Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry
Abstract
1. Introduction
2. Results
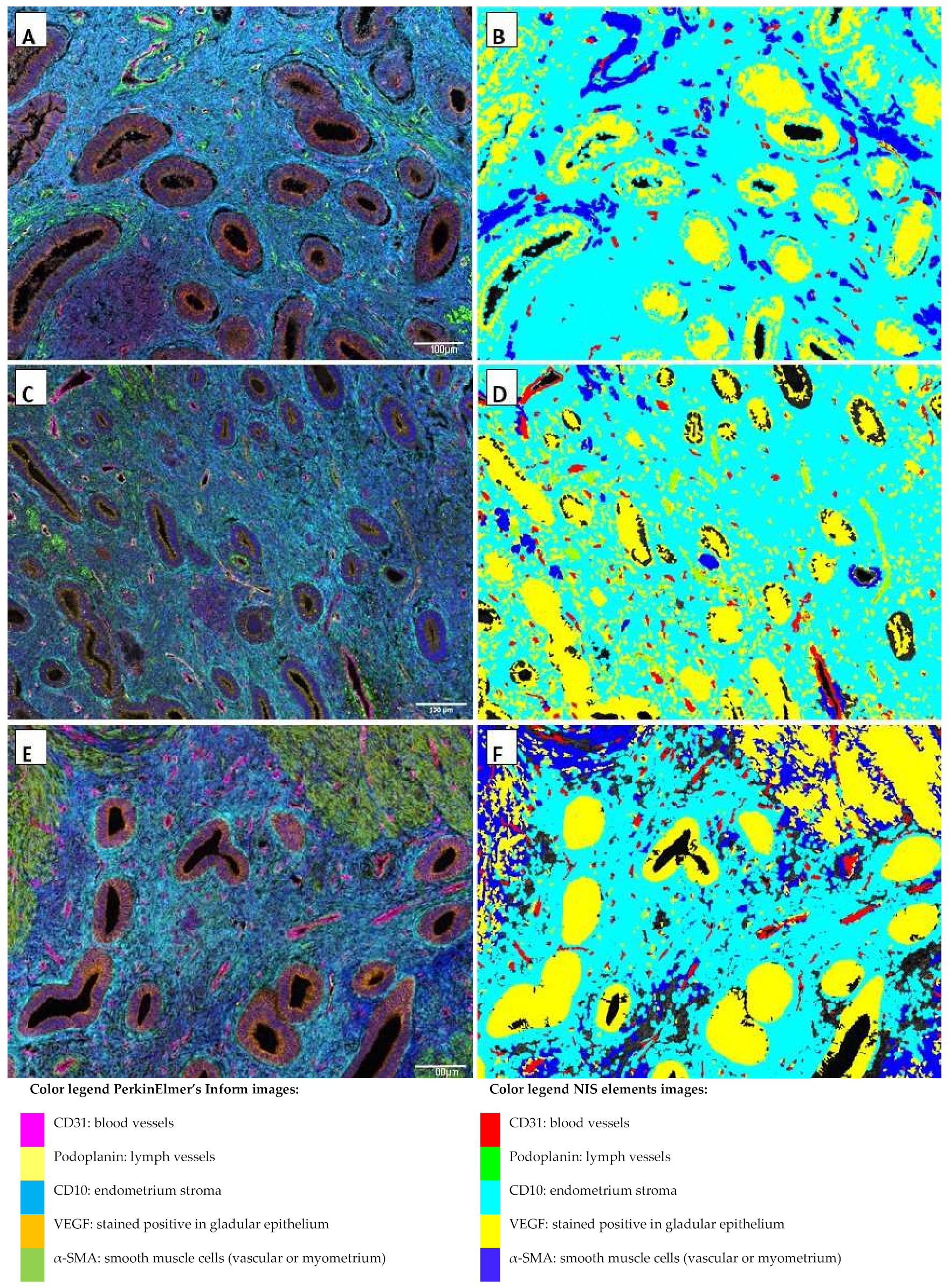
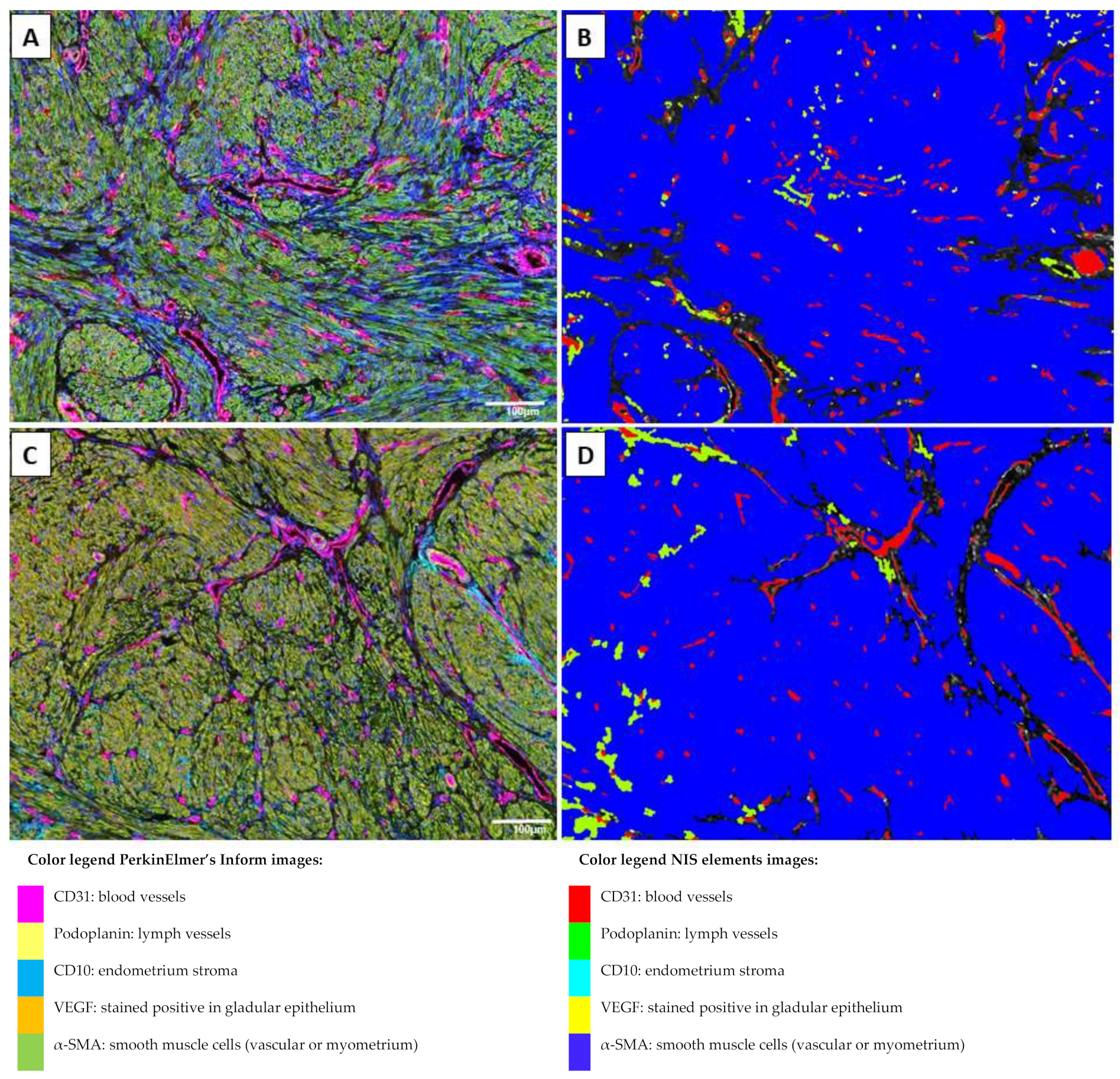
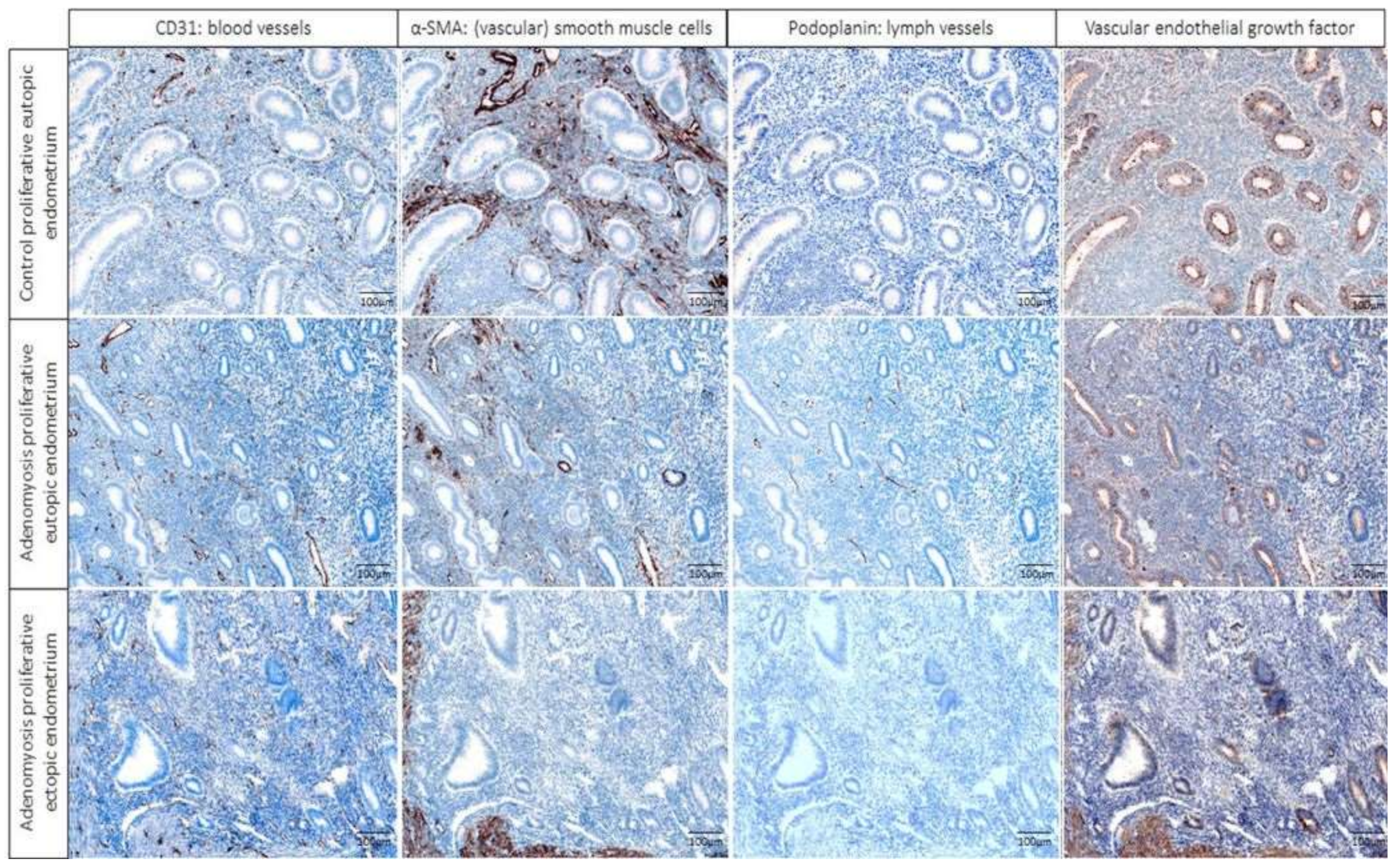
2.1. Multiplex Immunofluorescence
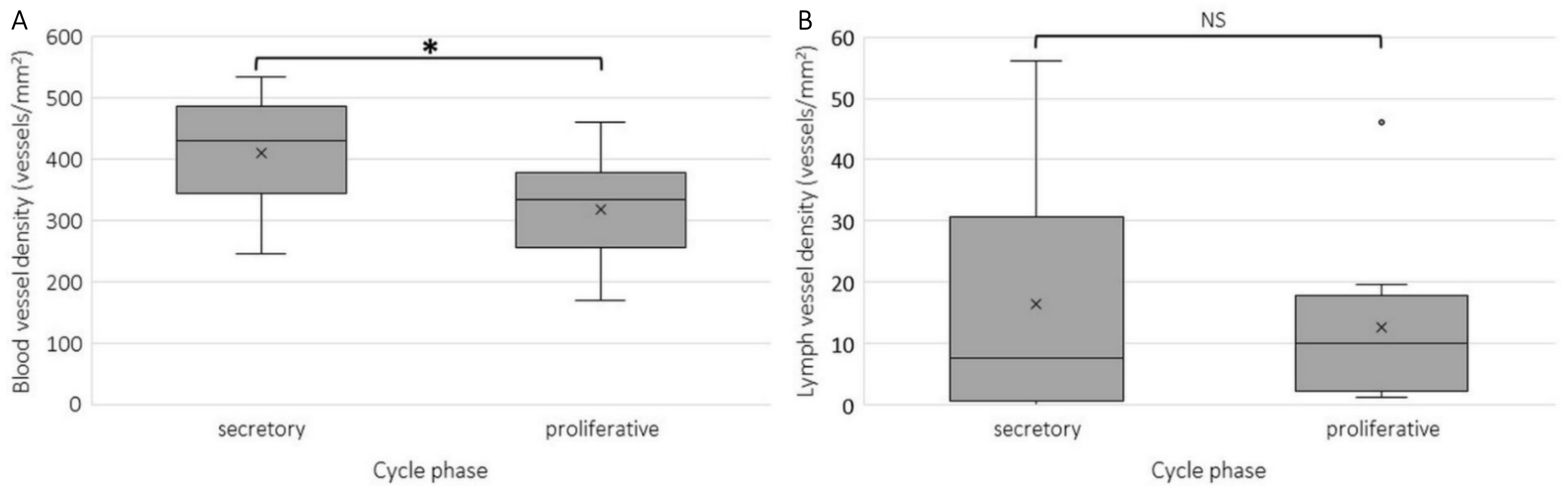
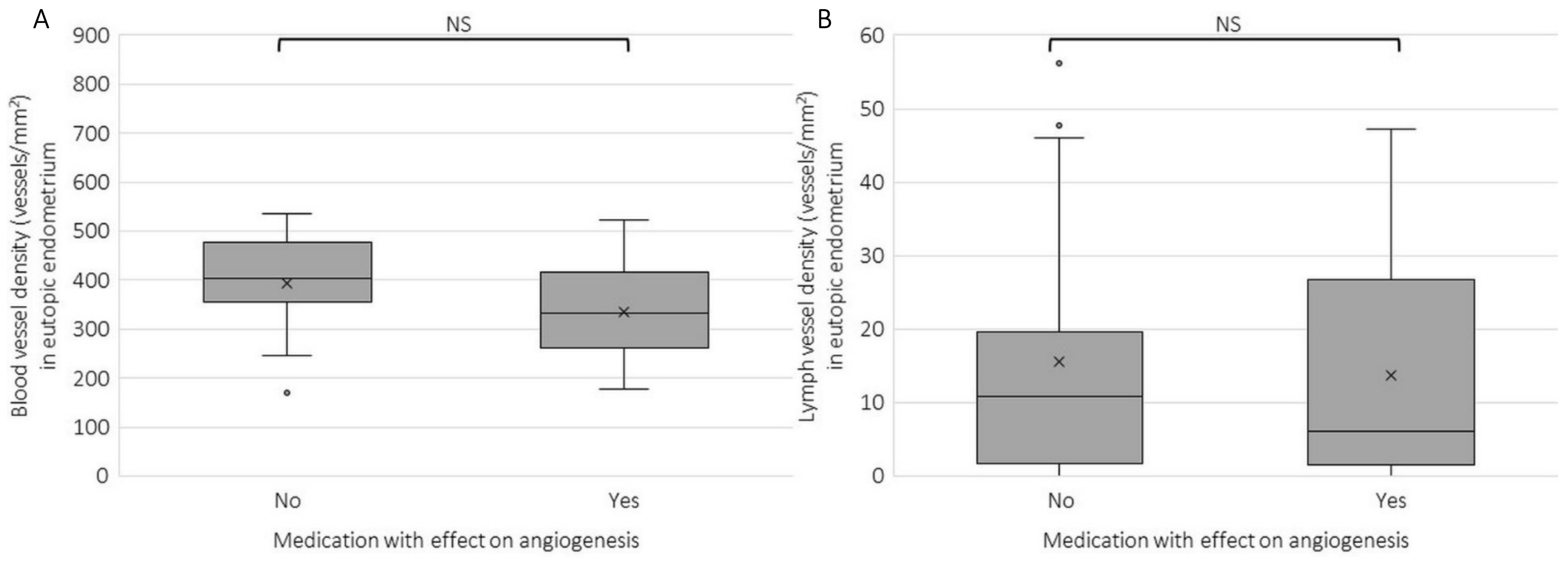
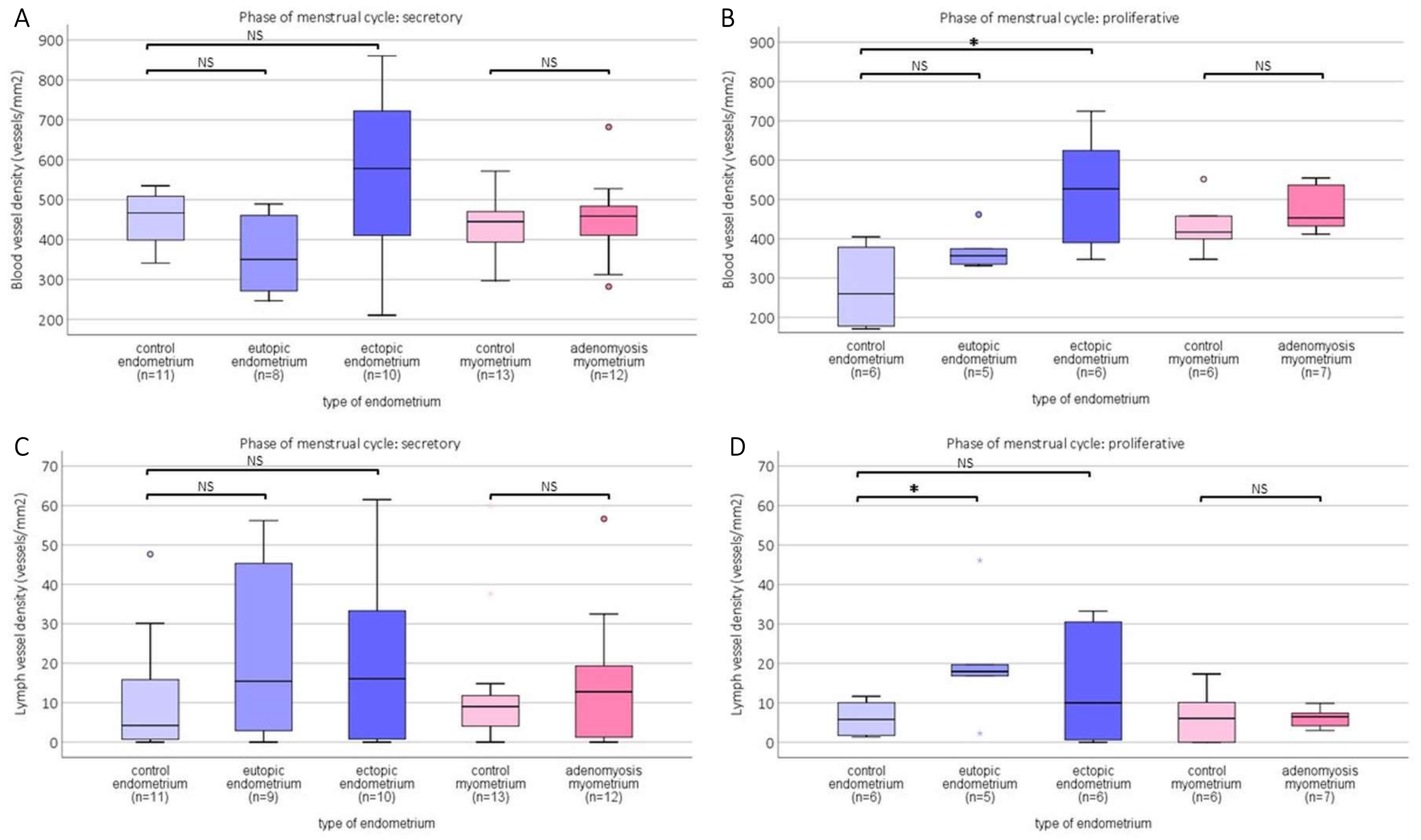
2.2. Blood and Lymph Vessel Density
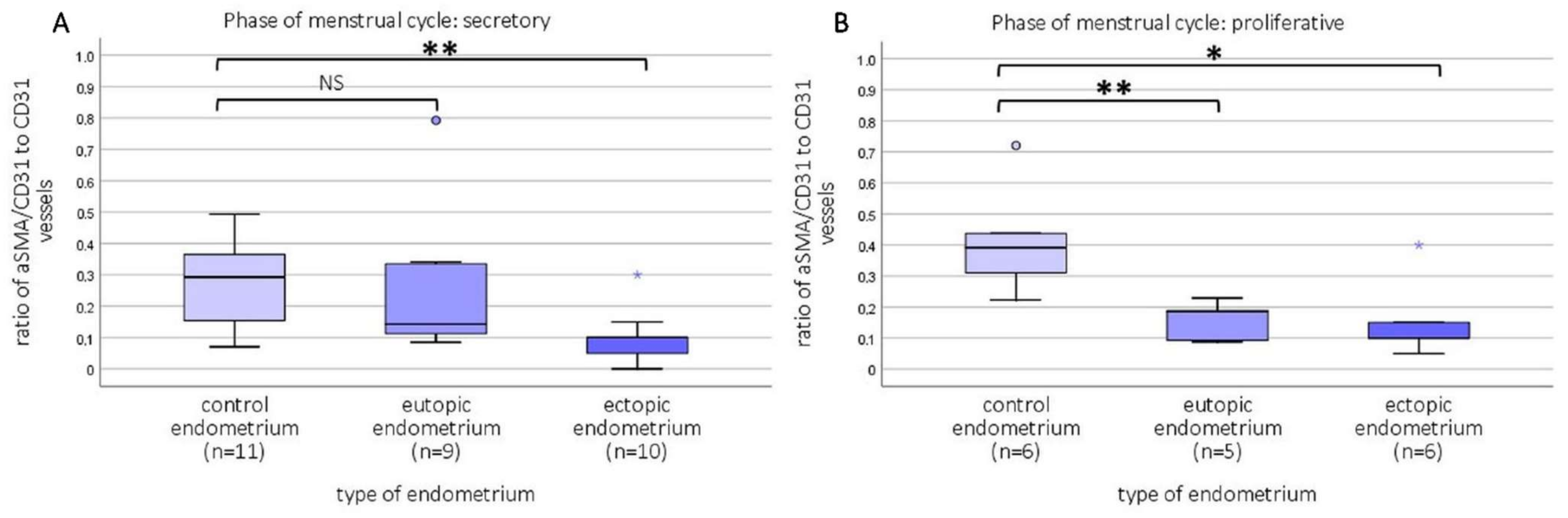
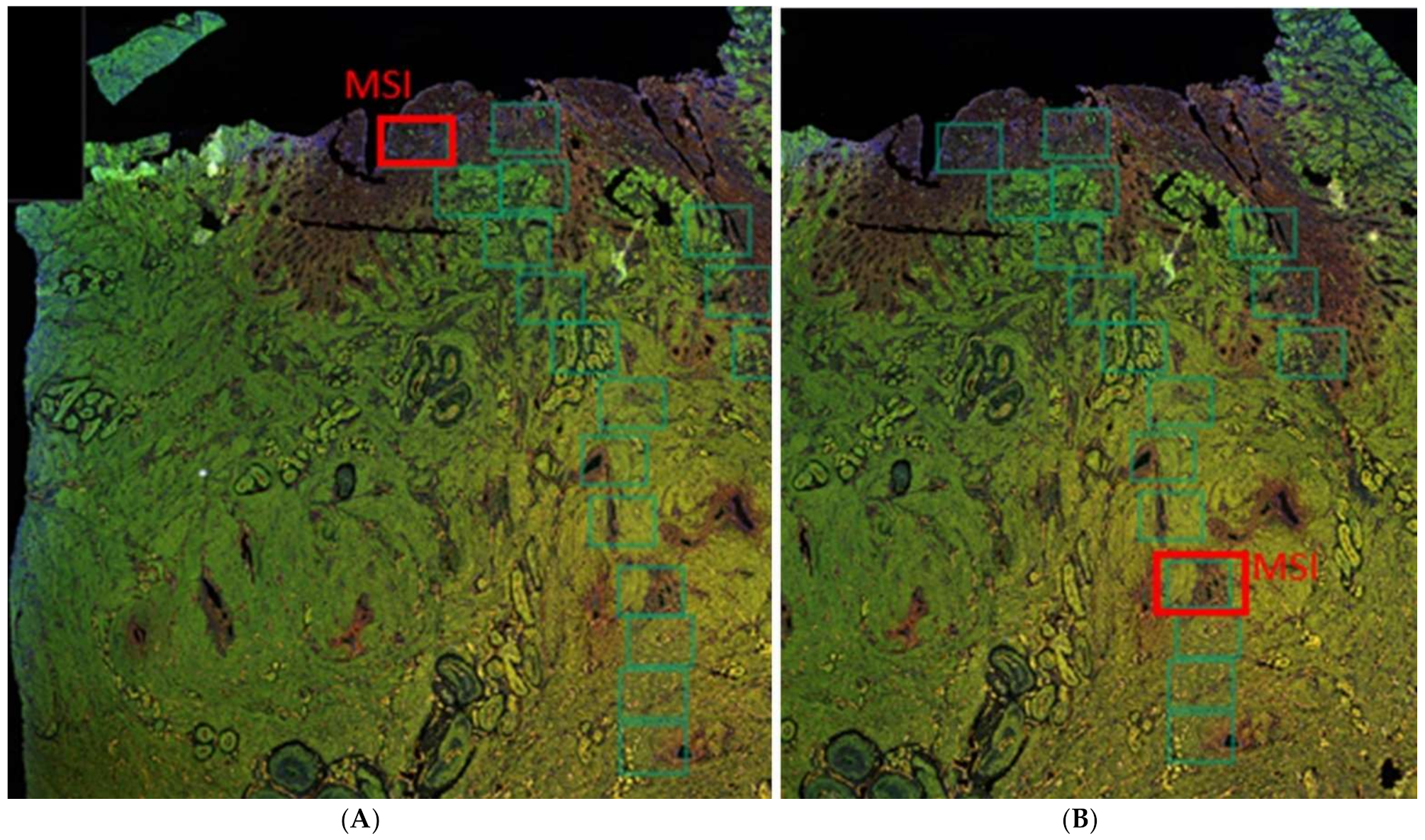
2.3. Relative Number of Capillaries and Newly Formed Vessels
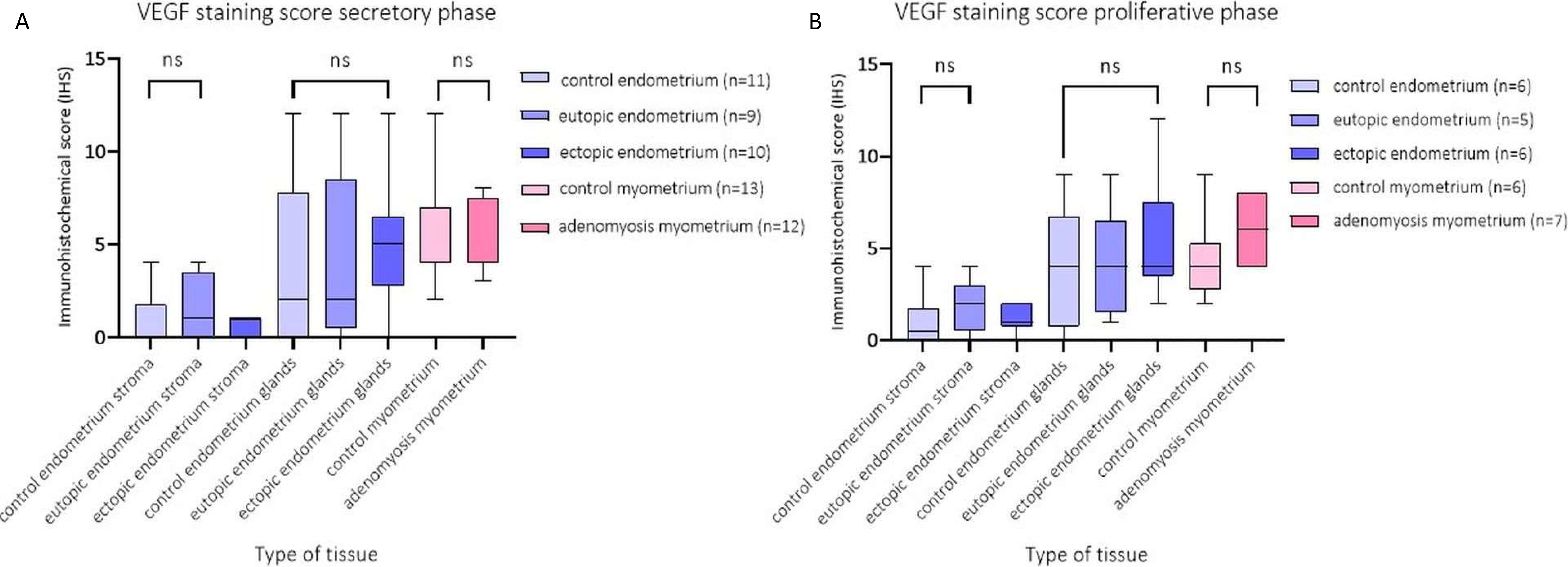
2.4. Immunohistochemical Score (IHS) of VEGF Expression per Tissue Type
3. Discussion
3.1. Strengths and Limitations
3.2. Wider Implications and Future Research
4. Materials and Methods
4.1. Tissue Samples
4.2. Multiplex Immunohistochemistry
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—Revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Benagiano, G.; Habiba, M.; Brosens, I. The pathophysiology of uterine adenomyosis: An update. Fertil. Steril. 2012, 98, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.J.; Wong, C.F.C.; Mijatovic, V.; Griffioen, A.W.; Groenman, F.A.; Hehenkamp, W.J.K.; Huirne, J.A.F. Role of angiogenesis in adenomyosis: A systematic review. Reprod. Sci. 2019, 26, 131A. [Google Scholar]
- Griffioen, A.W.; Molema, G. Angiogenesis: Potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 2000, 52, 237–268. [Google Scholar]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538. [Google Scholar] [CrossRef]
- Garcia-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Goteri, G.; Lucarini, G.; Montik, N.; Zizzi, A.; Stramazzotti, D.; Fabris, G.; Tranquilli, A.L.; Ciavattini, A. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis. Int. J. Gynecol. Pathol. 2009, 28, 157–163. [Google Scholar] [CrossRef]
- Huang, T.S.; Chen, Y.J.; Chou, T.Y.; Chen, C.Y.; Li, H.Y.; Huang, B.S.; Tsai, H.W.; Lan, H.Y.; Chang, C.H.; Twu, N.F.; et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J. Cell. Mol. Med. 2014, 18, 1358–1371. [Google Scholar] [CrossRef]
- Liu, X.; Shen, M.; Qi, Q.; Zhang, H.; Guo, S.W. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum. Reprod. 2016, 31, 734–749. [Google Scholar] [CrossRef]
- Tokyol, C.; Aktepe, F.; Dilek, F.H.; Sahin, O.; Arioz, D.T. Expression of cyclooxygenase-2 and matrix metalloproteinase-2 in adenomyosis and endometrial polyps and its correlation with angiogenesis. Int. J. Gynecol. Pathol. 2009, 28, 148–156. [Google Scholar] [CrossRef]
- Wang, J.; Deng, X.; Yang, Y.; Yang, X.; Kong, B.; Chao, L. Expression of GRIM-19 in adenomyosis and its possible role in pathogenesis. Fertil. Steril. 2016, 105, 1093–1101. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.F.; Mori, T.; Kudo, H.; Asakai, R.; Sassa, S.; Sakamoto, S. Effects of angiogenesis inhibitor TNP-470 on the development of uterine adenomyosis in mice. Fertil. Steril. 2003, 80 (Suppl. 2), 788–794. [Google Scholar] [CrossRef]
- Rogers, P.A.; Donoghue, J.F.; Walter, L.M.; Girling, J.E. Endometrial angiogenesis, vascular maturation, and lymphangiogenesis. Reprod. Sci. 2009, 16, 147–151. [Google Scholar] [CrossRef]
- Sheveleva, T.; Bejenar, V.; Komlichenko, E.; Dedul, A.; Malushko, A. Innovative approach in assessing the role of neurogenesis, angiogenesis, and lymphangiogenesis in the pathogenesis of external genital endometriosis. Gynecol. Endocrinol. 2016, 32 (Suppl. S2), 75–79. [Google Scholar] [CrossRef][Green Version]
- Jerman, L.F.; Hey-Cunningham, A.J. The role of the lymphatic system in endometriosis: A comprehensive review of the literature. Biol. Reprod. 2015, 92, 64. [Google Scholar] [CrossRef]
- Zhang, W.X.; Cao, L.B.; Zhao, Y.; Li, J.; Li, B.F.; Lv, J.N.; Yan, L.; Ma, J.L. Endometrial cavity fluid is associated with deleterious pregnancy outcomes in patients undergoing in vitro fertilization/intracytoplasmic sperm injection: A retrospective cohort study. Ann. Transl. Med. 2021, 9, 9. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X.; Zheng, Y.; Geng, J.G.; Guo, S.W. Increased immunoreactivity to SLIT/ROBO1 and its correlation with severity of dysmenorrhea in adenomyosis. Fertil. Steril. 2011, 95, 1164–1167. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Tanaka, T. Morphometric evaluation of stromal vascularization in the endometrium in adenomyosis. Hum. Reprod. 1998, 13, 715–719. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X. Immunoreactivity of CD68, granulocyte-macrophage colony-stimulating factors receptor and vonwillebrand factor and its association with dysmenorrhea severity and the amount of menses in adenomyosis. Int. J. Clin. Exp. Med. 2016, 9, 20856–20865. [Google Scholar]
- Schindl, M.; Birner, P.; Obermair, A.; Kiesel, L.; Wenzl, R. Increased microvessel density in adenomyosis uteri. Fertil. Steril. 2001, 75, 131–135. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.G.; Pu, D.M. Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis. Gynecol. Obstet. Investig. 2006, 62, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Moller, B.; Rasmussen, C.; Lindblom, B.; Olovsson, M. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol. Hum. Reprod. 2001, 7, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Girling, J.E.; Rogers, P.A. The endometrial lymphatic vasculature: Function and dysfunction. Rev. Endocr. Metab. Disord. 2012, 13, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Ibrahim, M.G.; Sillem, M.; Plendl, J.; Chiantera, V.; Sehouli, J.; Mechsner, S. Myofibroblasts Are Evidence of Chronic Tissue Microtrauma at the Endometrial-Myometrial Junctional Zone in Uteri With Adenomyosis. Reprod. Sci. 2017, 24, 1410–1418. [Google Scholar] [CrossRef]
- Hillen, F.; Griffioen, A.W. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis. Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005, 437, 497–504. [Google Scholar] [CrossRef]
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002, 160, 985–1000. [Google Scholar] [CrossRef]
- Griffioen, A.W.; Mans, L.A.; de Graaf, A.M.A.; Nowak-Sliwinska, P.; de Hoog, C.; de Jong, T.A.M.; Vyth-Dreese, F.A.; van Beijnum, J.R.; Bex, A.; Jonasch, E. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin. Cancer Res. 2012, 18, 3961–3971. [Google Scholar] [CrossRef]
- Abberton, K.M.; Healy, D.L.; Rogers, P.A. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum. Reprod. 1999, 14, 3095–3100. [Google Scholar] [CrossRef][Green Version]
- Runic, R.; Schatz, F.; Wan, L.; Demopoulos, R.; Krikun, G.; Lockwood, C.J. Effects of norplant on endometrial tissue factor expression and blood vessel structure. J. Clin. Endocrinol. Metab. 2000, 85, 3853–3859. [Google Scholar] [CrossRef]
- Cho, S.; Choi, Y.S.; Yun, B.H.; Chon, S.J.; Jung, Y.S.; Kim, H.; Park, J.H.; Seo, S.K.; Kim, S.H.; Lee, B.S. Effects of levonorgestrel-releasing intrauterine system on lymphangiogenesis of adenomyosis. Am. J. Clin. Pathol. 2015, 143, 352–361. [Google Scholar] [CrossRef]
- Hey-Cunningham, A.J.; Peters, K.M.; Zevallos, H.B.; Berbic, M.; Markham, R.; Fraser, I.S. Angiogenesis, lymphangiogenesis and neurogenesis in endometriosis. Front. Biosci. 2013, 5, 1033–1056. [Google Scholar] [CrossRef]
- Stratopoulou, C.A.; Camboni, A.; Donnez, J.; Dolmans, M.M. Identifying Common Pathogenic Features in Deep Endometriotic Nodules and Uterine Adenomyosis. J. Clin. Med. 2021, 10, 4585. [Google Scholar] [CrossRef]
- Hirschowitz, L.; Mayall, F.G.; Ganesan, R.; McCluggage, W.G. Intravascular adenomyomatosis: Expanding the morphologic spectrum of intravascular leiomyomatosis. Am. J. Surg. Pathol. 2013, 37, 1395–1400. [Google Scholar] [CrossRef]
- Balsat, C.; Blacher, S.; Signolle, N.; Beliard, A.; Munaut, C.; Goffin, F.; Noel, A.; Foidart, J.M.; Kridelka, F. Whole slide quantification of stromal lymphatic vessel distribution and peritumoral lymphatic vessel density in early invasive cervical cancer: A method description. ISRN Obstet. Gynecol. 2011, 2011, 354861. [Google Scholar] [CrossRef]
- Noyes, R.W.; Hertig, A.T.; Rock, J. Reprint of: Dating the Endometrial Biopsy. Fertil. Steril. 2019, 112 (Suppl. 1), e93–e115. [Google Scholar] [CrossRef]
- Heeren, A.M.; van Dijk, I.; Berry, D.; Khelil, M.; Ferns, D.; Kole, J.; Musters, R.J.P.; Thijssen, V.L.; Mom, C.H.; Kenter, G.G.; et al. Indoleamine 2,3-Dioxygenase Expression Pattern in the Tumor Microenvironment Predicts Clinical Outcome in Early Stage Cervical Cancer. Front. Immunol. 2018, 9, 1598. [Google Scholar] [CrossRef]
- IJsselsteijn, M.E.; Brouwer, T.P.; Abdulrahman, Z.; Reidy, E.; Ramalheiro, A.; Heeren, A.M.; Vahrmeijer, A.; Jordanova, E.S.; de Miranda, N.F. Cancer immunophenotyping by seven-colour multispectral imaging without tyramide signal amplification. J. Pathol. Clin. Res. 2019, 5, 3–11. [Google Scholar] [CrossRef]
- Shen, F.; Liu, X.; Geng, J.G.; Guo, S.W. Increased immunoreactivity to SLIT/ROBO1 in ovarian endometriomas: A likely constituent biomarker for recurrence. Am. J. Pathol. 2009, 175, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [PubMed]
- Biswas Shivhare, S.; Bulmer, J.N.; Innes, B.A.; Hapangama, D.K.; Lash, G.E. Altered vascular smooth muscle cell differentiation in the endometrial vasculature in menorrhagia. Hum. Reprod. 2014, 29, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Schicketanz, K.H. Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer. Computer-assisted image analysis (QIC score) vs. subjective grading (IRS). Pathol. Res. Pract. 1993, 189, 862–866. [Google Scholar] [CrossRef]
| Item | Patients without Adenomyosis (n = 19) | Patients with Adenomyosis (n = 19) | Statistical Significance |
|---|---|---|---|
| Median age (range) | 43.4 (28–48) | 45.4 (32–50) | 0.370 |
| Reason for hysterectomy (symptoms) | |||
| Prolapse | 11 (57.9%) | 0 (0%) | |
| Dysmenorrhea | 3 (15.8%) | 3 (15.8%) | |
| Cervical dysplasia | 3 (15.8%) | 1 (5.3%) | |
| Ovarian Cyst | 1 (5.3%) | 0 (0%) | |
| Abnormal uterine bleeding | 1 (5.3%) | 15 (78.9%) | |
| Menstrual phase | |||
| Proliferative | 6 (31.6%) | 7 (36.9%) | 0.732 |
| Secretory | 13 (68.5%) | 12 (63.2%) | |
| Number of patients with representative material | |||
| Eutopic endometrium | 17 | 14 | |
| Proliferative phase | 6 | 5 | |
| Secretory phase | 11 | 9 | |
| Ectopic endometrium | - | 16 | |
| Proliferative phase | 6 | ||
| Secretory phase | 10 | ||
| Myometrium | 19 | 19 | |
| Proliferative phase | 6 | 7 | |
| Secretory phase | 13 | 12 | |
| Parity | 0.118 | ||
| 0 | 0 (0%) | 2 (10.5%) | |
| 1 | 6 (31.6%) | 4 (21.1%) | |
| 2 | 8 (42.1%) | 12 (63.2%) | |
| 3 | 5 (26.3%) | 1 (5.3%) | |
| Previous cesarean section | 0.001 | ||
| No | 19 (100%) | 9 (47.4%) | |
| Yes | 10 (52.7%) | ||
| Medication | 0.119 | ||
| None | 14 (73.7%) | 11 (57.9%) | |
| Tranexamic acid | 0 (0%) | 1 (5.3%) | |
| NSAID (when needed) | 5 (26.3%) | 3 (15.8%) | |
| Other * | 0 (0%) | 4 (21.1%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmsen, M.J.; Arduç, A.; Bleeker, M.C.G.; Juffermans, L.J.M.; Griffioen, A.W.; Jordanova, E.S.; Huirne, J.A.F. Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry. Int. J. Mol. Sci. 2022, 23, 8434. https://doi.org/10.3390/ijms23158434
Harmsen MJ, Arduç A, Bleeker MCG, Juffermans LJM, Griffioen AW, Jordanova ES, Huirne JAF. Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry. International Journal of Molecular Sciences. 2022; 23(15):8434. https://doi.org/10.3390/ijms23158434
Chicago/Turabian StyleHarmsen, Marissa J., Arda Arduç, Maaike C. G. Bleeker, Lynda J. M. Juffermans, Arjan W. Griffioen, Ekaterina S. Jordanova, and Judith A. F. Huirne. 2022. "Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry" International Journal of Molecular Sciences 23, no. 15: 8434. https://doi.org/10.3390/ijms23158434
APA StyleHarmsen, M. J., Arduç, A., Bleeker, M. C. G., Juffermans, L. J. M., Griffioen, A. W., Jordanova, E. S., & Huirne, J. A. F. (2022). Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry. International Journal of Molecular Sciences, 23(15), 8434. https://doi.org/10.3390/ijms23158434







