Uterine Fibroids Causing Preterm Birth: A New Pathophysiological Hypothesis on the Role of Fibroid Necrosis and Inflammation
Abstract
:1. Introduction
2. Inflammation-Induced Preterm Birth
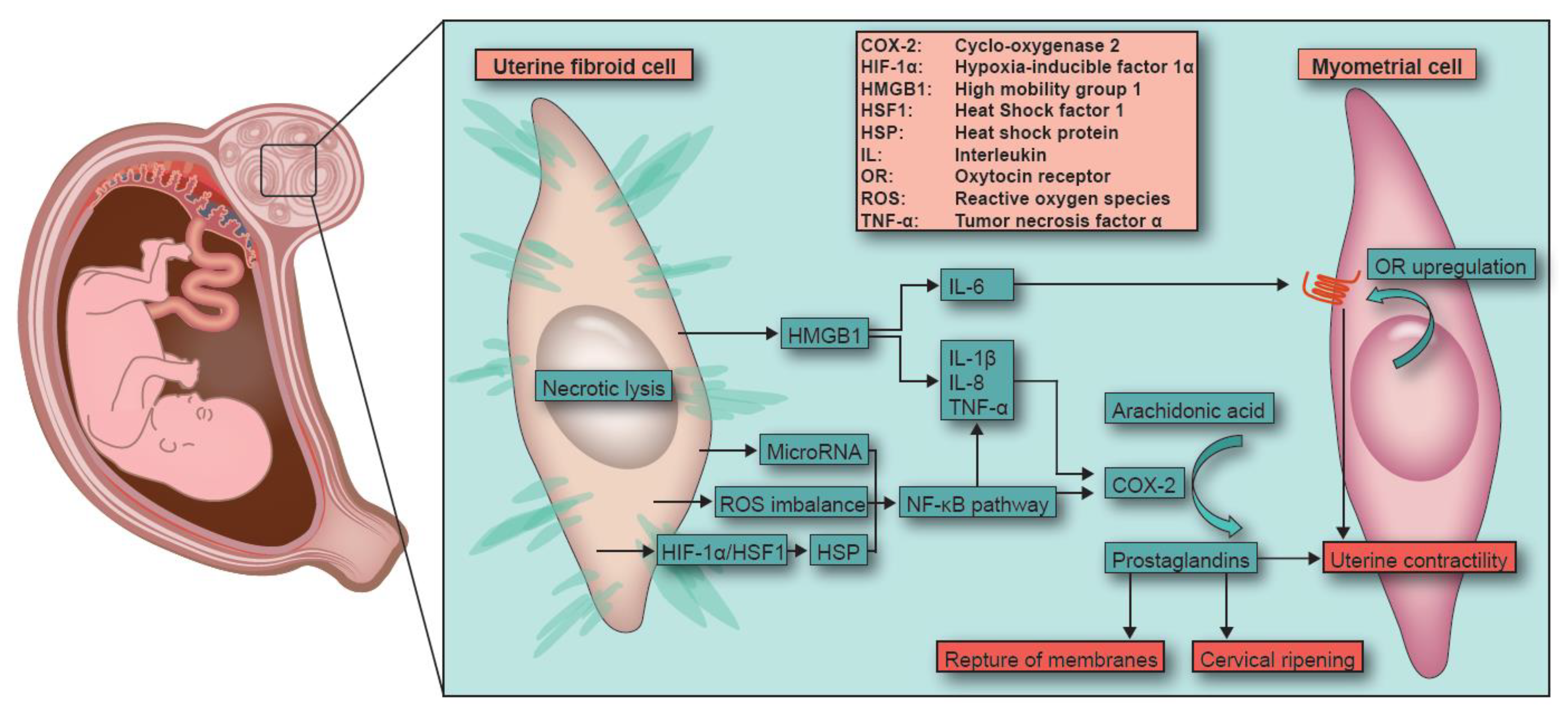
3. Fibroid Necrosis Initiated Spontaneous Preterm Birth
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, E.A. Uterine fibroids. Lancet 2001, 357, 293–298. [Google Scholar] [CrossRef]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.; Broder, M.S.; Fraser, I.S.; Disorders, F.W.G.o.M. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynaecol. Obstet. 2011, 113, 3–13. [Google Scholar] [CrossRef]
- Styer, A.K.; Rueda, B.R. The Epidemiology and Genetics of Uterine Leiomyoma. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 3–12. [Google Scholar] [CrossRef]
- Klatsky, P.C.; Tran, N.D.; Caughey, A.B.; Fujimoto, V.Y. Fibroids and reproductive outcomes: A systematic literature review from conception to delivery. Am. J. Obstet. Gynecol. 2008, 198, 357–366. [Google Scholar] [CrossRef]
- Pritts, E.A.; Parker, W.H.; Olive, D.L. Fibroids and infertility: An updated systematic review of the evidence. Fertil. Steril. 2009, 91, 1215–1223. [Google Scholar] [CrossRef]
- Olive, D.L.; Pritts, E.A. Fibroids and reproduction. Semin. Reprod. Med. 2010, 28, 218–227. [Google Scholar] [CrossRef]
- Spyropoulou, K.; Kosmas, I.; Tsakiridis, I.; Mamopoulos, A.; Kalogiannidis, I.; Athanasiadis, A.; Daponte, A.; Dagklis, T. Myomectomy during pregnancy: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 15–24. [Google Scholar] [CrossRef]
- Laughlin, S.K.; Baird, D.D.; Savitz, D.A.; Herring, A.H.; Hartmann, K.E. Prevalence of uterine leiomyomas in the first trimester of pregnancy: An ultrasound-screening study. Obstet. Gynecol. 2009, 113, 630–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Exacoustos, C.; Rosati, P. Ultrasound diagnosis of uterine myomas and complications in pregnancy. Obstet. Gynecol. 1993, 82, 97–101. [Google Scholar] [CrossRef]
- Faulkner, R.L. Red degeneration of uterine myomas. Am. J. Obstet. Gynecol. 1947, 53, 474–482. [Google Scholar] [CrossRef]
- Hasan, F.; Arumugam, K.; Sivanesaratnam, V. Uterine leiomyomata in pregnancy. Int. J. Gynaecol. Obstet. 1991, 34, 45–48. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [Green Version]
- Slattery, M.M.; Morrison, J.J. Preterm delivery. Lancet 2002, 360, 1489–1497. [Google Scholar] [CrossRef]
- Pérez-Roncero, G.R.; López-Baena, M.T.; Ornat, L.; Cuerva, M.J.; Garcia-Casarrubios, P.; Chedraui, P.; Pérez-López, F.R. Uterine fibroids and preterm birth risk: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2020, 46, 1711–1727. [Google Scholar] [CrossRef]
- Landman, A.J.E.M.C.; Don, E.E.; Vissers, G.; Ket, H.C.J.; Oudijk, M.A.; de Groot, C.J.M.; Huirne, J.A.F.; de Boer, M.A. The risk of preterm birth in women with uterine fibroids: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0269478. [Google Scholar] [CrossRef]
- Keelan, J.A. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J. Reprod. Immunol. 2018, 125, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.W. Preterm birth, intrauterine infection, and fetal inflammation. Front. Immunol. 2014, 5, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchirikov, M.; Schlabritz-Loutsevitch, N.; Maher, J.; Buchmann, J.; Naberezhnev, Y.; Winarno, A.S.; Seliger, G. Mid-trimester preterm premature rupture of membranes (PPROM): Etiology, diagnosis, classification, international recommendations of treatment options and outcome. J. Perinat. Med. 2018, 46, 465–488. [Google Scholar] [CrossRef]
- Dohbit, J.S.; Meka, E.N.U.; Tochie, J.N.; Kamla, I.; Danwang, C.; Tianyi, F.L.; Foumane, P.; Andze, G.O. Diagnostic ambiguity of aseptic necrobiosis of a uterine fibroid in a term pregnancy: A case report. BMC Pregnancy Childbirth 2019, 19, 9. [Google Scholar] [CrossRef]
- Zaima, A.; Ash, A. Fibroid in pregnancy: Characteristics, complications, and management. Postgrad. Med. J. 2011, 87, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Havelock, J.C.; Keller, P.; Muleba, N.; Mayhew, B.A.; Casey, B.M.; Rainey, W.E.; Word, R.A. Human myometrial gene expression before and during parturition. Biol. Reprod. 2005, 72, 707–719. [Google Scholar] [CrossRef]
- Fuchs, A.R.; Fuchs, F.; Husslein, P.; Soloff, M.S.; Fernstrom, M.J. Oxytocin receptors and human parturition: A dual role for oxytocin in the initiation of labor. Science 1982, 215, 1396–1398. [Google Scholar] [CrossRef]
- Kim, S.H.; Bennett, P.R.; Terzidou, V. Advances in the role of oxytocin receptors in human parturition. Mol. Cell Endocrinol. 2017, 449, 56–63. [Google Scholar] [CrossRef]
- Blanks, A.M.; Thornton, S. The role of oxytocin in parturition. BJOG 2003, 110 (Suppl. 20), 46–51. [Google Scholar] [CrossRef]
- Fuchs, A.R.; Fuchs, F.; Husslein, P.; Soloff, M.S. Oxytocin receptors in the human uterus during pregnancy and parturition. Am. J. Obstet. Gynecol. 1984, 150, 734–741. [Google Scholar] [CrossRef]
- Olson, D.M. The role of prostaglandins in the initiation of parturition. Best Pract Res. Clin. Obstet. Gynaecol. 2003, 17, 717–730. [Google Scholar] [CrossRef]
- Ravanos, K.; Dagklis, T.; Petousis, S.; Margioula-Siarkou, C.; Prapas, Y.; Prapas, N. Factors implicated in the initiation of human parturition in term and preterm labor: A review. Gynecol. Endocrinol. 2015, 31, 679–683. [Google Scholar] [CrossRef]
- Chiossi, G.; Costantine, M.M.; Bytautiene, E.; Kechichian, T.; Hankins, G.D.; Sbrana, E.; Saade, G.R.; Longo, M. The effects of prostaglandin E1 and prostaglandin E2 on in vitro myometrial contractility and uterine structure. Am. J. Perinatol. 2012, 29, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Parry, S.; Strauss, J.F., III. Premature rupture of the fetal membranes. N. Engl. J. Med. 1998, 338, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, M.; Gerriets, V. Prostaglandin E2 (Dinoprostone); StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Van Meir, C.A.; Sangha, R.K.; Walton, J.C.; Matthews, S.G.; Keirse, M.J.; Challis, J.R. Immunoreactive 15-hydroxyprostaglandin dehydrogenase (PGDH) is reduced in fetal membranes from patients at preterm delivery in the presence of infection. Placenta 1996, 17, 291–297. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Kelly, A.J.; Dowswell, T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst. Rev. 2009, 2009, CD003246. [Google Scholar] [CrossRef]
- Howarth, G.R.; Botha, D.J. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database Syst. Rev. 2001, 2001, CD003250. [Google Scholar] [CrossRef]
- Thomas, J.; Fairclough, A.; Kavanagh, J.; Kelly, A.J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014, 2014, CD003101. [Google Scholar] [CrossRef]
- Baumbusch, M.A.; Buhimschi, C.S.; Oliver, E.A.; Zhao, G.; Thung, S.; Rood, K.; Buhimschi, I.A. High Mobility Group-Box 1 (HMGB1) levels are increased in amniotic fluid of women with intra-amniotic inflammation-determined preterm birth, and the source may be the damaged fetal membranes. Cytokine 2016, 81, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Korzeniewski, S.J.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod Immunol 2014, 72, 458–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredeson, S.; Papaconstantinou, J.; Deford, J.H.; Kechichian, T.; Syed, T.A.; Saade, G.R.; Menon, R. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS ONE 2014, 9, e113799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Lopez, N.; Romero, R.; Plazyo, O.; Panaitescu, B.; Furcron, A.E.; Miller, D.; Roumayah, T.; Flom, E.; Hassan, S.S. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am. J. Reprod. Immunol. 2016, 75, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prairie, E.; Côté, F.; Tsakpinoglou, M.; Mina, M.; Quiniou, C.; Leimert, K.; Olson, D.; Chemtob, S. The determinant role of IL-6 in the establishment of inflammation leading to spontaneous preterm birth. Cytokine Growth Factor Rev. 2021, 59, 118–130. [Google Scholar] [CrossRef]
- Gilman-Sachs, A.; Dambaeva, S.; Salazar Garcia, M.D.; Hussein, Y.; Kwak-Kim, J.; Beaman, K. Inflammation induced preterm labor and birth. J. Reprod. Immunol. 2018, 129, 53–58. [Google Scholar] [CrossRef]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F., III; Petraglia, F. Inflammation and pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef]
- Fang, X.; Wong, S.; Mitchell, B.F. Effects of LPS and IL-6 on oxytocin receptor in non-pregnant and pregnant rat uterus. Am. J. Reprod. Immunol. 2000, 44, 65–72. [Google Scholar] [CrossRef]
- Rauk, P.N.; Friebe-Hoffmann, U.; Winebrenner, L.D.; Chiao, J.P. Interleukin-6 up-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am. J. Reprod. Immunol. 2001, 45, 148–153. [Google Scholar] [CrossRef]
- Cookson, V.J.; Chapman, N.R. NF-kappaB function in the human myometrium during pregnancy and parturition. Histol. Histopathol. 2010, 25, 945–956. [Google Scholar] [CrossRef]
- Peiris, H.N.; Vaswani, K.; Holland, O.; Koh, Y.Q.; Almughlliq, F.B.; Reed, S.; Mitchell, M.D. Altered productions of prostaglandins and prostamides by human amnion in response to infectious and inflammatory stimuli identified by mutliplex mass spectrometry. Prostaglandins Leukot Essent Fatty Acids 2020, 154, 102059. [Google Scholar] [CrossRef]
- Menon, R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front. Immunol. 2014, 5, 567. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.A.; Ahmad, I.M.; Zimmerman, M.C. Oxidative Stress and Preterm Birth: An Integrative Review. Biol. Res. Nurs. 2018, 20, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.r.C.; Renthal, N.E.; Gerard, R.D.; Mendelson, C.R. The microRNA (miR)-199a/214 Cluster Mediates Opposing Effects of Progesterone and Estrogen on Uterine Contractility during Pregnancy and Labor. Mol. Endocrinol. 2012, 26, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Renthal, N.E.; Chen, C.-C.; Williams, K.r.C.; Gerard, R.D.; Prange-Kiel, J.; Mendelson, C.R. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc. Natl. Acad. Sci. USA 2010, 107, 20828–20833. [Google Scholar] [CrossRef] [Green Version]
- Elovitz, M.A.; Anton, L.; Bastek, J.; Brown, A.G. Can microRNA profiling in maternal blood identify women at risk for preterm birth? Am. J. Obstet. Gynecol. 2015, 212, e781–e785. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Brown, A.G.; Anton, L.; Gilstrop, M.; Heiser, L.; Bastek, J. Distinct cervical microRNA profiles are present in women destined to have a preterm birth. Am. J. Obstet. Gynecol. 2014, 210, e1–e11. [Google Scholar] [CrossRef]
- Chill, H.H.; Karavani, G.; Rachmani, T.; Dior, U.; Tadmor, O.; Shushan, A. Growth pattern of uterine leiomyoma along pregnancy. BMC Womens Health 2019, 19, 100. [Google Scholar] [CrossRef] [Green Version]
- Vitagliano, A.; Noventa, M.; Di Spiezio Sardo, A.; Saccone, G.; Gizzo, S.; Borgato, S.; Vitale, S.G.; Lagana, A.S.; Nardelli, G.B.; Litta, P.S.; et al. Uterine fibroid size modifications during pregnancy and puerperium: Evidence from the first systematic review of literature. Arch. Gynecol. Obstet. 2018, 297, 823–835. [Google Scholar] [CrossRef] [Green Version]
- De Vivo, A.; Mancuso, A.; Giacobbe, A.; Savasta, L.M.; de Dominici, R.; Dugo, N.; Dugo, C.; Vaiarelli, A. Uterine myomas during pregnancy: A longitudinal sonographic study. Ultrasound Obstet. Gynecol. 2011, 37, 361–365. [Google Scholar] [CrossRef]
- Rice, J.P.; Kay, H.H.; Mahony, B.S. The clinical significance of uterine leiomyomas in pregnancy. Am. J. Obstet. Gynecol. 1989, 160, 1212–1216. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Huebener, P.; Pradere, J.P.; Hernandez, C.; Gwak, G.Y.; Caviglia, J.M.; Mu, X.; Loike, J.D.; Schwabe, R.F. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J. Clin. Investig. 2015, 125, 539–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Ganesh, S. Perinuclear mitochondrial clustering, increased ROS levels, and HIF1 are required for the activation of HSF1 by heat stress. J. Cell Sci. 2020, 133, jcs245589. [Google Scholar] [CrossRef]
- Lim, S.; MacIntyre, D.A.; Lee, Y.S.; Khanjani, S.; Terzidou, V.; Teoh, T.G.; Bennett, P.R. Nuclear factor kappa B activation occurs in the amnion prior to labour onset and modulates the expression of numerous labour associated genes. PLoS ONE 2012, 7, e34707. [Google Scholar]
- Martin, S.J.; Henry, C.M. Distinguishing between apoptosis, necrosis, necroptosis and other cell death modalities. Methods 2013, 61, 87–89. [Google Scholar] [CrossRef]
- Festjens, N.; Vanden Berghe, T.; Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: Signalling cascades, important mediators and concomitant immune response. BBA Bioenergetics 2006, 1757, 1371–1387. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Zhao, J.J.; Wu, Z.F.; Wang, L.; Feng, D.H.; Cheng, L. MicroRNA-145 Mediates Steroid-Induced Necrosis of the Femoral Head by Targeting the OPG/RANK/RANKL Signaling Pathway. PLoS ONE 2016, 11, e0159805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Sun, W.; Zheng, H.; Tian, F. Microrna-145 accelerates the inflammatory reaction through activation of NF-κB signaling in atherosclerosis cells and mice. Biomed. Pharmacother. 2018, 103, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Q.; Cao, S.; Xu, T.; Li, X.; Zhou, D.; Pan, L.; Li, C.; Huang, C.; Meng, X.; et al. MicroRNA-145 Increases the Apoptosis of Activated Hepatic Stellate Cells Induced by TRAIL through NF-κB Signaling Pathway. Front. Pharmacol. 2017, 8, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isomoto, H.; Matsushima, K.; Inoue, N.; Hayashi, T.; Nakayama, T.; Kunizaki, M.; Hidaka, S.; Nakayama, M.; Hisatsune, J.; Nakashima, M.; et al. Interweaving microRNAs and proinflammatory cytokines in gastric mucosa with reference to H. pylori infection. J. Clin. Immunol. 2012, 32, 290–299. [Google Scholar] [CrossRef]
- Rosenberg-Hasson, Y.; Hansmann, L.; Liedtke, M.; Herschmann, I.; Maecker, H.T. Effects of serum and plasma matrices on multiplex immunoassays. Immunol. Res. 2014, 58, 224–233. [Google Scholar] [CrossRef]
- Breslin, N.; Gyamfi-Bannerman, C. Current Preterm Birth Prevention Strategies. Clin. Perinatol. 2020, 47, 705–717. [Google Scholar] [CrossRef]
- Hall, O.J.; Klein, S.L. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal. Immunol. 2017, 10, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef] [Green Version]
- Landman, A.; de Boer, M.A.; Visser, L.; Nijman, T.A.J.; Hemels, M.A.C.; Naaktgeboren, C.N.; van der Weide, M.C.; Mol, B.W.; van Laar, J.; Papatsonis, D.N.M.; et al. Evaluation of low-dose aspirin in the prevention of recurrent spontaneous preterm labour (the APRIL study): A multicentre, randomised, double-blinded, placebo-controlled trial. PLoS Med. 2022, 19, e1003892. [Google Scholar] [CrossRef]
- Duley, L.; Meher, S.; Hunter, K.E.; Seidler, A.L.; Askie, L.M. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 29 February 2000. Identifier CT04535804, Aspirin in the Treatment of Pregnant Women With Adenomyosis on Reducing Preterm Delivery. 2 September 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04535804 (accessed on 13 June 2022).
- Kammerlander, H.; Nielsen, J.; Knudsen, T.; Kjeldsen, J.; Friedman, S.; Nørgård, B.M. Anti-TNF-α Use During the Third Trimester of Pregnancy in Women with Moderate-severe Inflammatory Bowel Disease and the Risk of Preterm Birth and Low Birth Weight. Inflamm. Bowel Dis. 2017, 23, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Moylan, H.E.C.; Nguyen-Ngo, C.; Lim, R.; Lappas, M. The short chain fatty acids butyrate and propionate protect against inflammation induced activation of mediators involved in active labor: Implications for preterm birth. Mol. Hum. Reprod. 2020, 26, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Miller, D.; Hassan, S.S.; Hsu, C.D.; Panaitescu, B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomesdagger. Biol. Reprod. 2019, 100, 1306–1318. [Google Scholar] [CrossRef]
- Kuroiwa, Y.; Takakusagi, Y.; Kusayanagi, T.; Kuramochi, K.; Imai, T.; Hirayama, T.; Ito, I.; Yoshida, M.; Sakaguchi, K.; Sugawara, F. Identification and characterization of the direct interaction between methotrexate (MTX) and high-mobility group box 1 (HMGB1) protein. PLoS ONE 2013, 8, e63073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollica, L.; de Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Kang, R.; Xiao, W.; Zhang, H.; Lotze, M.T.; Wang, H.; Xiao, X. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am. J. Respir. Cell Mol. Biol. 2009, 41, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Ciebiera, M.; Łukaszuk, K.; Męczekalski, B.; Ciebiera, M.; Wojtyła, C.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Alternative Oral Agents in Prophylaxis and Therapy of Uterine Fibroids-An Up-to-Date Review. Int. J. Mol. Sci. 2017, 18, 2586. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Don, E.E.; Landman, A.J.E.M.C.; Vissers, G.; Jordanova, E.S.; Post Uiterweer, E.D.; de Groot, C.J.M.; de Boer, M.A.; Huirne, J.A.F. Uterine Fibroids Causing Preterm Birth: A New Pathophysiological Hypothesis on the Role of Fibroid Necrosis and Inflammation. Int. J. Mol. Sci. 2022, 23, 8064. https://doi.org/10.3390/ijms23158064
Don EE, Landman AJEMC, Vissers G, Jordanova ES, Post Uiterweer ED, de Groot CJM, de Boer MA, Huirne JAF. Uterine Fibroids Causing Preterm Birth: A New Pathophysiological Hypothesis on the Role of Fibroid Necrosis and Inflammation. International Journal of Molecular Sciences. 2022; 23(15):8064. https://doi.org/10.3390/ijms23158064
Chicago/Turabian StyleDon, Emma E., Anadeijda J. E. M. C. Landman, Guus Vissers, Ekaterina S. Jordanova, Emiel D. Post Uiterweer, Christianne J. M. de Groot, Marjon A. de Boer, and Judith A. F. Huirne. 2022. "Uterine Fibroids Causing Preterm Birth: A New Pathophysiological Hypothesis on the Role of Fibroid Necrosis and Inflammation" International Journal of Molecular Sciences 23, no. 15: 8064. https://doi.org/10.3390/ijms23158064
APA StyleDon, E. E., Landman, A. J. E. M. C., Vissers, G., Jordanova, E. S., Post Uiterweer, E. D., de Groot, C. J. M., de Boer, M. A., & Huirne, J. A. F. (2022). Uterine Fibroids Causing Preterm Birth: A New Pathophysiological Hypothesis on the Role of Fibroid Necrosis and Inflammation. International Journal of Molecular Sciences, 23(15), 8064. https://doi.org/10.3390/ijms23158064






