 2016, 6, 21630. [Google Scholar] [CrossRef]
- Zhu, H.; Peng, B.; Klausen, C.; Yi, Y.; Li, Y.; Xiong, S.; von Dadelszen, P.; Leung, P.C.K. NPFF increases fusogenic proteins syncytin 1 and syncytin 2 via GCM1 in first trimester primary human cytotrophoblast cells. FASEB J. 2020, 34, 9419–9432. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Li, X.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef]
- Frendo, J.L.; Olivier, D.; Cheynet, V.; Blond, J.L.; Bouton, O.; Vidaud, M.; Rabreau, M.; Evain-Brion, D.; Mallet, F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell. Biol. 2003, 23, 3566–3574. [Google Scholar] [CrossRef]
- Esnault, C.; Priet, S.; Ribet, D.; Vernochet, C.; Bruls, T.; Lavialle, C.; Weissenbach, J.; Heidmann, T. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. USA 2008, 105, 17532–17537. [Google Scholar] [CrossRef]
- Lu, X.; Wang, R.; Zhu, C.; Wang, H.; Lin, H.Y.; Gu, Y.; Cross, J.C.; Wang, H. Fine-Tuned and Cell-Cycle-Restricted Expression of Fusogenic Protein Syncytin-2 Maintains Functional Placental Syncytia. Cell Rep. 2017, 21, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Drewlo, S.; Huppertz, B.; Armant, D.R. Trophoblast retrieval and isolation from the cervix: Origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum. Reprod. Update 2018, 24, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D. Expression of integrin alpha 6 beta 4 in human trophoblast and its loss from extravillous cells. Placenta 1993, 14, 203–215. [Google Scholar] [CrossRef]
- Rattila, S.; Dunk, C.E.E.; Im, M.; Grichenko, O.; Zhou, Y.; Yanez-Mo, M.; Blois, S.M.; Yamada, K.M.; Erez, O.; Gomez-Lopez, N.; et al. Interaction of Pregnancy-Specific Glycoprotein 1 With Integrin Alpha5beta1 Is a Modulator of Extravillous Trophoblast Functions. Cells 2019, 8, 1369. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Stefanoska, I.; Radojcic, L.; Vicovac, L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction 2010, 139, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Goffin, F.; Munaut, C.; Lai-Kuen, R.; Tricottet, V.; Foidart, J.M.; Vidaud, M.; Frankenne, F.; Evain-Brion, D. Effect of matrigel on human extravillous trophoblasts differentiation: Modulation of protease pattern gene expression. Biol. Reprod. 2002, 67, 1628–1637. [Google Scholar] [CrossRef]
- Harris, L.K.; Smith, S.D.; Keogh, R.J.; Jones, R.L.; Baker, P.N.; Knofler, M.; Cartwright, J.E.; Whitley, G.S.; Aplin, J.D. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am. J. Pathol. 2010, 177, 2103–2115. [Google Scholar] [CrossRef]
- Bjorn, S.F.; Hastrup, N.; Larsen, J.F.; Lund, L.R.; Pyke, C. Messenger RNA for membrane-type 2 matrix metalloproteinase, MT2-MMP, is expressed in human placenta of first trimester. Placenta 2000, 21, 170–176. [Google Scholar] [CrossRef]
- Fujiwara, H.; Higuchi, T.; Sato, Y.; Nishioka, Y.; Zeng, B.X.; Yoshioka, S.; Tatsumi, K.; Ueda, M.; Maeda, M. Regulation of human extravillous trophoblast function by membrane-bound peptidases. Biochim. Biophys. Acta 2005, 1751, 26–32. [Google Scholar] [CrossRef]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.Y.; Du, M.R.; Wu, X.; Wang, M.Y.; Li, D.J. Chemokine CXCL16, a scavenger receptor, induces proliferation and invasion of first-trimester human trophoblast cells in an autocrine manner. Hum. Reprod. 2006, 21, 1083–1091. [Google Scholar] [CrossRef]
- Drake, P.M.; Red-Horse, K.; Fisher, S.J. Reciprocal chemokine receptor and ligand expression in the human placenta: Implications for cytotrophoblast differentiation. Dev. Dyn. 2004, 229, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Varberg, K.M.; Iqbal, K.; Muto, M.; Simon, M.E.; Scott, R.L.; Kozai, K.; Choudhury, R.H.; Aplin, J.D.; Biswell, R.; Gibson, M.; et al. ASCL2 reciprocally controls key trophoblast lineage decisions during hemochorial placenta development. Proc. Natl. Acad. Sci. USA 2021, 118, e2016517118. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sharpe, E.E.; Maupin, A.B.; Teleron, A.A.; Pyle, A.L.; Carmeliet, P.; Young, P.P. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006, 20, 1495–1497. [Google Scholar] [CrossRef]
- Athanassiades, A.; Lala, P.K. Role of placenta growth factor (PIGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta 1998, 19, 465–473. [Google Scholar] [CrossRef]
- Demir, R.; Kayisli, U.A.; Seval, Y.; Celik-Ozenci, C.; Korgun, E.T.; Demir-Weusten, A.Y.; Huppertz, B. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: Differences between placental vasculogenesis and angiogenesis. Placenta 2004, 25, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Shore, V.H.; Wang, T.H.; Wang, C.L.; Torry, R.J.; Caudle, M.R.; Torry, D.S. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta 1997, 18, 657–665. [Google Scholar] [CrossRef]
- Bjoro, K., Jr. Gross pathology of the placenta in intrauterine growth retardation. Ann. Chir. Gynaecol. 1981, 70, 316–322. [Google Scholar] [PubMed]
- Barut, F.; Barut, A.; Gun, B.D.; Kandemir, N.O.; Harma, M.I.; Harma, M.; Aktunc, E.; Ozdamar, S.O. Intrauterine growth restriction and placental angiogenesis. Diagn. Pathol. 2010, 5, 24. [Google Scholar] [CrossRef]
- Williams, P.J.; Bulmer, J.N.; Searle, R.F.; Innes, B.A.; Robson, S.C. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: A comparison with late normal pregnancy. Reproduction 2009, 138, 177–184. [Google Scholar] [CrossRef]
- Amu, S.; Hahn-Zoric, M.; Malik, A.; Ashraf, R.; Zaman, S.; Kjellmer, I.; Hagberg, H.; Padyukov, L.; Hanson, L.A. Cytokines in the placenta of Pakistani newborns with and without intrauterine growth retardation. Pediatr. Res. 2006, 59, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Schiessl, B.; Kirkley, M.; Innes, B.A.; Cooper, A.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J. Leukoc. Biol. 2006, 80, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Jansson, N.; Palmberg, I.; Saljo, K.; Powell, T.L.; Jansson, T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007, 582, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Alvino, G. Intrauterine growth restriction: Implications for placental metabolism and transport. A review. Placenta 2009, 30 (Suppl. SA), S77–S82. [Google Scholar] [PubMed]
- Gaccioli, F.; Lager, S. Placental Nutrient Transport and Intrauterine Growth Restriction. Front. Physiol. 2016, 7, 40. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Cao, Y.; Jia, X.; Huang, Y.; Cai, M.; Lu, C.; Zhu, H. Downregulation of Placental Amino Acid Transporter Expression and mTORC1 Signaling Activity Contributes to Fetal Growth Retardation in Diabetic Rats. Int. J. Mol. Sci. 2020, 21, 1849. [Google Scholar] [CrossRef]
- Boyd, P.A.; Scott, A. Quantitative structural studies on human placentas associated with pre-eclampsia, essential hypertension and intrauterine growth retardation. Br. J. Obstet. Gynaecol. 1985, 92, 714–721. [Google Scholar] [CrossRef]
- Redman, C.W.; Tannetta, D.S.; Dragovic, R.A.; Gardiner, C.; Southcombe, J.H.; Collett, G.P.; Sargent, I.L. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012, 33, S48–S54. [Google Scholar] [CrossRef]
- Redman, C.W. Current topic: Pre-eclampsia and the placenta. Placenta 1991, 12, 301–308. [Google Scholar] [CrossRef]
- Wang, A.; Rana, S.; Karumanchi, S.A. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology 2009, 24, 147–158. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Dolati, S.; Hashemi, V.; Abdollahpour-Alitappeh, M.; Yousefi, M. Regulatory T and T helper 17 cells: Their roles in preeclampsia. J. Cell Physiol. 2018, 233, 6561–6573. [Google Scholar] [CrossRef] [PubMed]
- Eghbal-Fard, S.; Yousefi, M.; Heydarlou, H.; Ahmadi, M.; Taghavi, S.; Movasaghpour, A.; Jadidi-Niaragh, F.; Yousefi, B.; Dolati, S.; Hojjat-Farsangi, M.; et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J. Cell Physiol. 2019, 234, 5106–5116. [Google Scholar] [CrossRef]
- Duckitt, K.; Harrington, D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ 2005, 330, 565. [Google Scholar] [CrossRef]
- Avagliano, L.; Bulfamante, G.P.; Morabito, A.; Marconi, A.M. Abnormal spiral artery remodelling in the decidual segment during pregnancy: From histology to clinical correlation. J. Clin. Pathol. 2011, 64, 1064–1068. [Google Scholar] [CrossRef]
- Yoshida, K.; Yano, A.; Kusama, K.; Ishikawa, G.; Tamura, K. Alpha 1 Antitrypsin Regulates Trophoblast Syncytialization and Inflammatory Factor Expression. Int. J. Mol. Sci. 2022, 23, 1955. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamanaka-Tatematsu, M.; Fujita, N.; Koizumi, K.; Shima, T.; Yoshida, T.; Nikaido, T.; Okamoto, A.; Yoshimori, T.; Saito, S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy 2013, 9, 303–316. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A. A review of the mechanism for poor placentation in early-onset preeclampsia: The role of autophagy in trophoblast invasion and vascular remodeling. J. Reprod. Immunol. 2014, 101–102, 80–88. [Google Scholar] [CrossRef]
- Daskalakis, G.; Marinopoulos, S.; Krielesi, V.; Papapanagiotou, A.; Papantoniou, N.; Mesogitis, S.; Antsaklis, A. Placental pathology in women with gestational diabetes. Acta Obstet. Gynecol. Scand. 2008, 87, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Madazli, R.; Tuten, A.; Calay, Z.; Uzun, H.; Uludag, S.; Ocak, V. The incidence of placental abnormalities, maternal and cord plasma malondialdehyde and vascular endothelial growth factor levels in women with gestational diabetes mellitus and nondiabetic controls. Gynecol. Obstet. Investig. 2008, 65, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, T.; Varastehpour, A.; Catalano, P.; Hauguel-de Mouzon, S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 2003, 52, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Yee, K.; Permezel, M.; Rice, G.E. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J. Endocrinol. 2005, 186, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Magee, T.R.; Ross, M.G.; Wedekind, L.; Desai, M.; Kjos, S.; Belkacemi, L. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J. Diabetes Complicat. 2014, 28, 448–459. [Google Scholar] [CrossRef]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Hauguel-de Mouzon, S.; Jawerbaum, A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, H.; Geng, Q.; Ma, Q.; Long, Y.; Zhou, C.; Chen, M. Association of oxidative stress biomarkers with gestational diabetes mellitus in pregnant women: A case-control study. PLoS ONE 2015, 10, e0126490. [Google Scholar] [CrossRef]
- Yung, H.W.; Alnaes-Katjavivi, P.; Jones, C.J.; El-Bacha, T.; Golic, M.; Staff, A.C.; Burton, G.J. Placental endoplasmic reticulum stress in gestational diabetes: The potential for therapeutic intervention with chemical chaperones and antioxidants. Diabetologia 2016, 59, 2240–2250. [Google Scholar] [CrossRef]
- Gaither, K.; Quraishi, A.N.; Illsley, N.P. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J. Clin. Endocrinol. Metab. 1999, 84, 695–701. [Google Scholar] [CrossRef]
- Dekker Nitert, M.; Barrett, H.L.; Kubala, M.H.; Scholz Romero, K.; Denny, K.J.; Woodruff, T.M.; McIntyre, H.D.; Callaway, L.K. Increased placental expression of fibroblast growth factor 21 in gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2014, 99, E591–E598. [Google Scholar] [CrossRef]
- Sooranna, S.R.; Oteng-Ntim, E.; Meah, R.; Ryder, T.A.; Bajoria, R. Characterization of human placental explants: Morphological, biochemical and physiological studies using first and third trimester placenta. Hum. Reprod. 1999, 14, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, H.; Morozov, P.; Straus, A.; Sahasrabudhe, N.; Max, K.E.A.; Garzia, A.; Kustagi, M.; Tuschl, T.; Williams, Z. A single-cell survey of the human first-trimester placenta and decidua. Sci. Adv. 2018, 4, eaau4788. [Google Scholar] [CrossRef] [PubMed]
- Pattillo, R.A.; Gey, G.O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968, 28, 1231–1236. [Google Scholar] [PubMed]
- Kohler, P.O.; Bridson, W.E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J. Clin. Endocrinol. Metab. 1971, 32, 683–687. [Google Scholar] [CrossRef]
- Pattillo, R.A.; Hussa, R.O.; Garancis, J.C. Glycogen metabolism in human hormone-producing trophoblastic cells in continuous culture. I. Regulation of glycogen metabolism by glucose. In Vitro 1971, 7, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Straszewski-Chavez, S.L.; Abrahams, V.M.; Alvero, A.B.; Aldo, P.B.; Ma, Y.; Guller, S.; Romero, R.; Mor, G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta 2009, 30, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Abou-Kheir, W.; Barrak, J.; Hadadeh, O.; Daoud, G. HTR-8/SVneo cell line contains a mixed population of cells. Placenta 2017, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Amita, M.; Adachi, K.; Alexenko, A.P.; Sinha, S.; Schust, D.J.; Schulz, L.C.; Roberts, R.M.; Ezashi, T. Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc. Natl. Acad. Sci. USA 2013, 110, E1212–E1221. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.; Alexenko, A.P.; Amita, M.; Yang, Y.; Schust, D.J.; Sadovsky, Y.; Ezashi, T.; Roberts, R.M. Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas. Proc. Natl. Acad. Sci. USA 2016, 113, E2598–E2607. [Google Scholar] [CrossRef] [PubMed]
- Kojima, J.; Fukuda, A.; Taira, H.; Kawasaki, T.; Ito, H.; Kuji, N.; Isaka, K.; Umezawa, A.; Akutsu, H. Efficient production of trophoblast lineage cells from human induced pluripotent stem cells. Lab. Investig. 2017, 97, 1188–1200. [Google Scholar] [CrossRef]
- Maldonado-Estrada, J.; Menu, E.; Roques, P.; Barre-Sinoussi, F.; Chaouat, G. Evaluation of Cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry. J. Immunol. Methods 2004, 286, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, N.; Kojima, J.; Fukuda, A.; Oda, M.; Kawasaki, T.; Ito, H.; Kuji, N.; Isaka, K.; Nishi, H.; Umezawa, A.; et al. Transcriptomic features of trophoblast lineage cells derived from human induced pluripotent stem cells treated with BMP 4. Placenta 2020, 89, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell 2018, 22, 50–63.e6. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 2018, 564, 263–267. [Google Scholar] [CrossRef]
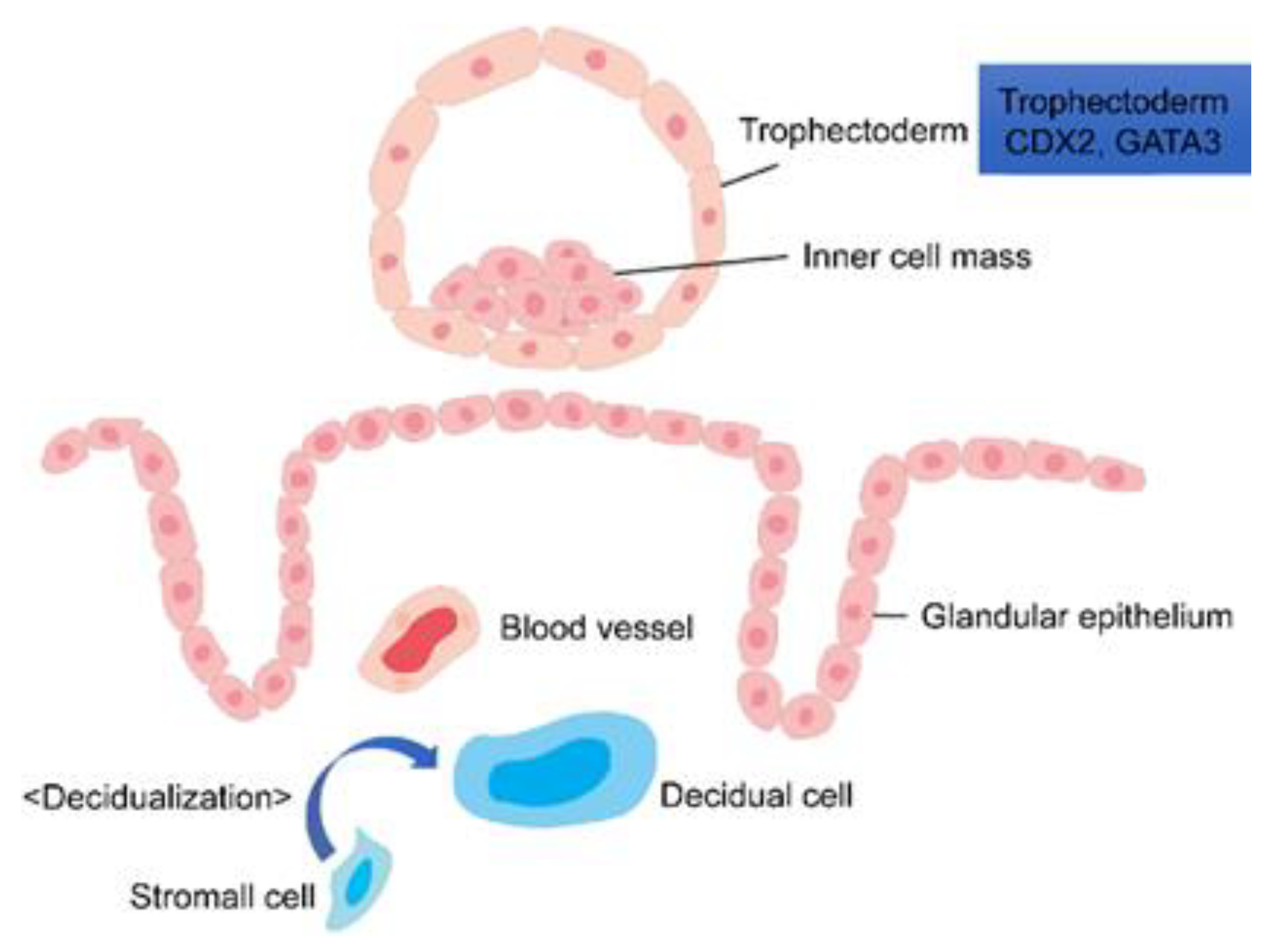
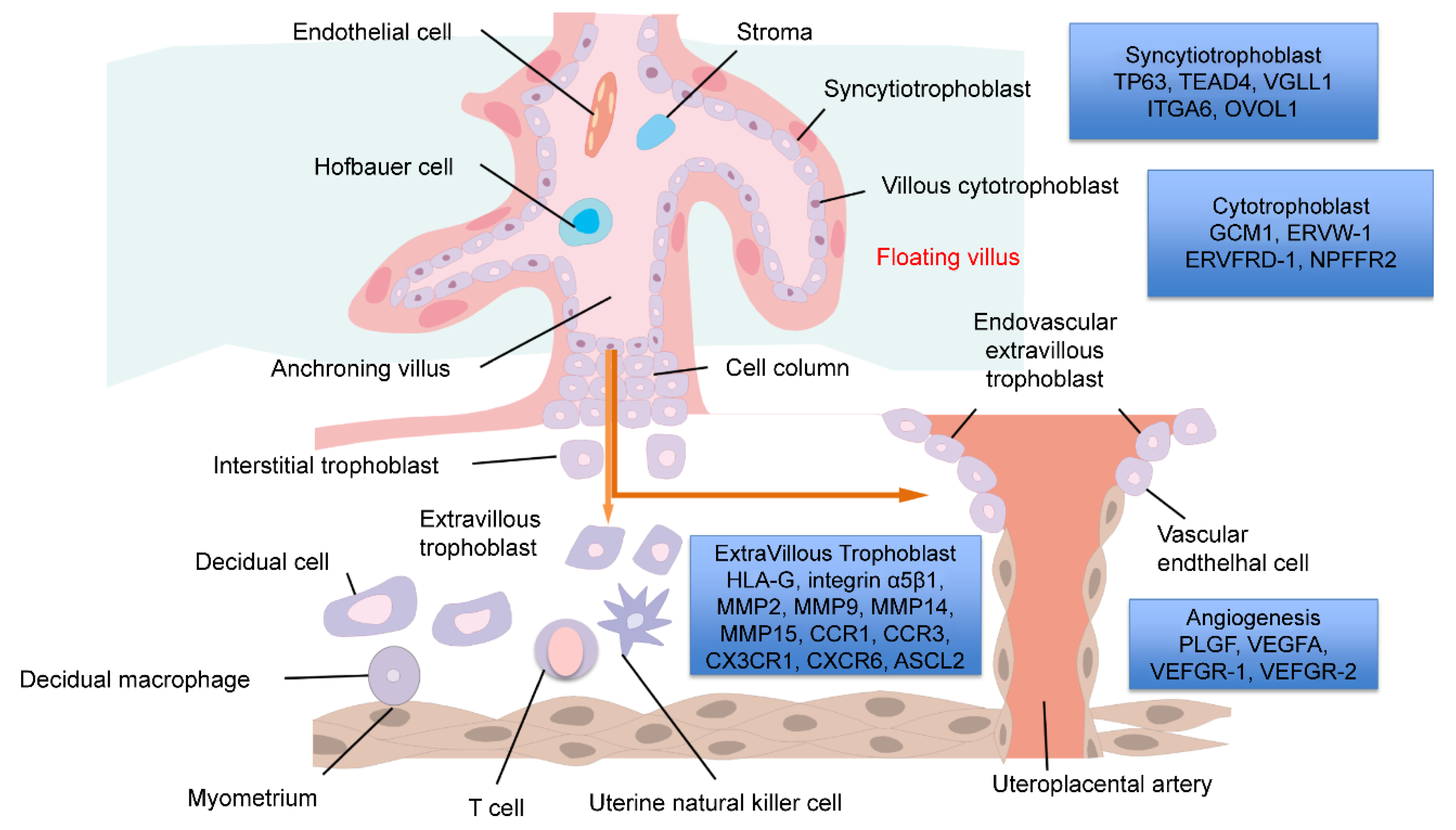
| Human First-Trimester Trophoblast Stage and Vascular Development | Gene Symbol | Gene Description |
|---|---|---|
| Trophectoderm (TE) | Caudal Type Homeobox 2 (CDX2) | The interaction of Oct4 and CDX2, which are typical undifferentiated markers that affect the inner cell mass, leads to the differentiation of the trophoblast and the inner cell mass. Although CDX2 is known to be expressed in the trophoblast, the detailed mechanism of trophoblast differentiation is unknown. |
| GATA Binding Protein 3 (GATA3) | GATA3, a transcription factor expressed in trophoblasts, is involved in the differentiation of trophoblasts into villi as well as the infiltration and migration of the villi into the maternal surface. It is thought to play an important role in the placental formation [85,86]. | |
| Cytotrophoblast (CT) | Tumor Protein P63 (TP63) | TP63 is a member of the P53 tumor suppressor family. Through the control of epithelial-mesenchymal transition, cell adhesion, and matrix degradation pathways, TP63 suppresses CT differentiation into EVT and maintains a proliferative CT state [87] |
| TEA Domain Transcription Factor 4 (TEAD4) | Transcription factors of the TEAD family are the ultimate intranuclear effectors of the Hippo pathway. Among these, TEAD4 regulates cell growth, proliferation, and homeostasis in CT [88,89,90]. | |
| Vestigial-Like Family Member 1 (VGLL1) | VGLL1 is a co-transcriptional activator of TEAD4; when VGLL1 expression decreases, the expression of TP63, which is a marker of CT, also decreases. For this reason, VGLL1 is thought to be involved in the maintenance of CT [91,92]. | |
| Integrin Subunit Alpha 6 (ITGA6) | ITGA6 is a cell surface protein that constitutes the major adhesive receptor for laminin. Abundant laminin is present in the stem cell niche, and ITGA6 is involved in cell proliferation and self-renewal [93]. | |
| Ovo-like Transcriptional Repressor 1 (OVOL1) | OVOL1 regulates TP63 expression. OVOL1 is necessary to suppress the differentiation of CT into ST and maintain its state by inhibiting the expression of syncytin1 and syncytin 2 [94]. | |
| Syncytiotrophoblast (ST) | Glial Cell Missing Transcription Factor 1 (GCM1) | GCM1 is a gene that inhibits the differentiation from CT to EVT and induces differentiation into ST by fusing cells. GCM1 is controlled by GATA3 [95,96]. |
| Neuropeptide FF-Amide Peptide Precursor (NPFF) | The role of neuropeptide FF (NPFF) is well known in the central nervous system. NPFF receptor 2 (NPFFR2) mRNA is abundant in the placenta; however, the function of NPFF-NPFFR2 in placental development is unknown. NPFF acts via NPFFR2, promotes the expression of syncytin 1 and 2 via GCM1, and is involved in ST formation [97]. | |
| Endogenous Retrovirus Group W Envelope Member 1, Envelope (ERVW-1) | ERVW-1, a gene encoding the syncytin-1 protein, is derived from an endogenous retrovirus. Syncytin-1 promotes cell fusion. Genes derived from retroviruses play an indispensable role in placental formation. The expression of ERVW-1 is regulated by GCM-1 [98,99]. | |
| Endogenous Retrovirus Group FRD Member 1, Envelope (ERVFRD-1) | ERVFRD-1 is a gene encoding the syncytin-2 protein. Like ERVW-1, ERVFRD-1 is also derived from an endogenous retrovirus. Syncytin-2 also promotes cell fusion and is also controlled by GCM1 [100,101] | |
| Extravillous trophoblast (EVT) | Major Histocompatibility Complex, Class I, G (HLA-G) | HLA-G is the most representative gene expressed in EVT. It is classified as a human leukocyte antigen, which is a human major histocompatibility complex. HLA-G contributes to immunosuppression to establish pregnancy and allow the fetus to escape maternal immunity [102]. |
| Integrin | Integrin is a protein on the cell surface and is a cell adhesion molecule. It is a heterodimer consisting of two subunits, the α and β chains. Integrin α5β1 is expressed in EVT [103]. Pregnancy-specific glycoproteins (PSGs) are secretory proteins present in the maternal placenta. There are 11 PSG genes in humans, and PSG1 interacts directly with integrin α5β1 [104]. | |
| Matrix Metalloproteinase (MMP) | The MMP family currently has 28 members (MMP 1 to 28), and the expression of MMP2, MMP9, MMP14, and MMP15 has been reported in EVT. MMPs degrade extracellular matrices and proteins expressed on the cell surface [105,106,107,108]. | |
| Chemokines and Chemokine Receptors (CCR) | Chemokines are small-molecule polypeptides involved in cell proliferation, differentiation, apoptosis, angiogenesis, hematopoiesis, tumor promotion, and inflammatory diseases (85,86). Chemokines play an important role in placental function and play a major role in the infiltration of EVT into the maternal decidua, as the chemokine receptors CCR1, CCR3, CX3CR1, and CXCR6 are localized in EVT [109,110,111,112]. | |
| Achaete-Scute Family BHLH Transcription Factor 2 (ASCL2) | ASCL2 is a member of the basic helix-loop-helix (BHLH) family of transcription factors. Its expression is observed in EVT in early pregnancy. While ASCL2 has been reported to be involved in tumor infiltration in breast cancer, it also plays an important role in human EVT formation [31,113]. | |
| Angiogenesis | Placental Growth Factor (PGF) | PGF belongs to the vascular endothelial growth factor (VEGF) family. PGF is expressed in vascular endothelial cells in the placenta and plays a role in vasodilation and angiogenesis [114,115]. |
| Vascular Endothelial Growth Facto A (VEGFA), Fms Related Receptor Tyrosine Kinase 1 (FLT1), and the Kinase Insert Domain Receptor (KDR) | VEGFA, a vascular endothelial growth factor, belongs to the VEGF family and is expressed in trophoblasts and vascular endothelial cells, and is also involved in angiogenesis in the early placenta. VEGFR-1/Flt-1 and VEGFR-2/KDR/Flk-1 have been identified in placental tissues as receptors for the VEGF family [116,117]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, J.; Ono, M.; Kuji, N.; Nishi, H. Human Chorionic Villous Differentiation and Placental Development. Int. J. Mol. Sci. 2022, 23, 8003. https://doi.org/10.3390/ijms23148003
Kojima J, Ono M, Kuji N, Nishi H. Human Chorionic Villous Differentiation and Placental Development. International Journal of Molecular Sciences. 2022; 23(14):8003. https://doi.org/10.3390/ijms23148003
Chicago/Turabian StyleKojima, Junya, Masanori Ono, Naoaki Kuji, and Hirotaka Nishi. 2022. "Human Chorionic Villous Differentiation and Placental Development" International Journal of Molecular Sciences 23, no. 14: 8003. https://doi.org/10.3390/ijms23148003
APA StyleKojima, J., Ono, M., Kuji, N., & Nishi, H. (2022). Human Chorionic Villous Differentiation and Placental Development. International Journal of Molecular Sciences, 23(14), 8003. https://doi.org/10.3390/ijms23148003






