Spectroscopic Analyses and Antimicrobial Activity of Novel Ciprofloxacin and 7-Hydroxy-4-methylcoumarin, the Plant-Based Natural Benzopyrone Derivative
Abstract
:1. Introduction
2. Results and Discussion
2.1. IR Absorption Spectra
2.2. Electronic Reflection Spectra
2.2.1. UV-Vis Spectra
2.2.2. 1H NMR Spectra
2.3. Thermal Studies
2.4. Thermodynamic Parameters
2.5. Antibacterial Investigation
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Coumarin
3.3. Synthesis of Ciprofloxacin/Coumarin Zr(IV) Complexes
3.4. Instruments
3.5. Antimicrobial Investigation and MIC Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camele, I.; Elshafie, H.S.; Caputo, L.; Sakr, S.H.; De Feo, V. Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. J. Biol. Res. 2019, 92, 39–45. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017, 8, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Elshafie, H.S.; Viggiani, L.; Mostafa, M.S.; El-Hashash, M.A.; Bufo, S.A.; Camele, I. Biological activity and chemical identification of ornithine lipid produced by Burkholderia gladioli pv. agaricicola ICMP 11096 using LC-MS and NMR analyses. J. Biol. Res. 2017, 90, 96–103. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Sofo, A.; Mazzone, G.; Caivano, M.; Masi, S.; Caniani, D. Mycoremediation effect of Trichoderma harzianum strain T22 combined with ozonation in diesel-contaminated sand. Chemosphere 2020, 252, 126597. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [Green Version]
- Phutdhawong, W.; Chuenchid, A.; Taechowisan, T.; Sirirak, J.; Phutdhawong, W.S. Synthesis and biological activity evaluation of Coumarin-3-Carboxamide derivatives. Molecules 2021, 26, 1653. [Google Scholar] [CrossRef]
- Kostova, I.; Manolov, I.; Nicolova, I.; Danchev, N. New metal complexes of 4-Methyl-7-hydroxycoumarin Sodium salt and their pharmacological activity. II Farm. 2001, 56, 707–713. [Google Scholar] [CrossRef]
- Kostova, I.; Manolov, I.; Nicolova, I.; Konstantinov, S.; Karaivanova, M. New lanthanide complexes of 4- Methyl-7-Hydroxycoumarin and their pharmacological activity. Eur. J. Med. Chem. 2001, 36, 339–347. [Google Scholar] [CrossRef]
- Patel, J.; Dholariya, H.; Patel, K.; Bhatt, J.; Patel, K. Cu(II) and Ni(II) complexes of coumarin derivatives with fourth generation flouroquinolone: Synthesis, characterization, microbicidal and antioxidant assay. Med. Chem. Res. 2014, 23, 3714–3724. [Google Scholar] [CrossRef]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Cytotoxic activity of new acetoxycoumarin derivatives in cancer cell lines. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef]
- Lin, H.C.; Tsai, S.H.; Chen, C.S.; Chang, Y.C.; Lee, C.M.; Lai, Z.Y.; Lin, C.M. Structure-activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem. Pharmacol. 2008, 75, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, Z.A.; Khalid, M.; Kumar, S.; Shahid, M.; Noor, S. Antimicrobial and SOD activities of novel transition metal complexes of Pyridine-2,6-dicarboxylic acid containing 4-Picoline as auxiliary ligand. Eur. J. Med. Chem. 2010, 45, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Turel, I. The interactions of metal ions with quinolone antibacterial agents. Coord. Chem. Rev. 2002, 32, 27–47. [Google Scholar] [CrossRef]
- Scully, B.E.; Nakatomi, M.; Ores, C.; Davidson, S.; Neu, H.C. Ciprofloxacin therapy in cystic fibrosis. Am. J. Med. 1987, 82, 196–201. [Google Scholar]
- El-Shwiniy, W.H.; El-Attar, M.S.; Sadeek, A.S. Metal Complexes of Enrofloxacin Part I: Preparation, spectroscopic, thermal analyses studies and antimicrobial evaluation. J. Korean Chem. Soc. 2013, 57, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Mononuclear dioxomolybdenum (VI) complexes with the quinolones enrofloxacin and sparfloxacin: Synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron 2008, 27, 349–356. [Google Scholar] [CrossRef]
- Shingnapurkar, D.; Butcher, R.; Afrasiabi, Z.; Sinn, E.; Ahmed, F.; Sarkar, F.; Padhye, S. Neutral dimeric copper–sparfloxacin conjugate having butterfly motif with antiproliferative effects against hormone independent BT20 breast cancer cell line. Inorg. Chem. Commun. 2007, 10, 459–462. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Katsarou, M.E.; Karaliota, A.; Psomas, G. Copper (II) complexes with sparfloxacin and nitrogen-donor heterocyclic ligands: Structure–activity relationship. J. Inorg. Biochem. 2008, 102, 910–920. [Google Scholar] [CrossRef]
- Sakr, S.H.; Elshafie, H.S.; Camele, I.; Sadeek, S.A. Synthesis, spectroscopic, and biological studies of mixed ligand complexes of gemifloxacin and glycine with Zn(II), Sn(II), and Ce(III). Molecules 2018, 23, 1182. [Google Scholar] [CrossRef] [Green Version]
- Elshafie, H.S.; Sakr, S.H.; Sadeek, S.A.; Camele, I. Biological investigations and spectroscopic studies of new Moxifloxacin/Glycine-Metal complexes. Chem. Biodiver. 2019, 16, e1800633. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Zordok, W.A.; Mohamed, A.A. Meloxicam and study of their antimicrobial effects against phyto and human pathogens. Molecules 2021, 26, 1480. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Metal complexes of the third-generation quinolone antimicrobial drug sparfloxacin: Structure and biological evaluation. J. Inorg. Biochem. 2010, 104, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Nguewa, P.A.; Fuertes, M.A.; Iborra, S.; Najajeh, Y.; Gibson, D.; Matínez, E.; Alonso, C.; Pérez, J.M. Water soluble cationic trans-platinum complexes which induce programmed cell death in the protozoan parasite Leishmania infantum. J. Inorg. Biochem. 2005, 99, 727–736. [Google Scholar] [CrossRef]
- Sultana, N.; Naz, A.; Arayne, M.S.; Mesaik, A.M. Synthesis, characterization, antibacterial, antifungal and immunomodulating activities of gatifloxacin–metal complexes. J. Mol. Struct. 2010, 969, 17–24. [Google Scholar] [CrossRef]
- Martindale, W. Martindale-The Extra Pharmacopeia, 30th ed.; The Pharmaceutical Press: London, UK, 1993. [Google Scholar]
- Ball, P. The Quinolones; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Sheehan, G.; Chew, N.S.Y. The history of quinolones. In Fluoroquinolone Antibiotics; Ronald, A.R., Low, D.E., Eds.; Birkhauser: Basel, Switzerland, 2003. [Google Scholar]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Mohamed, A.A. Biochemical characterization of new gemifloxacin schiff base (GMFX-o-phdn) metal complexes and evaluation of their antimicrobial activity against some phyto- or human pathogens. Int. J. Mol. Sci. 2022, 23, 2110. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.M.; Chiller, T.M.; Powers, J.H.; Angulo, F.J. Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinones from use in poultry: A public health success story. Clin. Infect. Dis. 2007, 44, 977–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sissi, C.; Palumbo, M.; Curr, C. The quinolone family: From antibacterial to anticancer agents. Med. Chem. Anticancer Agents 2003, 3, 439–450. [Google Scholar] [CrossRef]
- Zordok, W.A.; El-Shwiniy, W.H.; El-Attar, M.S.; Sadeek, S.A. Spectroscopic, thermal analyses, structural and antibacterial studies on the interaction of some metals with ofloxacin. J. Mol. Struct. 2013, 1047, 267–276. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Attar, M.S.; Abd El-Hamid, S.M. Preparation and characterization of new tetradentate Schiff base metal complexes and biological activity evaluation. J. Mol. Struct. 2013, 1051, 30–40. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Elshafie, H.S.; Sadeek, S.A.; Camele, I. Biochemical characterization, phytotoxic effect and antimicrobial activity against some phytopathogens of new Gemifloxacin schiff base metal complexes. Chem. Biodivers. 2021, 18, e2100365. [Google Scholar] [CrossRef]
- Sadeek, S.A.; EL-Shwiniy, A.W.H.; Zordok, W.A.; EL-Didamony, A.M. Spectroscopic, structure and antimicrobial activity of new Y(III) and Zr(IV) ciprofloxacin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Sadeek, S.A.; EL-Shwiniy, W.H. Metal complexes of the fourth generation quinolone antimicrobial drug gatifloxacin: Synthesis, structure and biological evaluation. J. Mol. Struct. 2010, 977, 243–253. [Google Scholar] [CrossRef]
- Sadeek, S.A.; EL-Shwiniy, W.H. Preparation, structure and microbial evaluation of metal complexes of the second generation quinolone antibacterial drug lomefloxacin. J. Mol. Struct. 2010, 981, 130–138. [Google Scholar] [CrossRef]
- Sadeek, S.A.; EL-Shwiniy, W.H. Metal complexes of the third generation quinolone antibacterial drug sparfloxacin: Preparation, structure, and microbial evaluation. J. Coord. Chem. 2010, 63, 3471–3482. [Google Scholar] [CrossRef]
- Sadeek, S.A.; EL-Shwiniy, W.A.; Essam, K. Spectroscopic studies, thermal analyses and biological evaluation of new V(IV), Zr(IV) and U(VI) moxifloxacin complexes. J. Mol. Struct. 2011, 1006, 192–209. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Sadeek, S.A. Ligational and biological studies of Fe(III), Co(II), Ni(II), Cu(II) and Zr(IV) complexes with carbamazepine as antiepileptic drug. Appl. Organomet. Chem. 2021, 35, e6178. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; El-Attar, M.S. Synthesis, characterization and antimicrobial investigation of some moxifloxacin metal complexes. J. Spectrochim. Acta A 2011, 84, 99–110. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; El-Attar, M.S.; Zordok, W.A. Spectroscopic, structural and antibacterial evaluation of some lomefloxacin metal complexes. Int. J. Adv. Res. 2014, 2, 158–208. [Google Scholar]
- Sadeek, S.A.; El-Attar, M.S.; Abd El-Hamid, S.M. Complexes and chelates of some bivalent and trivalent metals with Ciprofloxacin schiff base. J. Synth. React. Inorg. Metal. Organ. Nano-Metal Chem. 2015, 45, 1412–1426. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Attar, M.S.; Abd El-Hamid, S.M. Synthesis, characterization and antibacterial activity of some new transition metal complexes with ciprofloxacin-imine. Bull. Chem. Soc. Ethiop. 2015, 29, 259–274. [Google Scholar] [CrossRef] [Green Version]
- El-Attar, M.S. Preparation, spectroscopic, thermal analyses and antibacterial studies of levofloxacin vanadium (V) complexes. Int. J. Sci. Eng. Res. 2016, 7, 658–684. [Google Scholar]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Vogel, A.I. Qualitative Inorganic Analysis, 6th ed.; Svehla, G., Ed.; Wiley: New York, NY, USA, 1987; p. 174. [Google Scholar]
- Sadeek, S.A. Synthesis, thermogravimetric analysis, infrared, electronic and mass spectra of Mn (II), Co (II) and Fe (III) norfloxacin complexes. J. Mol. Struct. 2005, 753, 1–12. [Google Scholar] [CrossRef]
- El-Shwiniy, W.H.; Gamil, M.A.; Sadeek, S.A.; Zordok, W.A. Study molecular modeling and the effect of some biological metals on the efficiency of norfloxacin in presence of 3-(bromoacetyl)coumarin. Appl. Organomet. Chem. 2021, 35, e6448. [Google Scholar] [CrossRef]
- El-Samanody, E.A.; AbouEl-Enein, S.A.; Emara, E.M. Molecular modeling, spectral investigation and thermal studies of the new asymmetric Schiff base ligand; (E)-N’-(1-(4-((E)-2-hydroxybenzylideneamino) phenyl)ethylidene)morpholine-4-carbothiohydrazide and its metal complexes: Evaluation of their antibacterial and anti-molluscicidal activity. Appl. Organometal. Chem. 2018, 32, e4262. [Google Scholar]
- Sadeek, S.A.; Refat, M.S.; Hashem, H.A. Complexation and thermogravimetric investigation on tin (II) and tin (IV) with Norfloxacin as antibacterial agent. J. Coord. Chem. 2006, 59, 759–775. [Google Scholar] [CrossRef]
- Skauge, T.; Turel, I.; Sletten, E. Interaction between ciprofloxacin and DNA mediated by Mg2+-ions. Inorg. Chem. Acta. 2002, 339, 239–247. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Horowitz, H.W.; Metzger, G. A New analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Guzar, S.H.; Han, J.Q. Simple, selective, and sensitive spectrophotometric method for determination of trace amounts of nickel(II), copper (II), cobalt (II), and iron (III) with a novel reagent 2-Pyridine Carboxaldehyde Isonicotinyl Hydrazone. Chem. Res. Chin. Univ. 2008, 24, 143–147. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Munawar, A.; Supuran, C.T. Transition Metal Ion Complexes of schiff bases synthesis, characterization and antibacterial properties. Met. Based Drugs 2001, 8, 137–143. [Google Scholar] [CrossRef]
- Hanna, W.G.; Moawad, M.M. Synthesis, characterization and antimicrobial activity of cobalt(II), nickel(II) and copper(II) complexes with new asymmetrical Schiff base ligands derived from 7-formyanil-substituted diamine-sulphoxine and acetylacetone. Transit. Met. Chem. 2001, 26, 644–651. [Google Scholar] [CrossRef]
- Iqbal, J.; Tirmizi, S.; Watto, F.; Imran, M.; Watto, M.H.; Sharfuddin, S.; Latif, S. Biological Properties of Chloro-salicylidene Aniline and Its Complexes with Co(II) and Cu(II). Turk. J. Biol. 2006, 30, 1–4. [Google Scholar]
- Singh, V.P.; Katiyar, A.; Singh, S. Synthesis, characterization of some transition metal(II) complexes of acetone p-amino acetophenone salicyloyl hydrazone and their antimicrobial activity. Bio. Met. 2008, 21, 491–501. [Google Scholar]
- Furniss, B.S.; Hannaford, A.J.; Rogres, V.; Smith, P.W.G.; Tachell, A.R. Vogel’s Texetbook of Practical Organic Chemistry, 4th ed.; Longman Group: London, UK, 1976. [Google Scholar]
- Beecher, D.J.; Wong, A.C. Identification of hemolysin Bl-producing Bacillus cereus isolated by a discontinuous hemolytic pattern in blood agar. Appl. Environ. Microbiol. 1994, 60, 1646–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Sakr, S.H.; De Feo, V.; Camele, I. Study of bio-pharmaceutical and antimicrobial properties of pomegranate (Punica granatum L.) leathery exocarp extract. Plants 2021, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.T.; Cleirigh, C.O.; Walsh, P.K.; Shea, D.G.O. Development of a robust microtiter plate-based assay method for assessment of bioactivity. J. Microbiol. Methods 2004, 58, 327–334. [Google Scholar] [CrossRef] [PubMed]
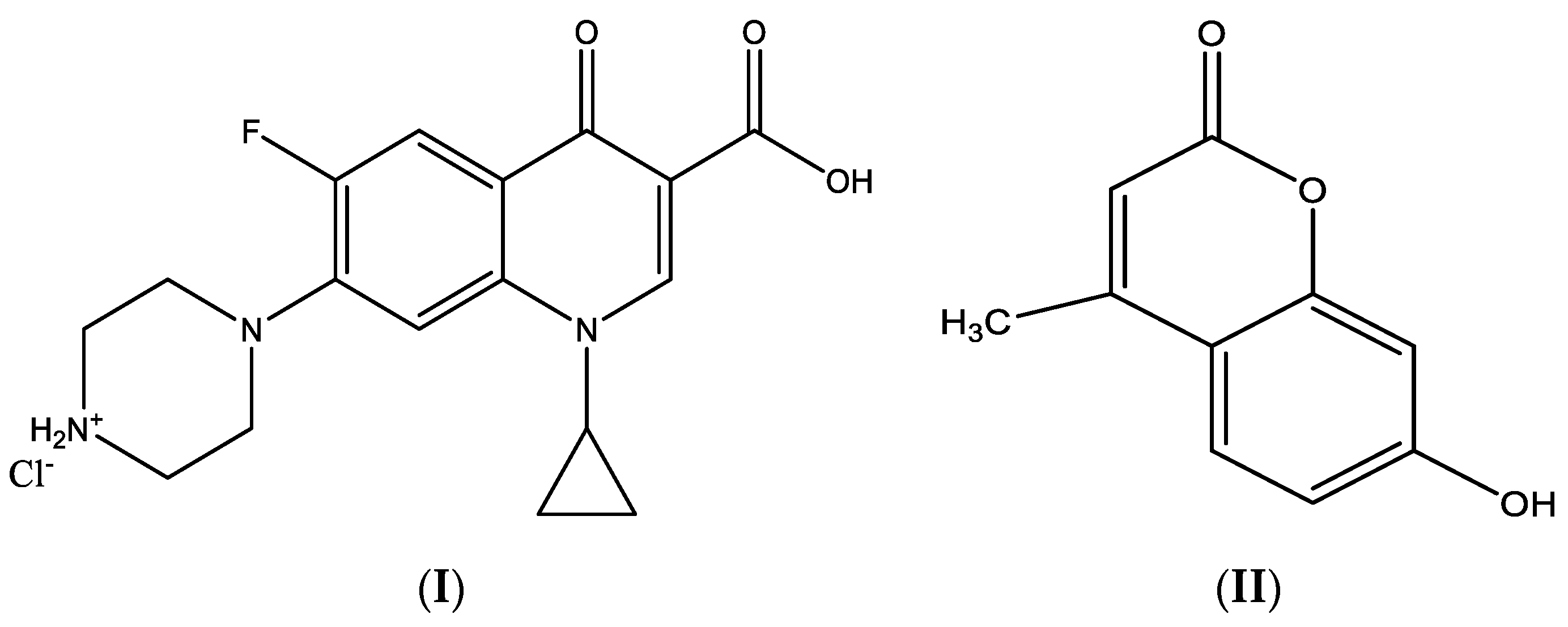
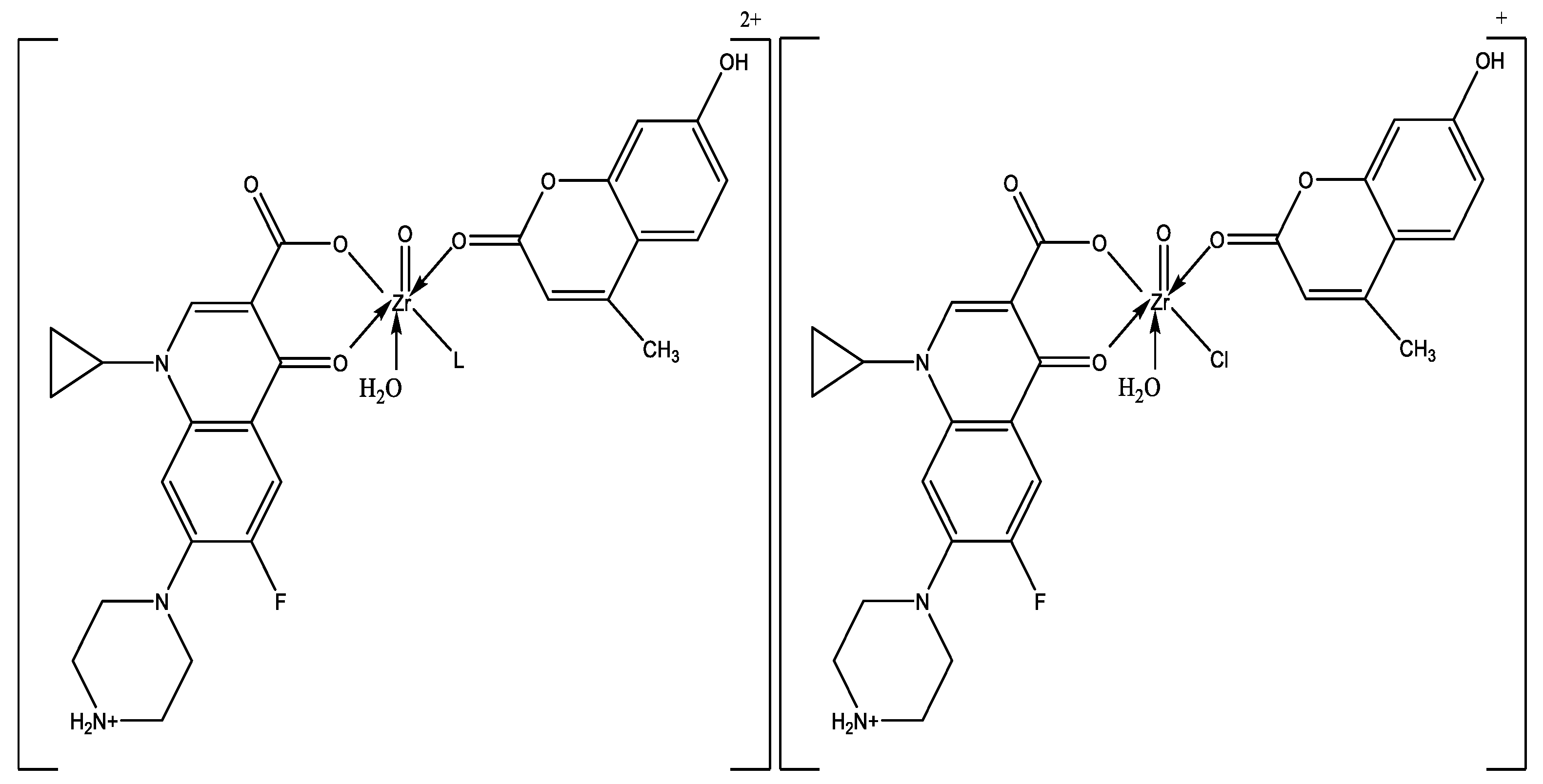
| Compounds M.Wt. (M.F.) | Yield% | mp/°C | Color | Content (calc.) Found (%) | Λ (S cm2 mol−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | M | Cl | |||||
| CIP 367.45 (C17H19N3O3FCl) | - | 305 | White | (55.52) 55.48 | (5.17) 5.10 | (11.43) 11.34 | - | (9.65) 9.56 | 70.20 |
| HMC 203.00 (C10H11O4.5) | 79 | 190 | Yellow | (59.11) 59.03 | (5.42) 5.36 | - - | - - | - - | 15.12 |
| (A) 793.12 (C27H38N3O13FCl2Zr) | 75 | 290 | Faint brown | (40.85) 40.73 | (4.79) 4.70 | (5.30) 5.23 | (11.50) 11.42 | (8.94) 8.88 | 76.23 |
| (B) 956.12 (C30H55N4O19FCl2Zr) | 80 | 200 | Green | (37.65) 37.56 | (5.75) 5.69 | (5.86) 5.76 | (9.54) 9.45 | (7.42) 7.35 | 122.15 |
| (C) 962.12 (C32H53N4O18FCl2Zr) | 78 | 340 | Greenish yellow | (39.91) 39.81 | (5.51) 5.45 | (5.82) 5.73 | (9.48) 9.40 | (7.37) 7.29 | 125.26 |
| (D) 858.12 (C33H49N4O11FCl2Zr) | 85 | 160 | Brownish yellow | (46.15) 46.02 | (5.71) 5.63 | (6.53) 6.45 | (10.63) 10.55 | (8.26) 8.17 | 120.30 |
| Compounds | ν(O-H); H2O; COOH | ν(C=O); COOH | νas(COO−) | ν(C=O) | νs(COO−) | ν (Zr = O) | ν(M-O) and ν(M-N) |
|---|---|---|---|---|---|---|---|
| CIP | 3530 m | 1706 vs | - | 1620 vs | - | - | - |
| HMC | 3499 ms | - | - | 1674 vs | - | - | - |
| (A) | 3489 w | - | 1670 ms | 1609 vs 1521 w | 1389 vs | 845 s | 625 m, 540 m |
| (B) | 3533 w 3411 w | - | 1628 s,br | 1539 w | 1389 s | 848 w | 637 ms, 560 wbr |
| (C) | 3444 w | - | 1678 m | 1624 s 1572 m | 1389 vs | 845 s | 679 ms, 539 w |
| (D) | 3517 mbr | - | 1686 m | 1620 mbr 1522 m | 1389 vs | 849 vs | 629 s, 539 w |
| Assignments (nm) | CIP | HMC | (A) | (B) | (C) | (D) |
|---|---|---|---|---|---|---|
| π–π* transitions | 243, 298 | 258 | 252, 304 | 277, 312 | 282, 302 | 282, 302 |
| n–π* transitions | 338 | 410 | 385 | 404, 431 | 323, 407, 431 | 323, 407, 442 |
| Ligand-metal charge transfer | - | - | 525, 574 | 522, 545, 567 | 514, 572 | 514, 572 |
| Assignments (ppm) | CIP | HMC | (A) | (B) | (C) | (D) |
|---|---|---|---|---|---|---|
| δH, -CH and -CH3 | 1.33 | - | 0.96–1.35 | 1.19–1.31 | 0.79–1.33 | 1.13–1.24 |
| δH, -NH; piperazine | 2.00 | - | - | - | - | - |
| δH, -+NH2 | - | - | 2.11–2.35 | 2.36 | 2.12–2.36 | 2.36 |
| δH, -CH2 aliphatic | 2.78, 3.46 | 2.35–2.50 | 2.41–2.51 | 2.50 | 2.45–2.56 | 2.49–2.51 |
| δH, H2O | - | 3.79 | 3.15–3.86 | 3.37, 3.57 | 3.04–3.63 | 3.01–3.36 |
| δH, -CH2 aromatic | 6.04–8.66 | 6.12–7.60 | 5.09–9.88 | 6.12–8.80 | 6.12–9.91 | 6.12–7.60 |
| δH, -COOH and -OH | 11.00 | 10.50 | 10.69 | 10.67 | 10.68 | 10.20 |
| Compounds | Decomposition | Tmax (°C) | Weight Loss (%) | Lost Species | |
|---|---|---|---|---|---|
| Calc. | Found | ||||
| HMC | First step Second step Total loss | 60 267, 463 | 13.30 86.70 100 | 13.25 86.59 99.84 | 1.5H2O 4C2H2+CO+CO2 |
| (A) | First step Second step Third step Total loss, Residue | 100 324 431, 547 | 11.35 33.40 38.20 82.95, 17.05 | 11.27 33.35 38.15 82.77, 17.23 | 5H2O 4C2H2+2CO2+2HCl 7C2H2+6CO+NH3+N2+HF ZrO2+C |
| (B) | First step Second step Third step Total loss, Residue | 65 280 493 | 18.82 35.34 32.95 87.11, 12.89 | 18.78 35.29 32.89 86.96, 13.04 | 10H2O 2C2H2+2C2H4+5CO+NH3+2HCl 7C2H2+C2N2+NH3+HF+CO2 ZrO2 |
| (C) | First step Second step Third step Total loss, Residue | 60 210 497 | 18.71 35.74 27.75 82.20, 17.80 | 18.69 35.70 27.69 82.08, 17.92 | 10H2O 4C2H2+C2H4+4CO+HCN+2HCl 5C2H2+C2H4+CO2+NH3+HF+N2 ZrO2+4C |
| (D) | First step Second step Third step Total loss, Residue | 50 183 495 | 6.29 42.64 36.71 85.64, 14.36 | 6.26 42.60 36.69 85.55, 14.45 | 3H2O 2C2H2+4C2H4+4CO+ NH3+2HCl 7C2H2+C2N2+ NH3+HF+CO2 ZrO2 |
| Compounds | Decomposition Range (K) | Ts (K) | Method | Parameter | R a | SD b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol−1) | A (s−1) | ΔS* (kJ/mol.K) | ΔH* (kJ/mol) | ΔG* (kJ/mol) | ||||||
| HMC | 313–393 | 333 | CR HM | 53.26 55.33 | 1.14 × 106 2.39 × 105 | −0.1297 −0.1156 | 50.52 52.59 | 93.21 90.64 | 0.999 0.998 | 0.03 0.81 |
| 471–593 | 540 | CR HM | 68.67 85.48 | 1.27 × 105 8.04 × 106 | −0.1513 −0.1169 | 64.57 81.38 | 139.17 139.00 | 0.995 0.995 | 0.05 0.11 | |
| (A) | 673–754 | 704 | CR HM | 106.80 115.20 | 1.8 × 108 2.5 × 109 | −0.092 −0.070 | 102.42 110.87 | 150.49 147.55 | 0.990 0.990 | 0.13 0.15 |
| (B) | 693–803 | 766 | CR HM | 61.43 67.39 | 1.21 × 104 1.08 × 105 | −0.1708 −0.1526 | 57.41 63.36 | 140.08 137.21 | 0.990 0.990 | 0.11 0.14 |
| (C) | 708–823 | 770 | CR HM | 177.80 193.31 | 5.96 × 1010 7.91 × 1011 | −0.046 −0.025 | 171.8 187.32 | 204.90 204.90 | 0.998 0.998 | 0.05 0.05 |
| (D) | 423–493 | 456 | CR HM | 45.13 57.53 | 5.4 × 102 8.3 × 103 | −0.217 −0.175 | 40.45 52.86 | 162.34 151.39 | 0.994 0.997 | 0.074 0.07 |
| 743–818 | 768 | CR HM | 77.66 92.41 | 1.46 × 102 2.16 × 103 | −0.2119 −0.1895 | 70.85 85.60 | 244.39 240.77 | 0.990 0.980 | 0.15 0.20 | |
| Tested Compounds | Tested G+ve Bacterial Strains | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | B. cereus | Br. otitidis | ||||||||
| D.iz a (mm) | AI b (%) | MIC c (μg/mL) | D.iz (mm) | AI (%) | MIC (μg/mL) | D.iz (mm) | AI (%) | MIC (μg/mL) | ||
| CIP | 26 ± 0.58 | - | 0.50 ± 0.005 | 34 ± 0.43 | - | 0.50 ± 0.007 | 22 ± 0.29 | - | 0.75 ± 0.005 | |
| HMC | 18 ± 0.22 | 0.69 | 0.25 ± 0.03 | 8 ± 0.15 | 0.24 | 0.25 ± 0.01 | 7 ± 0.11 | 0.32 | 0.25 ± 0.006 | |
| (A) | 66+3 ± 0.26 | 2.5 | 0.75 ± 0.01 | 27 ± 0.36 | 0.79 | 0.75 ± 0.006 | 27+1 ± 0.65 | 1.23 | 0.50 ± 0.01 | |
| (B) | 61+3 ± 0.25 | 2.35 | 0.75 ± 0.006 | 24 ± 0.24 | 0.71 | 0.25 ± 0.02 | 19 ± 0.21 | 0.86 | 0.50 ± 0.03 | |
| (C) | 58+2 ± 0.45 | 2.23 | 0.50 ± 0.007 | 26 ± 0.32 | 0.76 | 0.50 ± 0.01 | 19 ± 0.17 | 0.86 | 0.75 ± 0.007 | |
| (D) | 49+2 ± 0.49 | 1.88 | 0.50 ± 0.01 | 22 ± 0.15 | 0.65 | 0.75 ± 0.03 | 16 ± 0.09 | 0.73 | 0.25 ± 0.01 | |
| ZrOCl2·8H2O | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | |
| Control (DMF) | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | |
| Standards | Moxifloxacin | 40 ± 0.5 | 1.54 | - | 36 ± 1.2 | 1.06 | - | 25 ± 0.24 | 1.14 | - |
| Lomefloxacin | 24 ± 0.2 | 0.92 | - | 25 ± 0.5 | 0.74 | - | 26 ± 0.31 | 1.18 | - | |
| Tested Compounds | Tested G-ve Bacterial Strains | |||||||||
| E. coli | P. aeruginosa | K. pneumoniae | ||||||||
| D.iz a (mm) | AI b (%) | MIC c (μg/mL) | D.iz (mm) | AI (%) | MIC (μg/mL) | D.iz (mm) | AI (%) | MIC (μg/mL) | ||
| CIP | 28 ± 0.3 | - | 0.75 ± 0.01 | 23 ± 0.3 | - | 0.50 ± 0.02 | 20 ± 0.4 | - | 0.75 ± 0.03 | |
| HMC | 7 ± 0.05 | 0.25 | 0.50 ± 0.007 | ND | - | 0.25 ± 0.01 | 12 ± 0.31 | 0.60 | 0.25 ± 0.007 | |
| (A) | 31+1 ± 0.81 | 1.1 | 1.0 ± 0.02 | - | - | 1.0 ± 0.007 | 16 ± 0.19 | 0.8 | 1.0 ± 0.02 | |
| (B) | 26 ± 0.23 | 0.93 | 0.50 ± 0.005 | 29+1 ± 0.92 | 1.26 | 0.50 ± 0.005 | 27+1 ± 0.88 | 1.35 | 1.0 ± 0.01 | |
| (C) | 28 ± 0.09 | 1 | 0.75 ± 0.03 | 33+2 ± 0.68 | 1.43 | 0.75 ± 0.01 | 21NS ± 0.60 | 1.05 | 0.75 ± 0.005 | |
| (D) | 35+2 ± 0.45 | 1.25 | 1.0 ± 0.006 | 34+2 ± 0.76 | 1.48 | 1.0 ± 0.03 | 21NS ± 0.51 | 1.05 | 0.50 ± 0.02 | |
| ZrOCl2·8H2O | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | |
| Control (DMF) | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | |
| Standards | Moxifloxacin | 22 ± 1.7 | 0.78 | - | 22 ± 0.3 | 0.96 | - | 16 ± 0.1 | - | - |
| Lomefloxacin | 17 ± 0.1 | 0.61 | - | 13 ± 0.3 | 0.56 | - | 19 ± 0.1 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Attar, M.S.; Sadeek, S.A.; Abd El-Hamid, S.M.; Elshafie, H.S. Spectroscopic Analyses and Antimicrobial Activity of Novel Ciprofloxacin and 7-Hydroxy-4-methylcoumarin, the Plant-Based Natural Benzopyrone Derivative. Int. J. Mol. Sci. 2022, 23, 8019. https://doi.org/10.3390/ijms23148019
El-Attar MS, Sadeek SA, Abd El-Hamid SM, Elshafie HS. Spectroscopic Analyses and Antimicrobial Activity of Novel Ciprofloxacin and 7-Hydroxy-4-methylcoumarin, the Plant-Based Natural Benzopyrone Derivative. International Journal of Molecular Sciences. 2022; 23(14):8019. https://doi.org/10.3390/ijms23148019
Chicago/Turabian StyleEl-Attar, Mohamed S., Sadeek A. Sadeek, Sherif M. Abd El-Hamid, and Hazem S. Elshafie. 2022. "Spectroscopic Analyses and Antimicrobial Activity of Novel Ciprofloxacin and 7-Hydroxy-4-methylcoumarin, the Plant-Based Natural Benzopyrone Derivative" International Journal of Molecular Sciences 23, no. 14: 8019. https://doi.org/10.3390/ijms23148019







