NO Synthesis but Not Apoptosis, Mitosis or Inflammation Can Explain Correlations between Flow Directionality and Paracellular Permeability of Cultured Endothelium
Abstract
:1. Introduction
2. Results
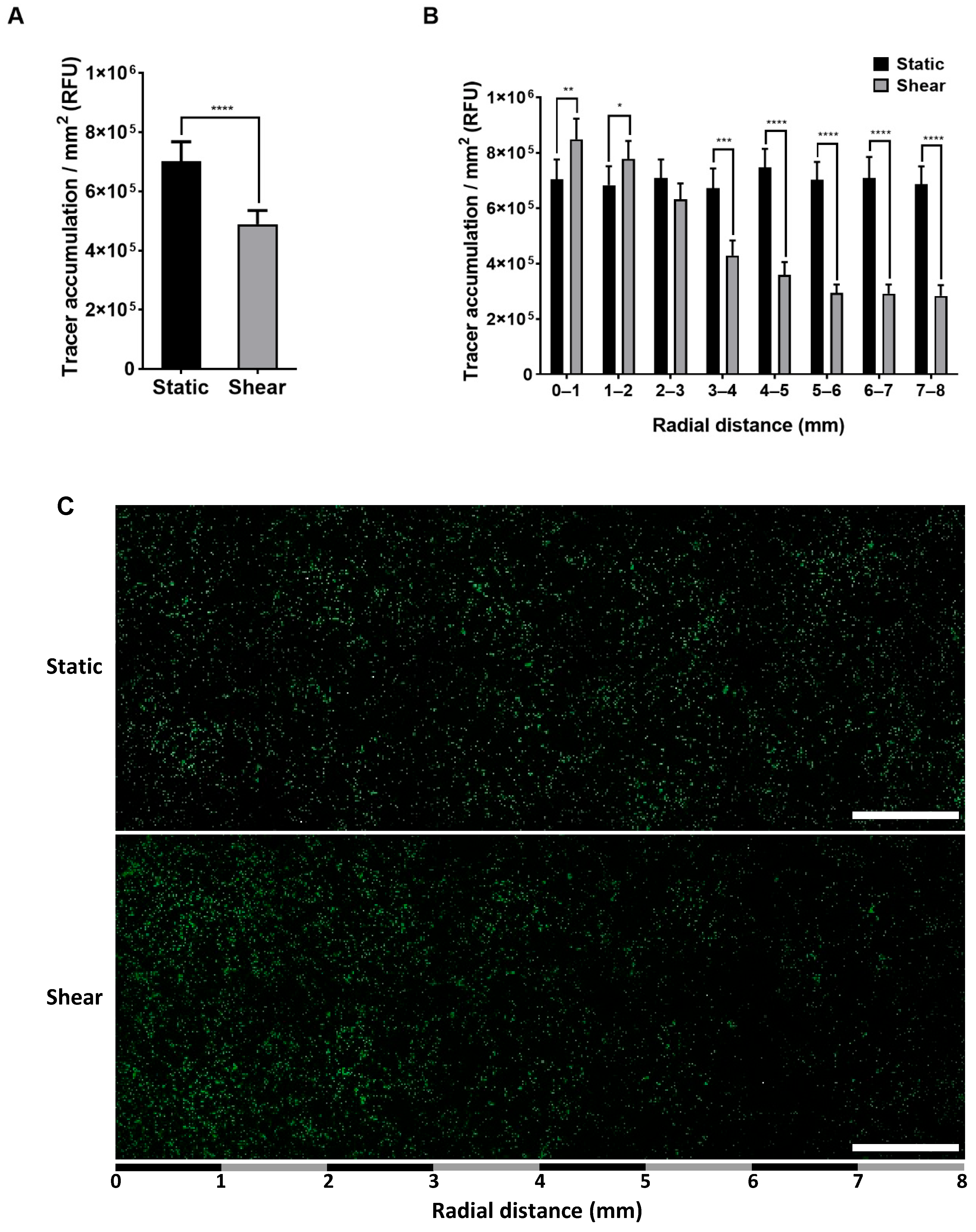
2.1. Effect of Shear on Permeability
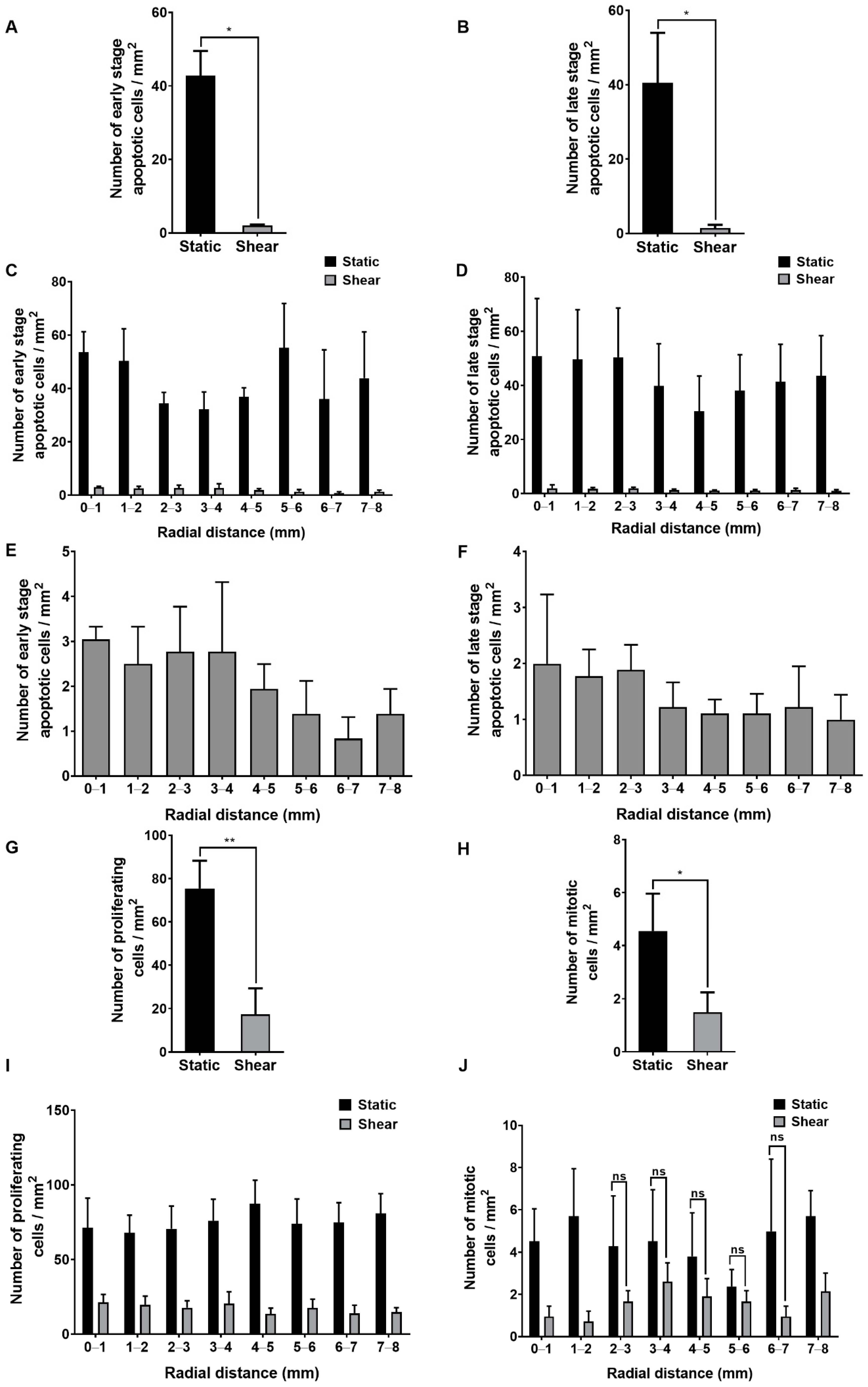
2.2. Effect of Shear on Apoptosis, Proliferation and Mitosis
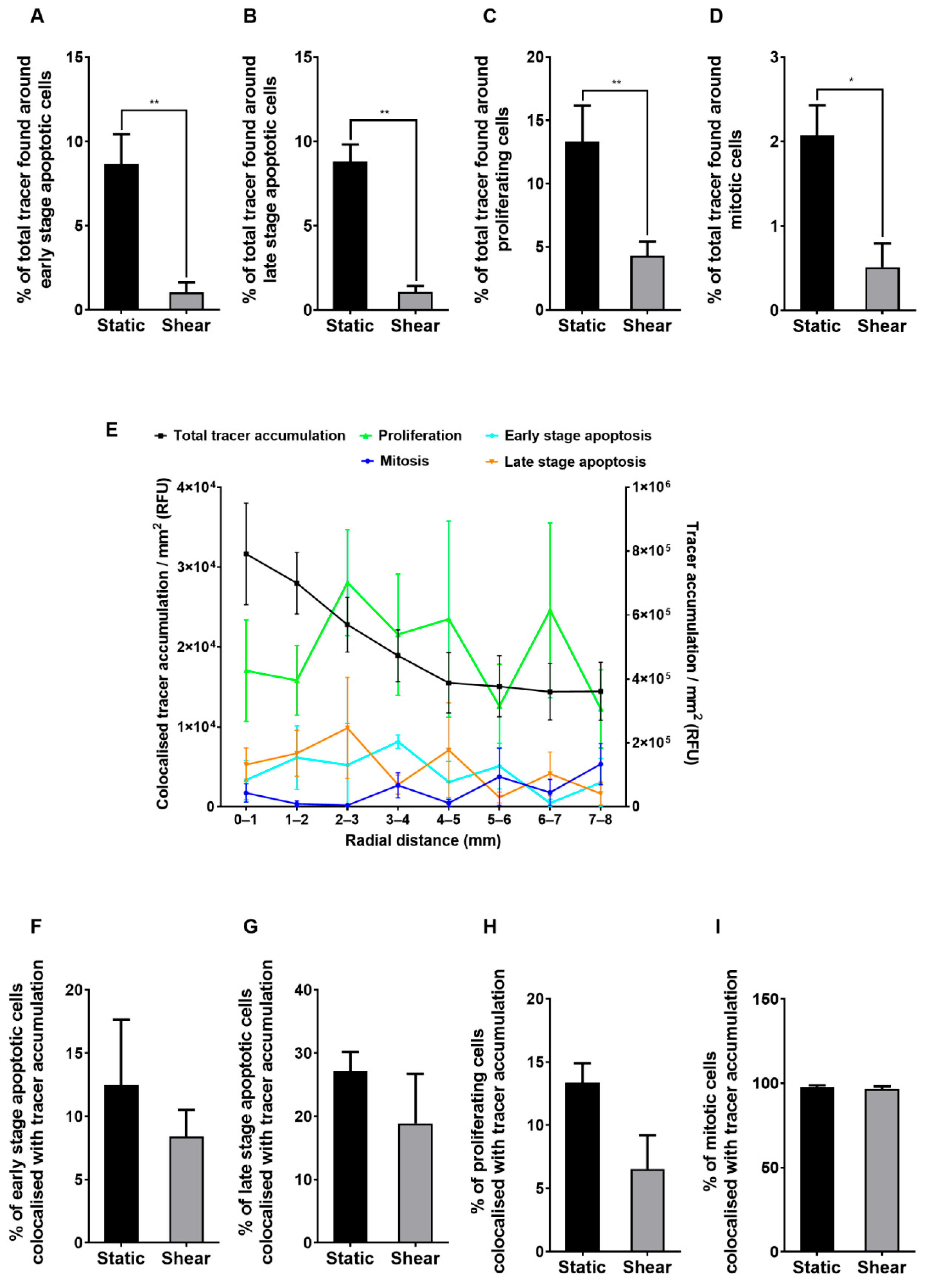
2.3. Correlation of Permeability with the Number of Apoptotic, Proliferating and Mitotic Cells
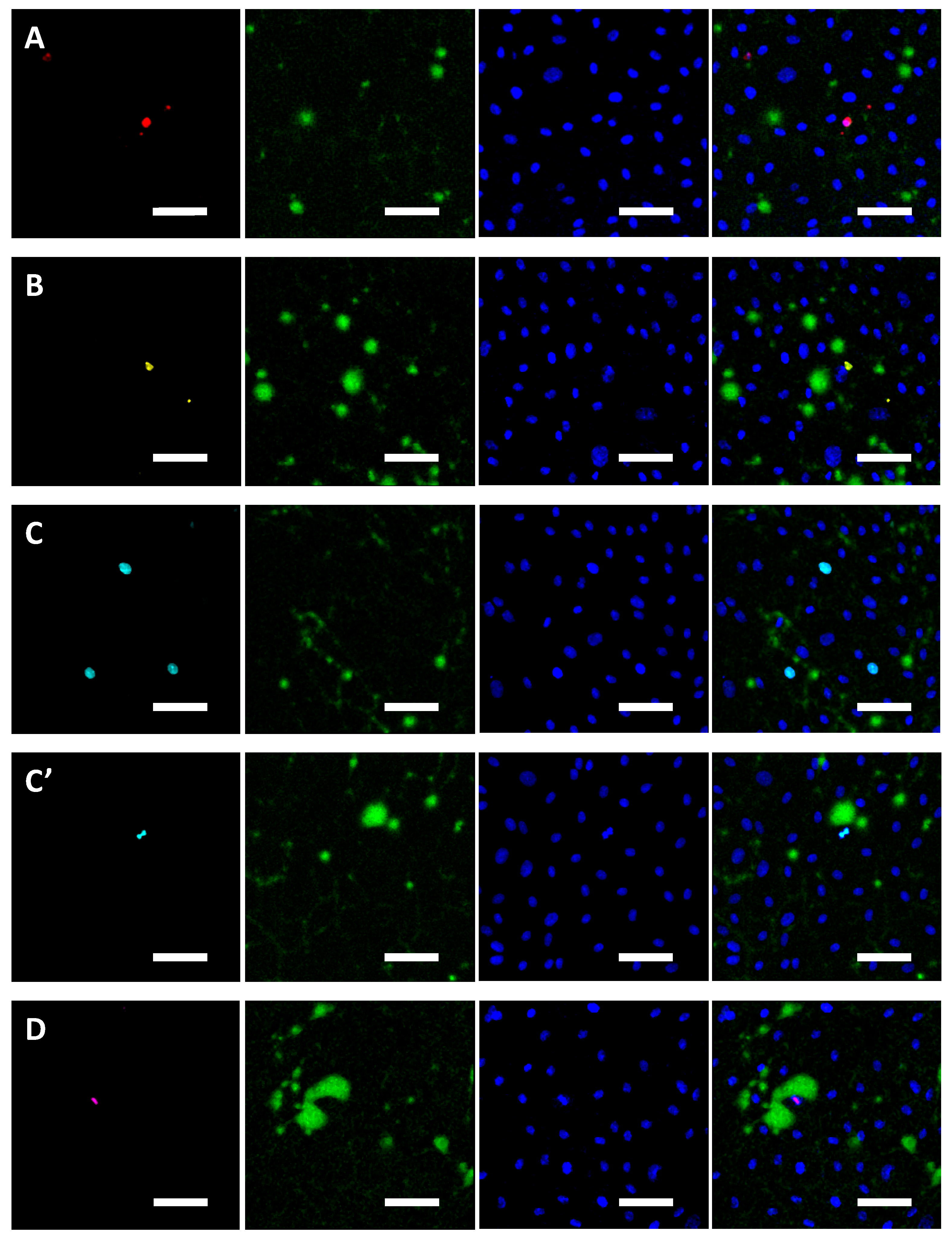
2.4. Colocalisation Studies
2.5. Effect of Shear on Inflammation and Tracer Uptake
2.6. Effect of L-NAME on Permeability with and without Shear
3. Discussion
4. Methods and Materials
4.1. Cell Culture
4.2. Application of Shear Stress
4.3. Application of Tracers
4.4. Staining
4.5. Inhibition of Nitric Oxide Production
4.6. Imaging and Image Processing
4.6.1. Quantification of Tracer Accumulation and Frequency of Apoptotic and Mitotic Cells
4.6.2. Quantification of NF-κB p65 Translocation
4.6.3. Quantification of Endothelial Tricellular Junctions
4.7. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staughton, T.J.; Lever, M.J.; Weinberg, P.D. Effect of altered flow on the pattern of permeability around rabbit aortic branches. Am. J. Physiol. Circ. Physiol. 2001, 281, H53–H59. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.G.; Fitz-Gerald, J.M.; Schroter, R.C. Atheroma and arterial wall shear—Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc. R. Soc. Lond. Ser. B Boil. Sci. 1971, 177, 109–133. [Google Scholar] [CrossRef]
- Ku, D.N.; Giddens, D.P.; Zarins, C.K.; Glagov, S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler. Thromb. Vasc. Biol. 1985, 5, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Mohamied, Y.; Rowland, E.M.; Bailey, E.L.; Sherwin, S.; Schwartz, M.A.; Weinberg, P.D. Change of Direction in the Biomechanics of Atherosclerosis. Ann. Biomed. Eng. 2015, 43, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwenke, D.C.; Carew, T.E. Quantification in vivo of increased LDL content and rate of LDL degradation in normal rabbit aorta occurring at sites susceptible to early atherosclerotic lesions. Circ. Res. 1988, 62, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Berceli, S.A.; Warty, V.S.; Sheppeck, R.A.; Mandarino, W.A.; Tanksale, S.K.; Borovetz, H.S. Hemodynamics and low density lipoprotein metabolism. Rates of low density lipoprotein incorporation and degradation along medial and lateral walls of the rabbit aorto-iliac bifurcation. Arteriosclerosis 1990, 10, 686–694. [Google Scholar] [CrossRef]
- Sebkhi, A.; Weinberg, P.D. Age-related variations in transport properties of the rabbit arterial wall near branches. Atherosclerosis 1994, 106, 1–8. [Google Scholar] [CrossRef]
- Weinbaum, S.; Tzeghai, G.; Ganatos, P.; Pfeffer, R.; Chien, S. Effect of cell turnover and leaky junctions on arterial macromolecular transport. Am. J. Physiol. 1985, 248, H945–H960. [Google Scholar] [CrossRef]
- Lin, S.-J.; Jan, K.-M.; Schuessler, G.; Weinbaum, S.; Chien, S. Enhanced macromolecular permeability of aortic endothelial cells in association with mitosis. Atherosclerosis 1988, 73, 223–232. [Google Scholar] [CrossRef]
- Lin, S.J.; Jan, K.M.; Weinbaum, S.; Chien, S. Transendothelial transport of low density lipoprotein in association with cell mitosis in rat aorta. Arteriosclerosis 1989, 9, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.J.; Jan, K.M.; Chien, S. Role of dying endothelial cells in transendothelial macromolecular transport. Arteriosclerosis 1990, 10, 703–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, P.T.; Cheng, H.J.; Lin, S.J.; Jan, K.M.; Lee, M.M.; Chien, S. Macromolecular transport across arterial and venous endothelium in rats. Studies with Evans blue-albumin and horseradish peroxidase. Arteriosclerosis 1990, 10, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truskey, G.A.; Roberts, W.L.; Herrmann, R.A.; Malinauskas, R.A. Measurement of endothelial permeability to 125I-low density lipoproteins in rabbit arteries by use of en face preparations. Circ. Res. 1992, 71, 883–897. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-L.; Jan, K.-M.; Lin, H.-S.; Chien, S. Ultrastructural studies on macromolecular permeability in relation to endothelial cell turnover. Atherosclerosis 1995, 118, 89–104. [Google Scholar] [CrossRef]
- Dardik, A.; Chen, L.; Frattini, J.; Asada, H.; Aziz, F.; Kudo, F.A.; Sumpio, B.E. Differential effects of orbital and laminar shear stress on endothelial cells. J. Vasc. Surg. 2005, 41, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, A.; Chakraborty, S.; Jala, V.R.; Haribabu, B.; Sharp, M.K.; Berson, R.E. Effects of biaxial oscillatory shear stress on endothelial cell proliferation and morphology. Biotechnol. Bioeng. 2012, 109, 695–707. [Google Scholar] [CrossRef]
- Serbanovic-Canic, J.; de Luca, A.; Warboys, C.; Ferreira, P.F.; Luong, L.A.; Hsiao, S.; Gauci, I.; Mahmoud, M.; Feng, S.; Souilhol, C.; et al. Zebrafish Model for Functional Screening of Flow-Responsive Genes. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Cancel, L.M.; Fitting, A.; Tarbell, J.M. In vitro study of LDL transport under pressurized (convective) conditions. Am. J. Physiol. Circ. Physiol. 2007, 293, H126–H132. [Google Scholar] [CrossRef] [Green Version]
- Cancel, L.M.; Tarbell, J.M. The role of apoptosis in LDL transport through cultured endothelial cell monolayers. Atherosclerosis 2010, 208, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Warboys, C.M.; Berson, R.E.; Mann, G.E.; Pearson, J.D.; Weinberg, P.D. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H1850–H1856. [Google Scholar] [CrossRef]
- Cancel, L.M.; Tarbell, J.M. The role of mitosis in LDL transport through cultured endothelial cell monolayers. Am. J. Physiol. Circ. Physiol. 2011, 300, H769–H776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Cancel, L.M.; Tarbell, J.M. Effect of shear stress on water and LDL transport through cultured endothelial cell monolayers. Atherosclerosis 2014, 233, 682–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghim, M.; Alpresa, P.; Yang, S.-W.; Braakman, S.T.; Gray, S.G.; Sherwin, S.J.; van Reeuwijk, M.; Weinberg, P.D. Visualization of three pathways for macromolecule transport across cultured endothelium and their modification by flow. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, H959–H973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghim, M.; Pang, K.T.; Burnap, S.A.; Baig, F.; Yin, X.; Arshad, M.; Mayr, M.; Weinberg, P.D. Endothelial cells exposed to atheroprotective flow secrete follistatin-like 1 protein which reduces transcytosis and inflammation. Atherosclerosis 2021, 333, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ghim, M.; Mohamied, Y.; Weinberg, P.D. The Role of Tricellular Junctions in the Transport of Macromolecules across Endothelium. Cardiovasc. Eng. Technol. 2021, 12, 101–113. [Google Scholar] [CrossRef]
- Busse, R.; Fleming, I. Endothelial dysfunction in atherosclerosis. J. Vasc. Res. 1996, 33, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Rubanyi, G.M.; Romero, J.C.; Vanhoutte, P.M. Flow-induced release of endothelium-derived relaxing factor. Am. J. Physiol. 1986, 250 Pt 2, H1145–H1149. [Google Scholar] [CrossRef]
- Boo, Y.C.; Jo, H. Flow-dependent regulation of endothelial nitric oxide synthase: Role of protein kinases. Am. J. Physiol.-Cell Physiol. 2003, 285, C499–C508. [Google Scholar] [CrossRef] [Green Version]
- Partridge, J.; Carlsen, H.; Enesa, K.; Chaudhury, H.; Zakkar, M.; Luong, L.; Kinderlerer, A.; Johns, M.; Blomhoff, R.; Mason, J.C.; et al. Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB J. 2007, 21, 3553–3561. [Google Scholar] [CrossRef] [Green Version]
- Forster, B.A.; Weinberg, P.D. Changes with age in the influence of endogenous nitric oxide on transport properties of the rabbit aortic wall near branches. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1361–1368. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Shimizu, J.; Miyawaki-Shimizu, K.; Vogel, S.M.; Bair, A.M.; Minshall, R.D.; Predescu, D.; Malik, A.B. Role of NF-κB-dependent caveolin-1 expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J. Biol. Chem. 2008, 283, 4210–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warboys, C.M.; de Luca, A.; Amini, N.; Luong, L.; Duckles, H.; Hsiao, S.; White, A.; Biswas, S.; Khamis, R.; Chong, C.K.; et al. Disturbed Flow Promotes Endothelial Senescence via a p53-Dependent Pathway. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 985–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshad, M.; Rowland, E.M.; Riemer, K.; Sherwin, S.J.; Weinberg, P.D. Improvement and validation of a computational model of flow in the swirling well cell culture model. Biotechnol. Bioeng. 2022, 119, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskyi, O.; Birukova, A.A.; Birukov, K.G. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab. Investig. 2013, 93, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Computation in the rabbit aorta of a new metric—The transverse wall shear stress—To quantify the multidirectional character of disturbed blood flow. J. Biomech. 2013, 46, 2651–2658. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.; Ghim, M.; Mohamied, Y.; Sherwin, S.J.; Weinberg, P.D. Endothelial cells do not align with the mean wall shear stress vector. J. R. Soc. Interface 2021, 18, 20200772. [Google Scholar] [CrossRef]
- Schnitzer, J.; Oh, P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J. Biol. Chem. 1994, 269, 6072–6082. [Google Scholar] [CrossRef]
- Chooi, K.Y.; Comerford, A.; Cremers, S.J.; Weinberg, P.D. Role of endothelial permeability hotspots and endothelial mitosis in determining age-related patterns of macromolecule uptake by the rabbit aortic wall near branch points. Atherosclerosis 2016, 250, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, P.D. Haemodynamic Wall Shear Stress, Endothelial Permeability and Atherosclerosis-A Triad of Controversy. Front. Bioeng. Biotechnol. 2022, 10, 836680. [Google Scholar] [CrossRef]
- Staughton, T.J.; Weinberg, P.D. Investigation of the role of endogenous nitric oxide synthesis in determining patterns of arterial wall permeability and diet-induced lipid deposition in the rabbit. In Trends in Atherosclerosis Research; Clark, L.V., Ed.; Nova Biomedical Books: Hauppauge, NY, USA, 2004; pp. 123–144. [Google Scholar]
- Bailey, E.L.; Bazigou, E.; Sowinski, P.S.J.; Weinberg, P.D. Mass Transport Properties of the Rabbit Aortic Wall. PLoS ONE 2015, 10, e0120363. [Google Scholar] [CrossRef] [Green Version]
- Bogle, R.G.; Baydoun, A.R.; Pearson, J.D.; Mann, G.E. Regulation of L-arginine transport and nitric oxide release in superfused porcine aortic endothelial cells. J. Physiol. 1996, 490, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warboys, C.M.; Ghim, M.; Weinberg, P.D. Understanding mechanobiology in cultured endothelium: A review of the orbital shaker method. Atherosclerosis 2019, 285, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, L.; Tarbell, J.M. Solute transport to the endothelial intercellular cleft: The effect of wall shear stress. Ann. Biomed. Eng. 2002, 30, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.M.; Hildebrand, D.G.C.; Jeong, W.-K. FusionNet: A Deep Fully Residual Convolutional Neural Network for Image Segmentation in Connectomics. arXiv 2016, arXiv:1612.05360. [Google Scholar] [CrossRef]
- Lin, G.; Milan, A.; Shen, C.; Reid, I. Refinenet: Multi-path refinement networks for high-resolution semantic segmentation. arXiv 2016, arXiv:1611.06612. [Google Scholar]




| Early-Stage Apoptosis | Late-Stage Apoptosis | Proliferation | Mitosis | |
|---|---|---|---|---|
| r | 0.33 | 0.48 | 0.01 | −0.48 |
| R squared | 0.11 | 0.23 | 0.21 | 0.23 |
| p-value | 0.43 | 0.23 | 0.98 | 0.22 |
| 2 h | 4 h | 8 h | 1 Day | 4 Days | 5 Days | |
|---|---|---|---|---|---|---|
| r | 0.73 | 0.34 | 0.30 | 0.30 | 0.21 | 0.35 |
| R squared | 0.53 | 0.12 | 0.0092 | 0.089 | 0.042 | 0.13 |
| p | 0.039 | 0.41 | 0.47 | 0.47 | 0.62 | 0.39 |
| Antigen | Primary Antibody | Secondary Antibody |
|---|---|---|
| Cleaved caspase-9 | Rabbit monoclonal anti-cleaved caspase 9, 1:800 (Cell Signalling Technology, Danvers, MA, USA) | Goat anti-rabbit Alexa Fluor 546, 1:1200 (Invitrogen, Waltham, MA, USA) |
| Cleaved caspase-3 | Rabbit polyclonal anti-cleaved caspase 3, 1:1000 (Cell Signalling Technology) | Goat anti-rabbit Alexa Fluor 546, 1:1500 (Invitrogen) |
| KI67 | Rabbit polyclonal anti-Ki67, 1:1000 (Abcam, Cambridge, UK) | Goat anti-rabbit Alexa Fluor 546, 1:1500 (Invitrogen) |
| Phospho-Ser/Thr–Pro MPM-2 | Mouse monoclonal anti-phospho-Ser/Thr–Pro conjugated to Cy5, 1:800 (Merk, Darmstadt, Germany) | N/A |
| NF-κB p65 | Rabbit polyclonal anti-NF-κB p65, 1:200 (Santa Cruz Biotechnology, Dallas, TX, USA) | Goat anti-rabbit Alexa Fluor 546, 1:300 (Invitrogen) |
| VE-cadherin | Goat polyclonal anti-VE-cadherin, 1:200 (Santa Cruz Biotechnology) | Donkey anti-goat Alexa Fluor 568, 1:300 (Invitrogen) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghim, M.; Yang, S.-W.; David, K.R.Z.; Eustaquio, J.; Warboys, C.M.; Weinberg, P.D. NO Synthesis but Not Apoptosis, Mitosis or Inflammation Can Explain Correlations between Flow Directionality and Paracellular Permeability of Cultured Endothelium. Int. J. Mol. Sci. 2022, 23, 8076. https://doi.org/10.3390/ijms23158076
Ghim M, Yang S-W, David KRZ, Eustaquio J, Warboys CM, Weinberg PD. NO Synthesis but Not Apoptosis, Mitosis or Inflammation Can Explain Correlations between Flow Directionality and Paracellular Permeability of Cultured Endothelium. International Journal of Molecular Sciences. 2022; 23(15):8076. https://doi.org/10.3390/ijms23158076
Chicago/Turabian StyleGhim, Mean, Sung-Wook Yang, Kamilah R. Z. David, Joel Eustaquio, Christina M. Warboys, and Peter D. Weinberg. 2022. "NO Synthesis but Not Apoptosis, Mitosis or Inflammation Can Explain Correlations between Flow Directionality and Paracellular Permeability of Cultured Endothelium" International Journal of Molecular Sciences 23, no. 15: 8076. https://doi.org/10.3390/ijms23158076





