Halochromic Behavior and Anticancer Effect of New Synthetic Anthocyanidins Complexed with β-Cyclodextrin Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Measurements
2.2. Methods
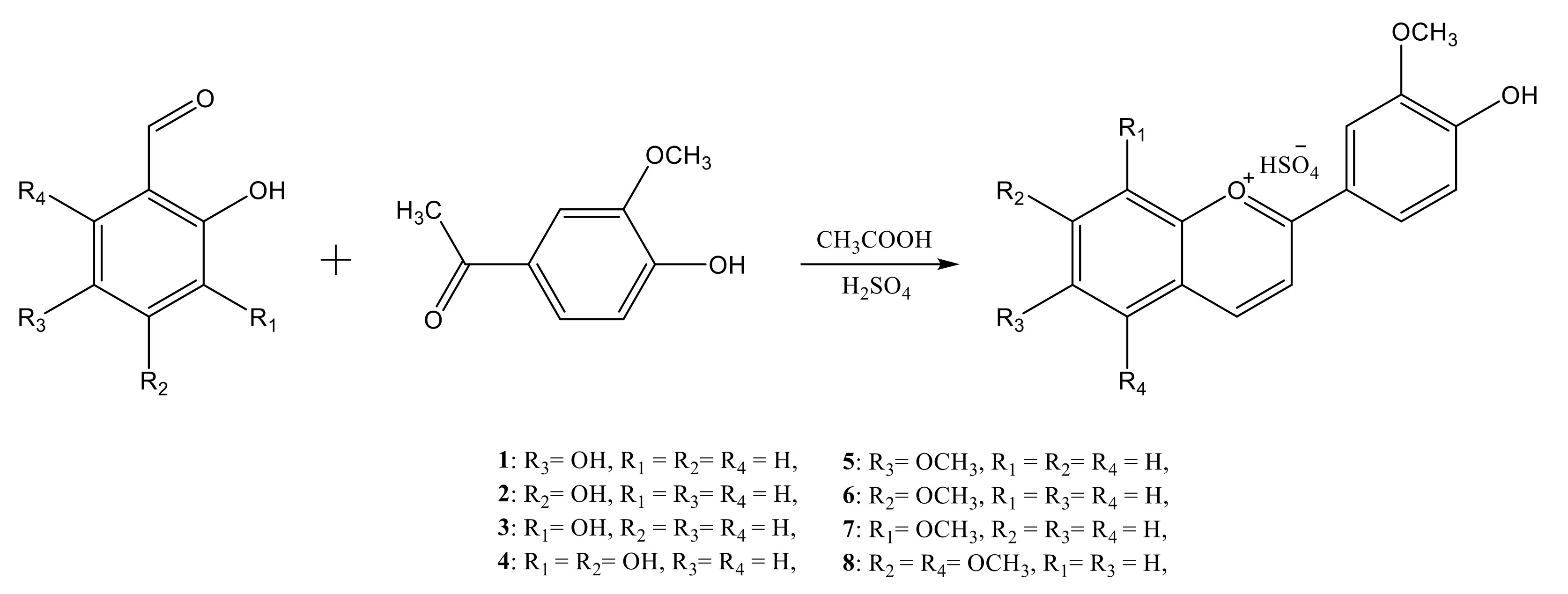
2.2.1. Synthesis of the Bio-Inspired Anthocyanidins/Flavylium Compounds
2.2.2. Spectroscopic Study of Halochromic Properties
2.2.3. In Vitro Cytotoxicity Study
2.2.4. Investigation of Molecular Encapsulation by Cyclodextrins
2.2.5. Data Processing
3. Results and Discussion
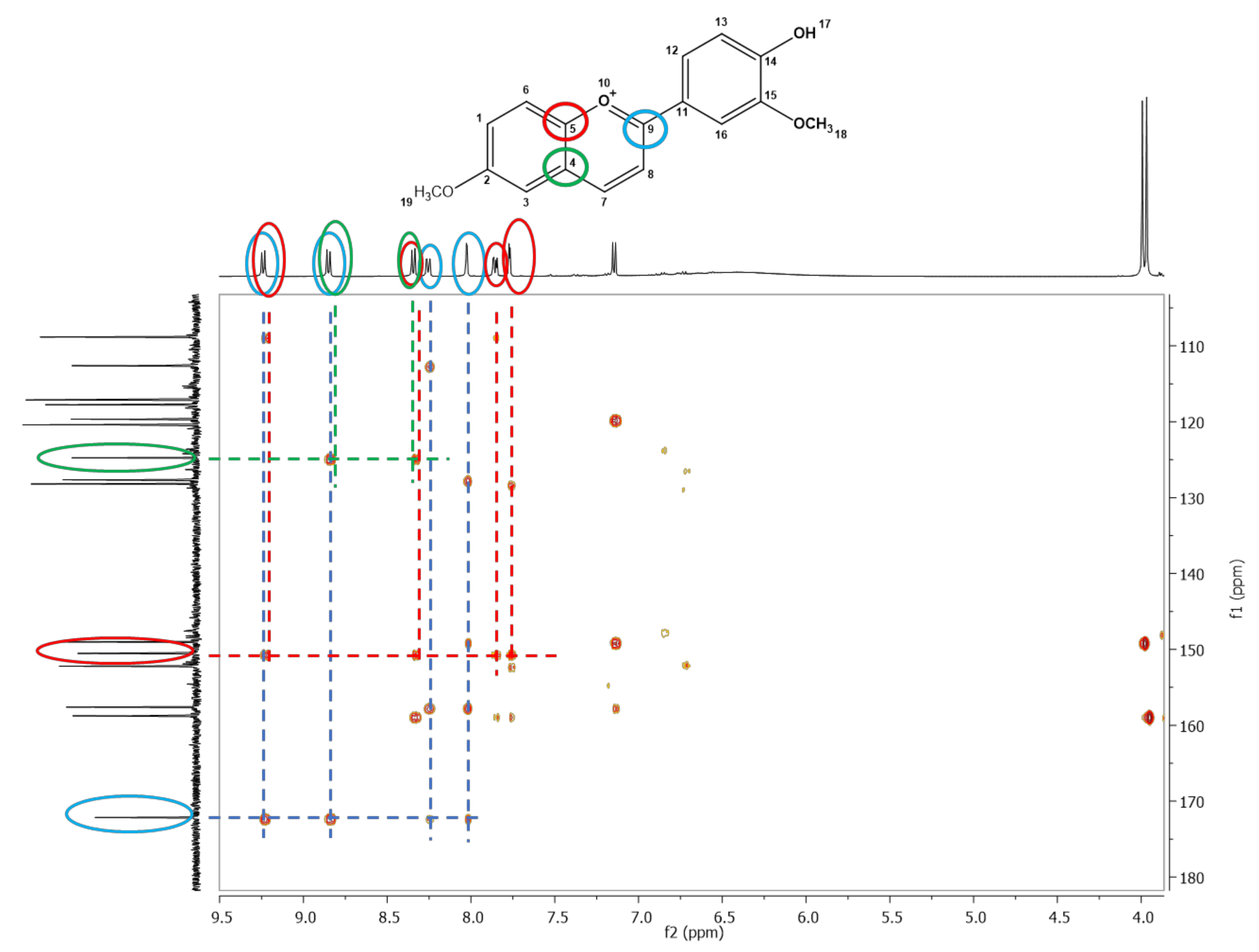
3.1. Synthesis and Characterization of the Anthocyanidins
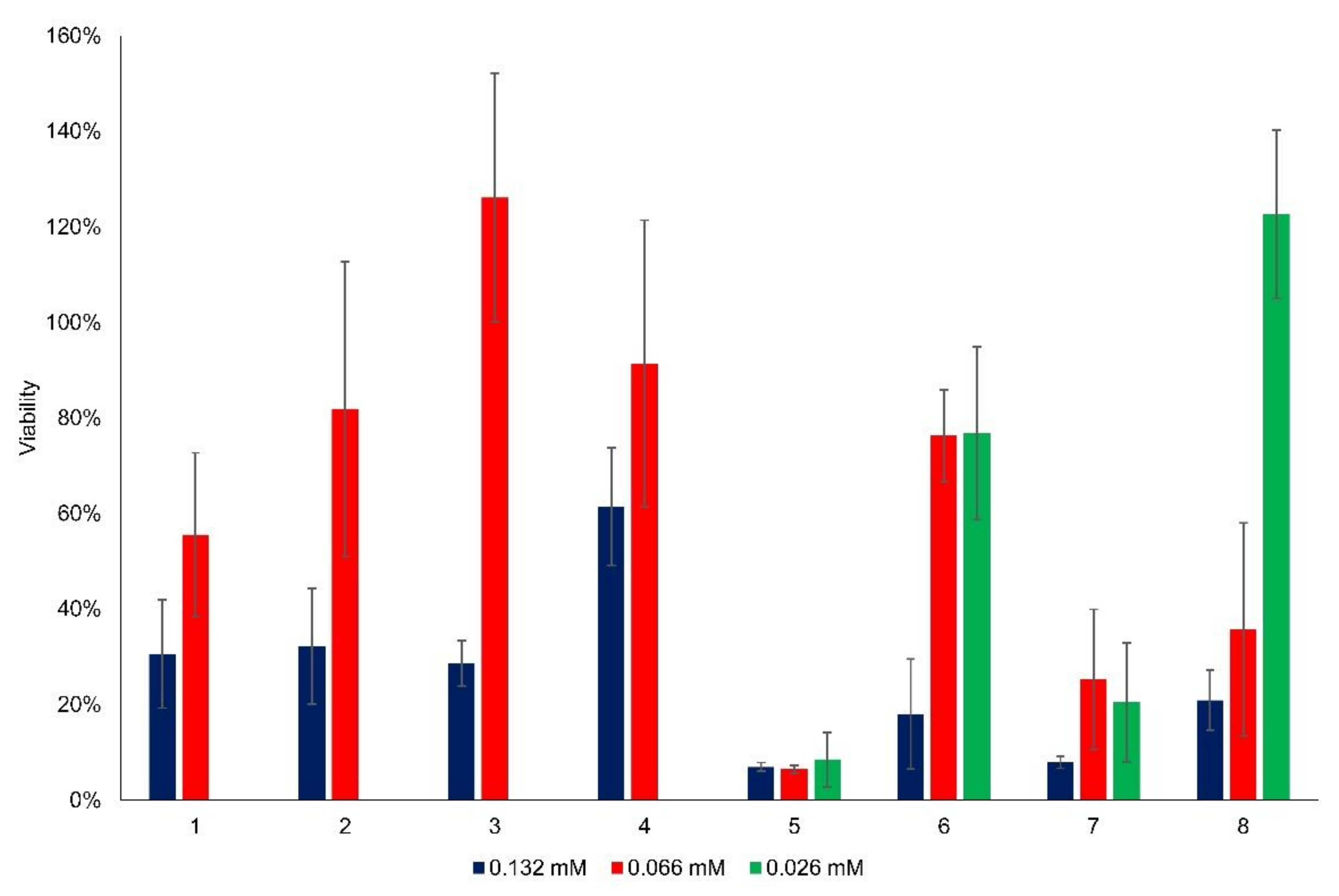
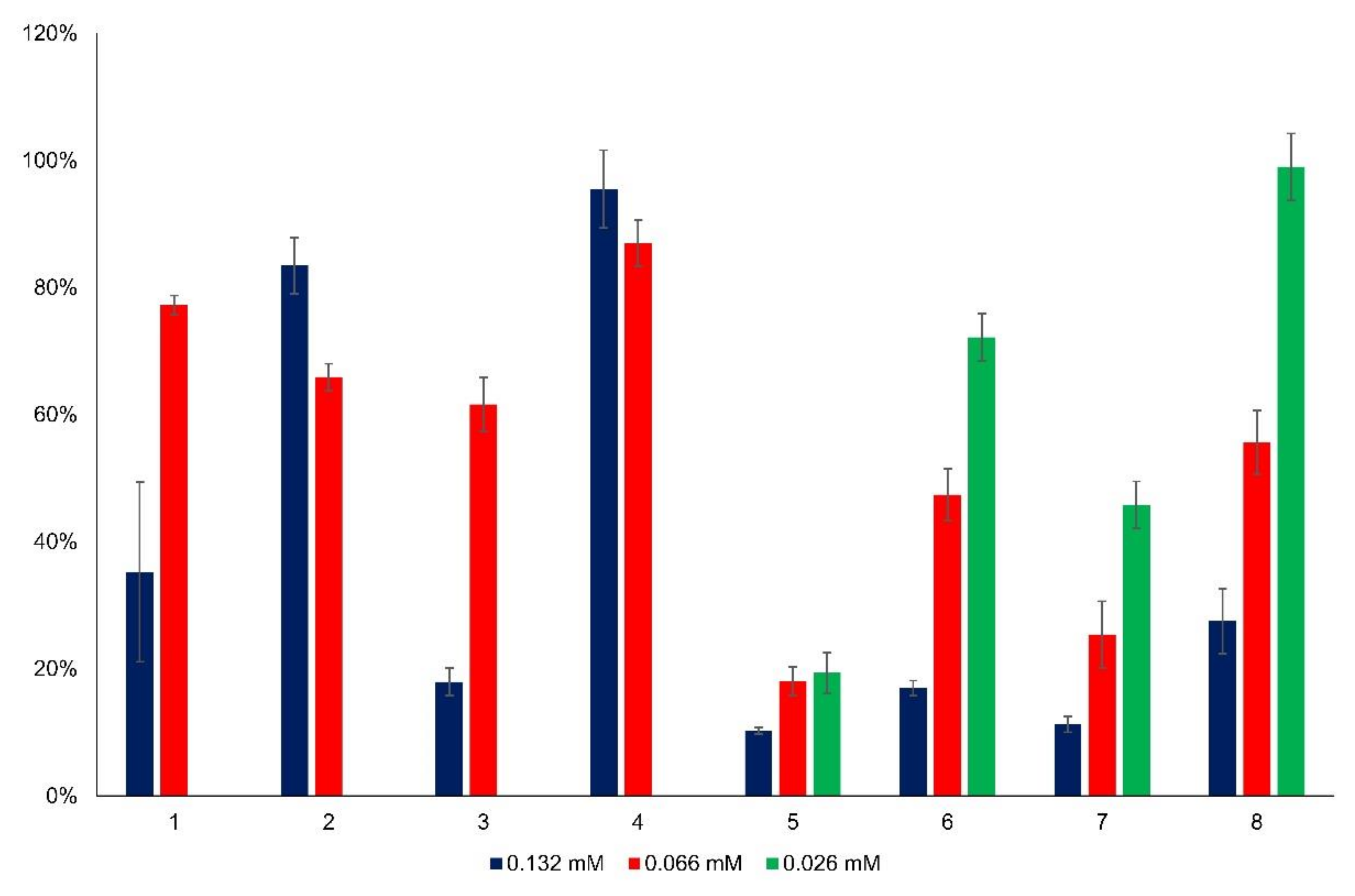
3.2. Cytotoxicity of the New Flavylium Compounds
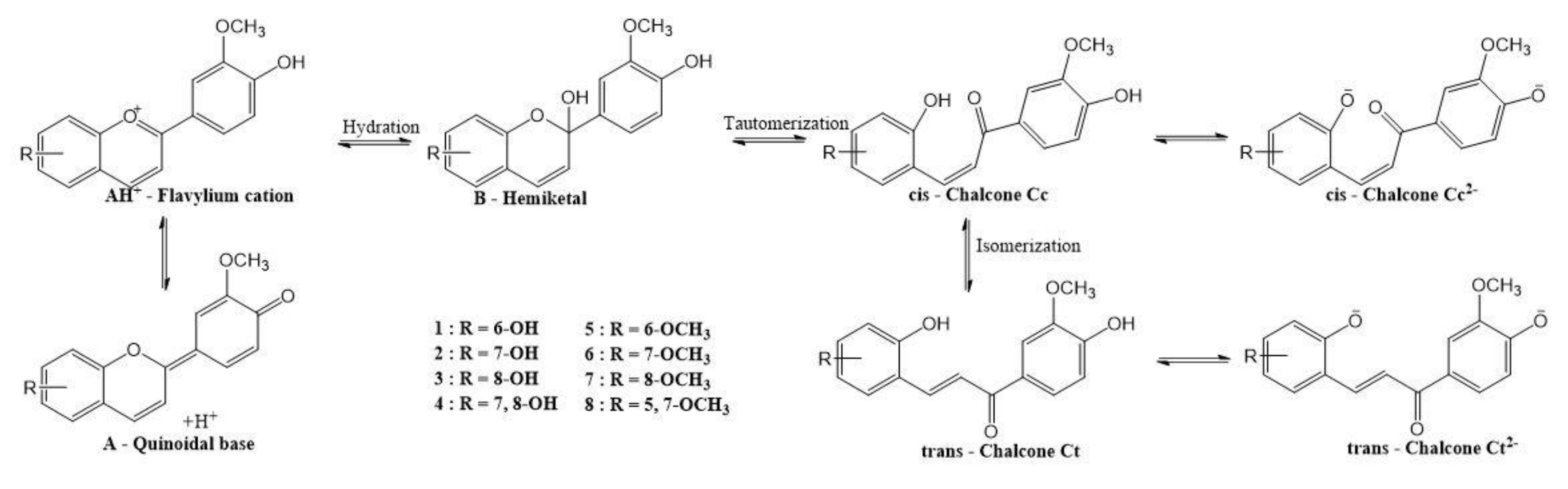
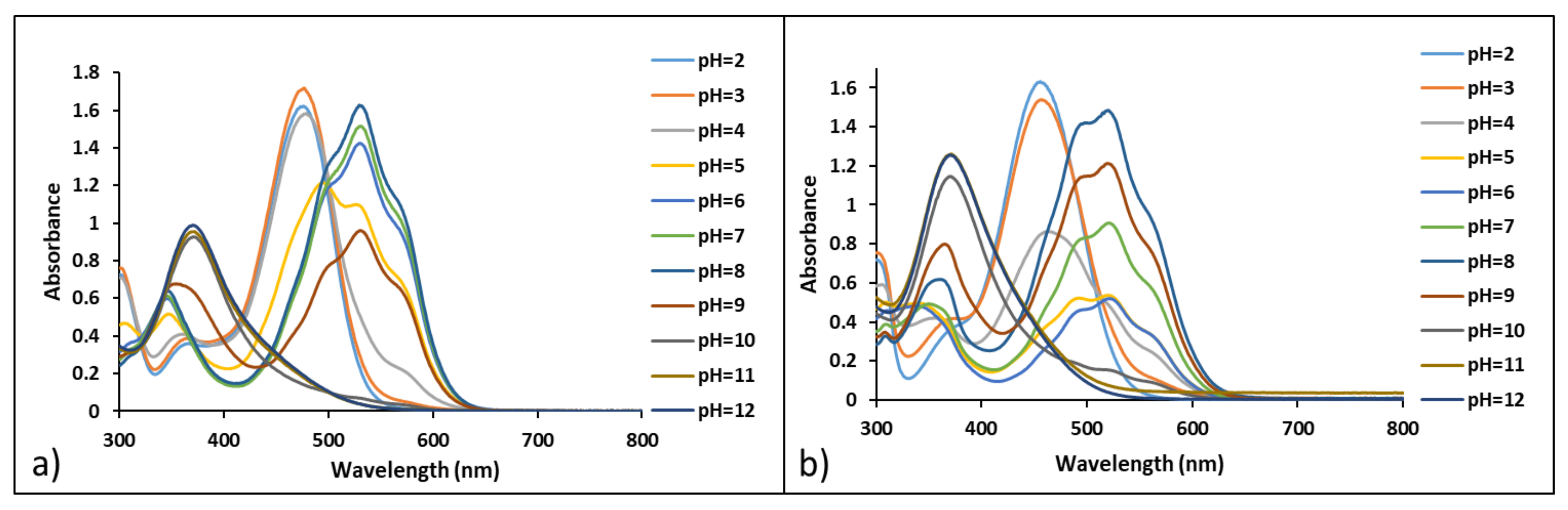
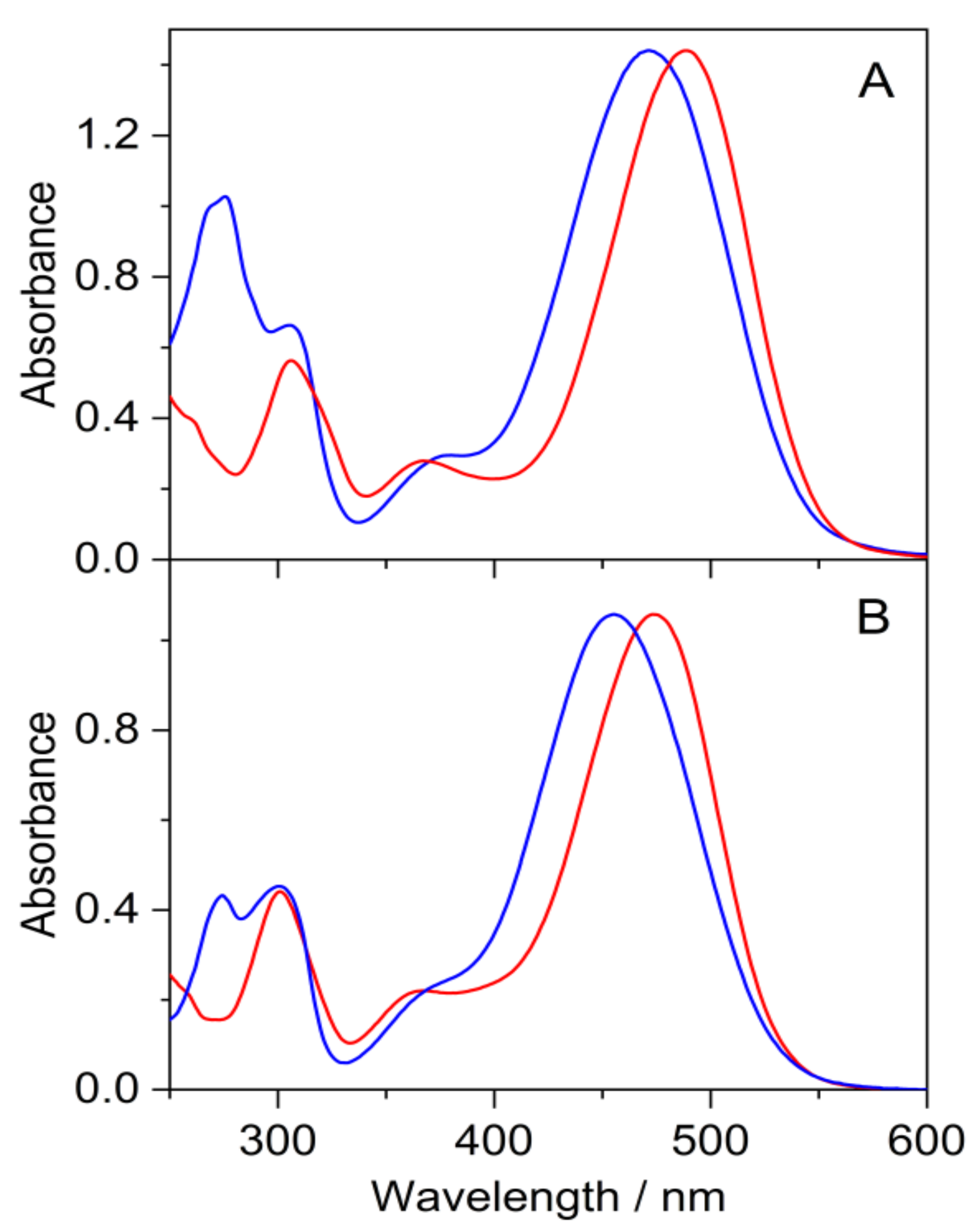
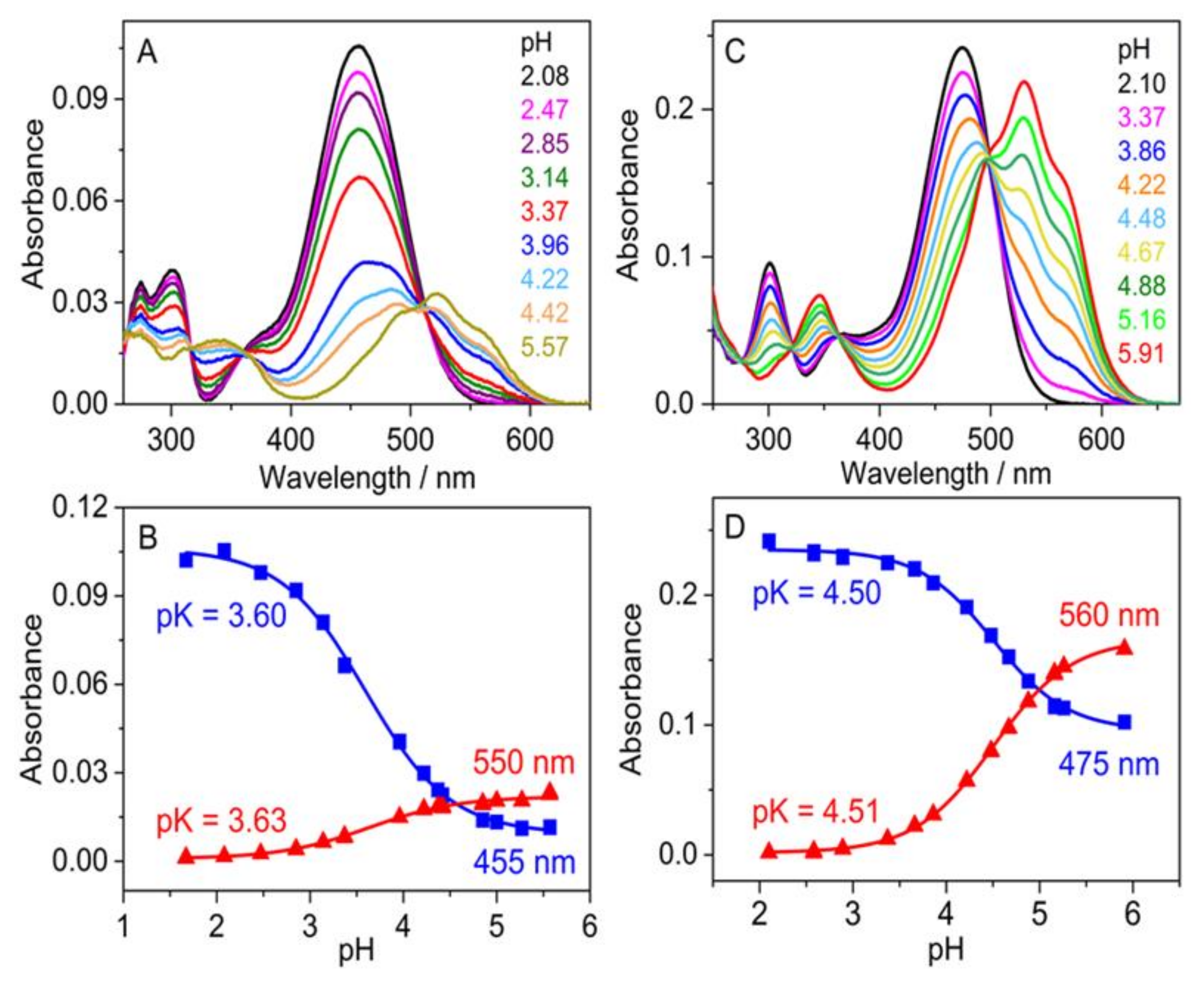
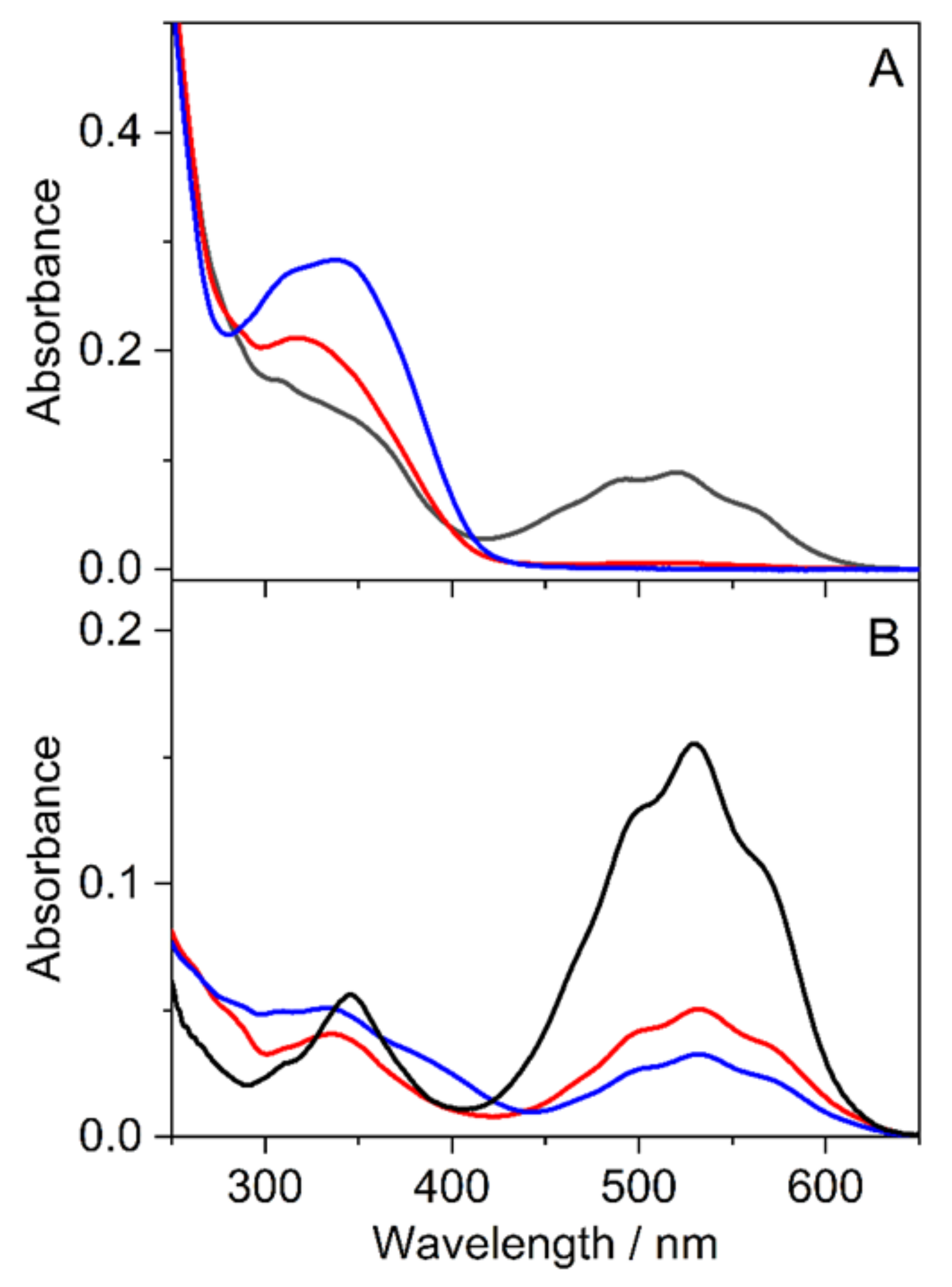
3.3. Study of Halochromic Properties
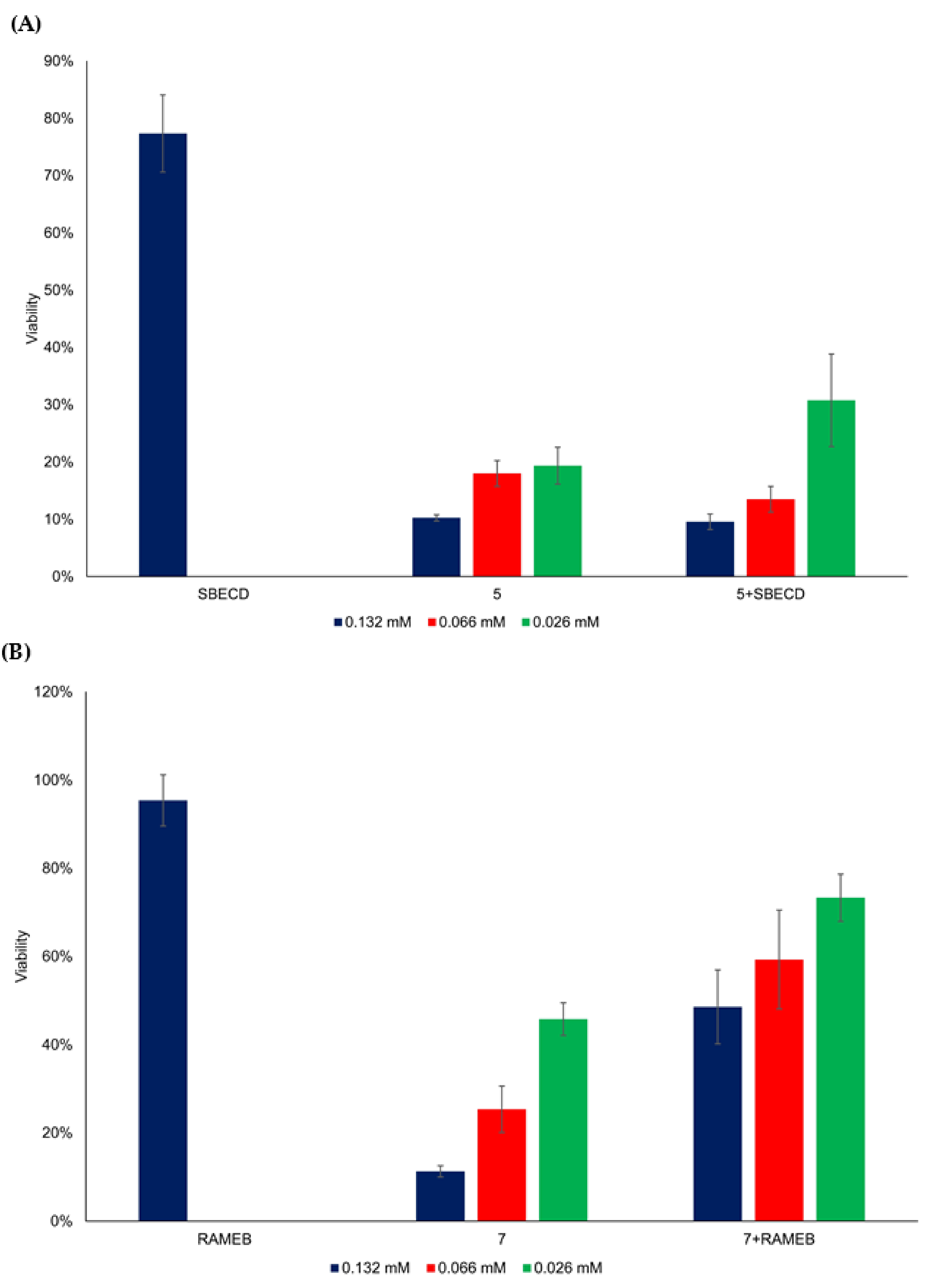
3.4. Cytotoxicity of Flavylium Complexes with Cyclodextrins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Wang, L.; Bai, W.; Chen, W.; Chen, F.; Shu, C. Scope and progress on anthocyanins. In Anthocyanins; Springer: Singapore, 2021; pp. 2–17. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, H.; Wu, N.; Zhang, Q.; Wang, J.; Oliveira, J.; de Freitas, V.; Mateus, N.; He, J.; Fernandes, I. Bioavailability studies and anticancer properties of malvidin based anthocyanins, pyranoanthocyanins and non-oxonium derivatives. Food Funct. 2016, 7, 2462–2468. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of anthocyanins—Critical review of techniques and wall materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, K.; Pathak, M.P.; Saikia, R.; Gogoi, U.; Sahariah, J.J.; Zothantluanga, J.H.; Samanta, A.; Das, A. Cancer chemotherapy via natural bioactive compounds. Curr. Drug Discov. Technol. 2022, 19, 4–23. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25 (Suppl. 2), 41–59. [Google Scholar] [CrossRef]
- de Arruda Nascimento, E.; de Lima Coutinho, L.; da Silva, C.J.; de Lima, V.L.A.G.; dos Santos Aguiar, J. In vitro anticancer properties of anthocyanins: A systematic review. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188748. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Doustimotlagh, A.H.; Irajie, C.; Tanideh, N.; Barzegar, A.; Iraji, A. The promising therapeutic and preventive properties of anthocyanidins/anthocyanins on prostate cancer. Cells 2022, 11, 1070. [Google Scholar] [CrossRef]
- Sorrenti, V.; Vanella, L.; Acquaviva, R.; Cardile, V.; Giofre, S.; Di Giacomo, C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015, 47, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Medic, N.; Tramer, F.; Passamonti, S. Anthocyanins in colorectal cancer prevention. a systematic review of the literature in search of molecular oncotargets. Front. Pharmacol. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Charepalli, V.; Reddivari, L.; Radhakrishnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K.P. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Hao, Y.; Liu, Q.; Wu, Y.; Liu, X.; Luo, J.; Zhou, T.; Sun, B.; Luo, X.; et al. Cyanidin curtails renal cell carcinoma tumorigenesis. Cell Physiol. Biochem. 2018, 46, 2517–2531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ujvarosi, V.; Munteanu, A.C.; Sharma, A.; Singh Tuli, H. Metal complexation and patent studies of flavonoid. In Current Aspects of Flavonoids: Their Role in Cancer Treatment; Singh Tuli, H., Ed.; Springer: Singapore, 2019; pp. 39–89. [Google Scholar] [CrossRef]
- Anwar, S.; Fratantonio, D.; Ferrari, D.; Saija, A.; Cimino, F.; Speciale, A. Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase-3 activation and p21 upregulation. Mol. Med. Rep. 2016, 14, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, K.; Ferguson, L.R.; Philpott, M.; Karunasinghe, N. Cancer-preventive properties of an anthocyanin-enriched sweet potato in the APCMIN mouse model. J. Cancer Prev. 2017, 22, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Kraus, G.A.; Geraskin, I.M. Synthetic anthocyanidins from natural benzopyrans. Nat. Prod. Commun. 2016, 11, 1649–1650. [Google Scholar] [CrossRef] [Green Version]
- Gago, S.; Basilio, N.; Fernandes, A.; Freitas, V.; Quintas, A.; Pina, F. Photochromism of the complex between 4′-(2-hydroxyethoxy)-7-hydroxyflavylium and β-cyclodextrin, studied by 1H NMR, UV-Vis, continuous irradiation and circular dichroism. Dyes Pigment. 2014, 110, 106–112. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Differential effects of synthesized 2’-oxygenated chalcone derivatives: Modulation of human cell cycle phase distribution. Bioorg. Med. Chem. 2004, 12, 2679–2686. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar] [CrossRef]
- Iwata, S.; Nishino, T.; Inoue, H.; Nagata, N.; Satomi, Y.; Nishino, H.; Shibata, S. Antitumorigenic activities of chalcones (II). Photo-isomerization of chalcones and the correlation with their biological activities. Biol. Pharm. Bull. 1997, 20, 1266–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capelezzo, A.P.; Mohr, L.C.; Dalcanton, F.; de Mello, J.M.M.; Fiori, M.A. β-Cyclodextrins as encapsulating agents of essential oils. In Cyclodextrin—A Versatile Ingredien; Arora, P., Dhingra, N., Eds.; IntechOpen: London, UK, 2018; pp. 169–200. [Google Scholar]
- Chakraborty, S.; Basu, S.; Lahiri, A.; Basak, S. Inclusion of chrysin in β cyclodextrin nanocavity and its effect on antioxidant potential of chrysin: A spectroscopic and molecular modeling approach. J. Mol. Struct. 2010, 977, 180–188. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Baron-Cangrejo, O.G.; Salgado-Rivera, P.A. Encapsulation of carotenoids as food colorants via formation of cyclodextrin inclusion complexes: A review. Polysaccharides 2021, 2, 454–476. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Ivanova, G.; Brás, N.F.; Mateus, N.; Ramos, M.J.; Rangel, M.; de Freitas, V. Structural characterization of inclusion complexes between cyanidin-3-O-glucoside and β-cyclodextrin. Carbohyd. Polym. 2014, 102, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, F.; Nguyen, T.L.P.; Haimhoffer, Á.; Rusznyák, Á.; Vasvári, G.; Bácskay, I.; Vecsernyés, M.; Ignat, S.R.; Dinescu, S.; Costache, M.; et al. Cyclodextrin complexation improves the solubility and caco-2 permeability of chrysin. Materials 2020, 13, 3618. [Google Scholar] [CrossRef]
- Ahmad, M.; Ashraf, B.; Gani, A.; Gani, A. Microencapsulation of saffron anthocyanins using β glucan and β cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in-vitro digestion. Int. J. Biol. Macromol. 2018, 109, 435–442. [Google Scholar] [CrossRef]
- Fernandes, A.; Rocha, M.A.A.; Santos, L.M.N.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry anthocyanins: β-cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018, 245, 426–431. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yannakopoulou, K.; Gioxari, A.; Chiou, A.; Makris, D.P. Polyphenol characterization and encapsulation in β-cyclodextrin of a flavonoid-rich Hypericum perforatum (St John’s wort) extract. LWT—Food Sci. Technol. 2010, 43, 882–889. [Google Scholar] [CrossRef]
- Vilanova, N.; Solans, C. Vitamin A Palmitate-β-cyclodextrin inclusion complexes: Characterization, protection and emulsification properties. Food Chem. 2015, 175, 529–535. [Google Scholar] [CrossRef]
- Gago, S.; Basílio, N.; Quintas, A.; Pina, F. Effect of β-cyclodextrin on the multistate species distribution of 3-methoxy-4′,7-dihydroxyflavylium. Discrimination of the two hemiketal enantiomers. J. Agric. Food Chem. 2017, 65, 6346–6358. [Google Scholar] [CrossRef] [PubMed]
- Calogero, G.; Sinopoli, A.; Citro, I.; Di Marco, G.; Petrov, V.; Diniz, A.M.; Parola, A.J.; Pina, F. Synthetic analogues of anthocyanins as sensitizers for dye-sensitized solar cells. Photochem. Photobiol. Sci. 2013, 12, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Carmody, W.R. Easily prepared wide range buffer series. J. Chem. Educ. 1961, 38, 559–560. [Google Scholar] [CrossRef]
- Brouillard, R.; Dubois, J.-E. Mechanism of the structural transformations of anthocyanins in acidic media. J. Am. Chem. Soc. 1977, 99, 1359–1364. [Google Scholar] [CrossRef]
- Brouillard, R.; Delaporte, B. Chemistry of anthocyanin pigments. 2. Kinetic and thermodynamic study of proton transfer, hydration, and tautomeric reactions of malvidin 3-glucoside. J. Am. Chem. Soc. 1977, 99, 8461–8468. [Google Scholar] [CrossRef]
- Cruz, L.; Basílio, N.; Mateus, N.; de Freitas, V.; Pina, F. Natural and synthetic flavylium-based dyes: The chemistry behind the color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef]
- Pina, F.; Petrov, V.; Laia, C.A.T. Photochromism of flavylium systems. An overview of a versatile multistate system. Dyes Pigment. 2012, 92, 877–889. [Google Scholar] [CrossRef]
- Mendoza, J.; Basílio, N.; Dangles, O.; Mora, N.; Al Bittar, S.; Pina, F. Binding of the five multistate species of the anthocyanin analog 7-β-d-glucopyranosyloxy-4′-hydroxyflavylium to the β-cyclodextrin derivative captisol. Dyes Pigment. 2017, 143, 479–487. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdottir, D.; Masson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Poorghorban, M.; Das, U.; Alidi, O.; Chitanda, J.M.; Michel, D.; Dimmock, J.; Verrall, R.; Grochulski, P.; Badea, I. Characterization of the host-guest complex of a curcumin analog with β-cyclodextrin and β-cyclodextrin-gemini surfactant and evaluation of its anticancer activity. Int. J. Nanomed. 2015, 10, 503–515. [Google Scholar]
- Basílio, N.; Pina, F. Chemistry and photochemistry of anthocyanins and related compounds: A thermodynamic and kinetic approach. Molecules 2016, 21, 1502. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păușescu, I.; Kántor, I.; Babos, G.; May, Z.; Fodor-Kardos, A.; Miskolczy, Z.; Biczók, L.; Péter, F.; Medeleanu, M.; Feczkó, T. Halochromic Behavior and Anticancer Effect of New Synthetic Anthocyanidins Complexed with β-Cyclodextrin Derivatives. Int. J. Mol. Sci. 2022, 23, 8103. https://doi.org/10.3390/ijms23158103
Păușescu I, Kántor I, Babos G, May Z, Fodor-Kardos A, Miskolczy Z, Biczók L, Péter F, Medeleanu M, Feczkó T. Halochromic Behavior and Anticancer Effect of New Synthetic Anthocyanidins Complexed with β-Cyclodextrin Derivatives. International Journal of Molecular Sciences. 2022; 23(15):8103. https://doi.org/10.3390/ijms23158103
Chicago/Turabian StylePăușescu, Iulia, Izolda Kántor, György Babos, Zoltán May, Andrea Fodor-Kardos, Zsombor Miskolczy, László Biczók, Francisc Péter, Mihai Medeleanu, and Tivadar Feczkó. 2022. "Halochromic Behavior and Anticancer Effect of New Synthetic Anthocyanidins Complexed with β-Cyclodextrin Derivatives" International Journal of Molecular Sciences 23, no. 15: 8103. https://doi.org/10.3390/ijms23158103
APA StylePăușescu, I., Kántor, I., Babos, G., May, Z., Fodor-Kardos, A., Miskolczy, Z., Biczók, L., Péter, F., Medeleanu, M., & Feczkó, T. (2022). Halochromic Behavior and Anticancer Effect of New Synthetic Anthocyanidins Complexed with β-Cyclodextrin Derivatives. International Journal of Molecular Sciences, 23(15), 8103. https://doi.org/10.3390/ijms23158103








