Hematites Precipitated in Alkaline Precursors: Comparison of Structural and Textural Properties for Methane Oxidation
Abstract
:1. Introduction
2. Results and Discussion
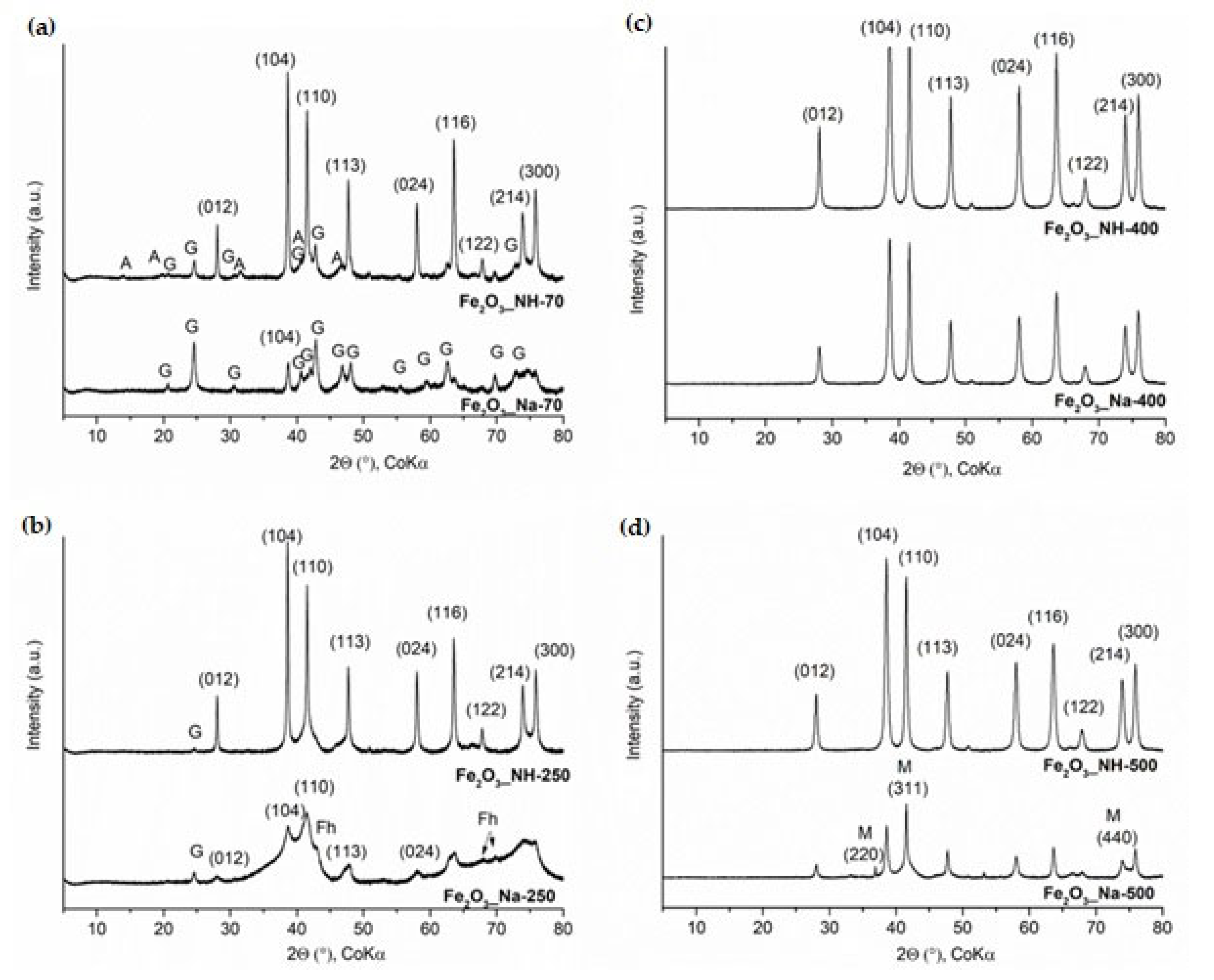
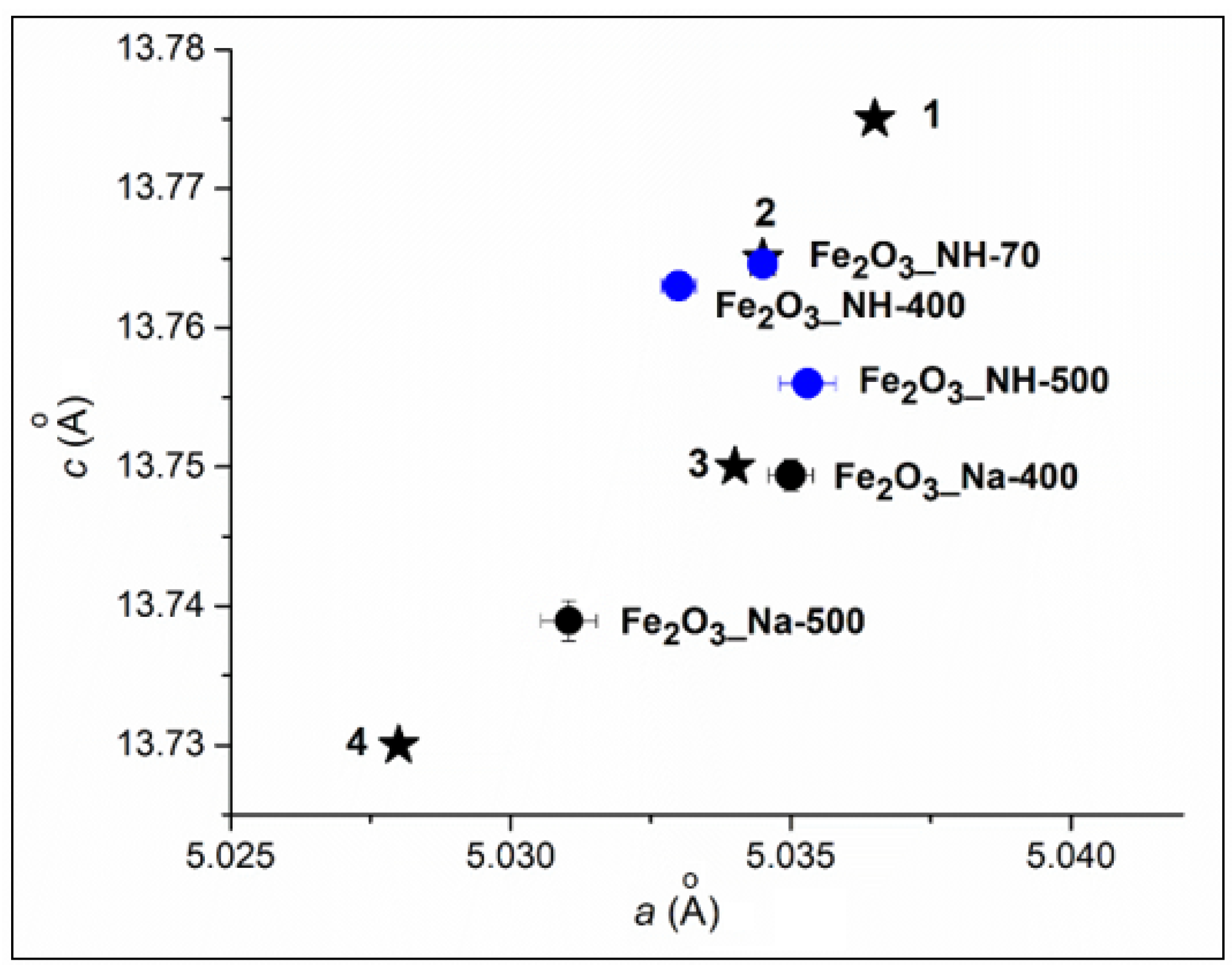
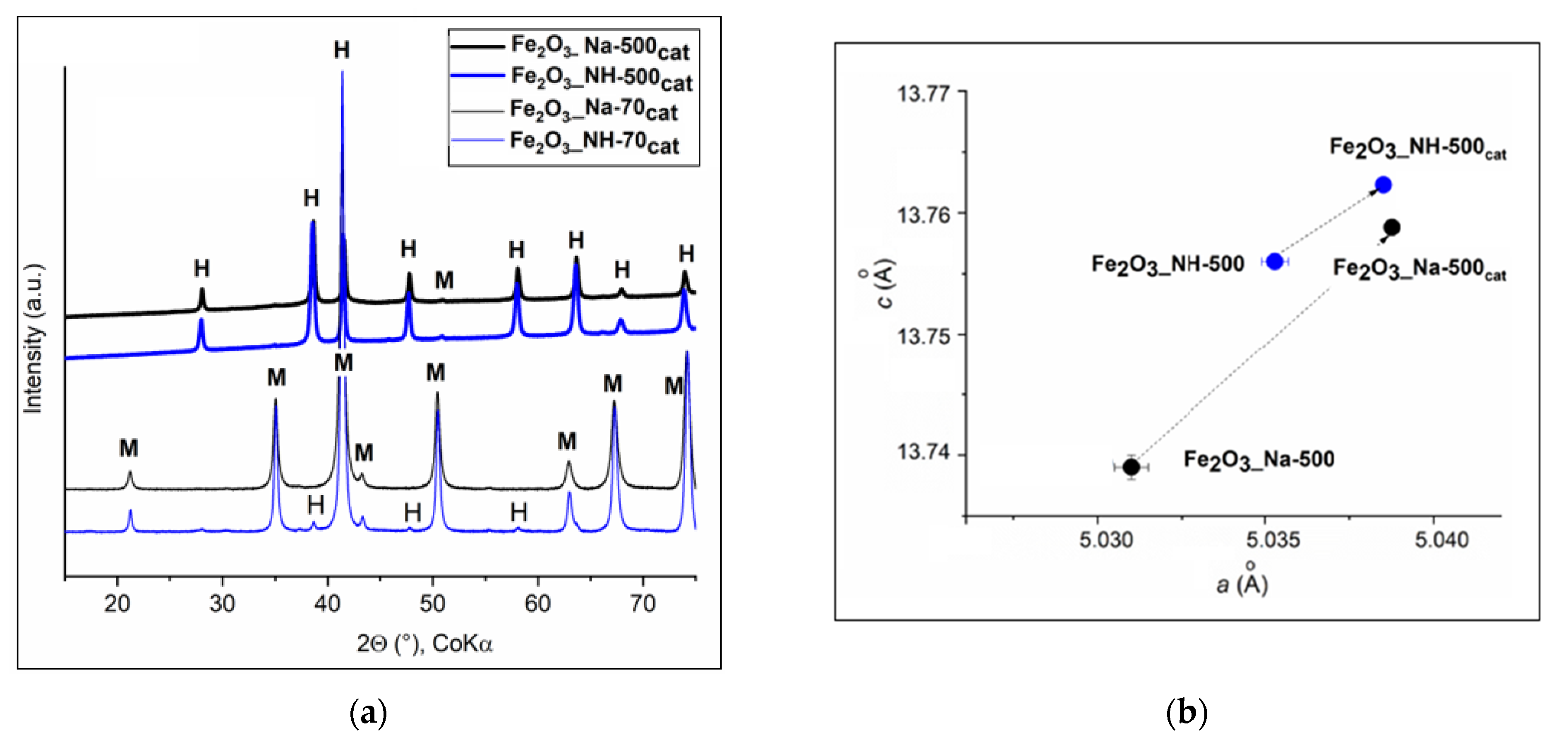
2.1. X-ray Diffraction Analysis of NPs
2.1.1. XRD Phase Analysis of Precipitates
2.1.2. XRD Phase Analysis of Calcined Precipitates
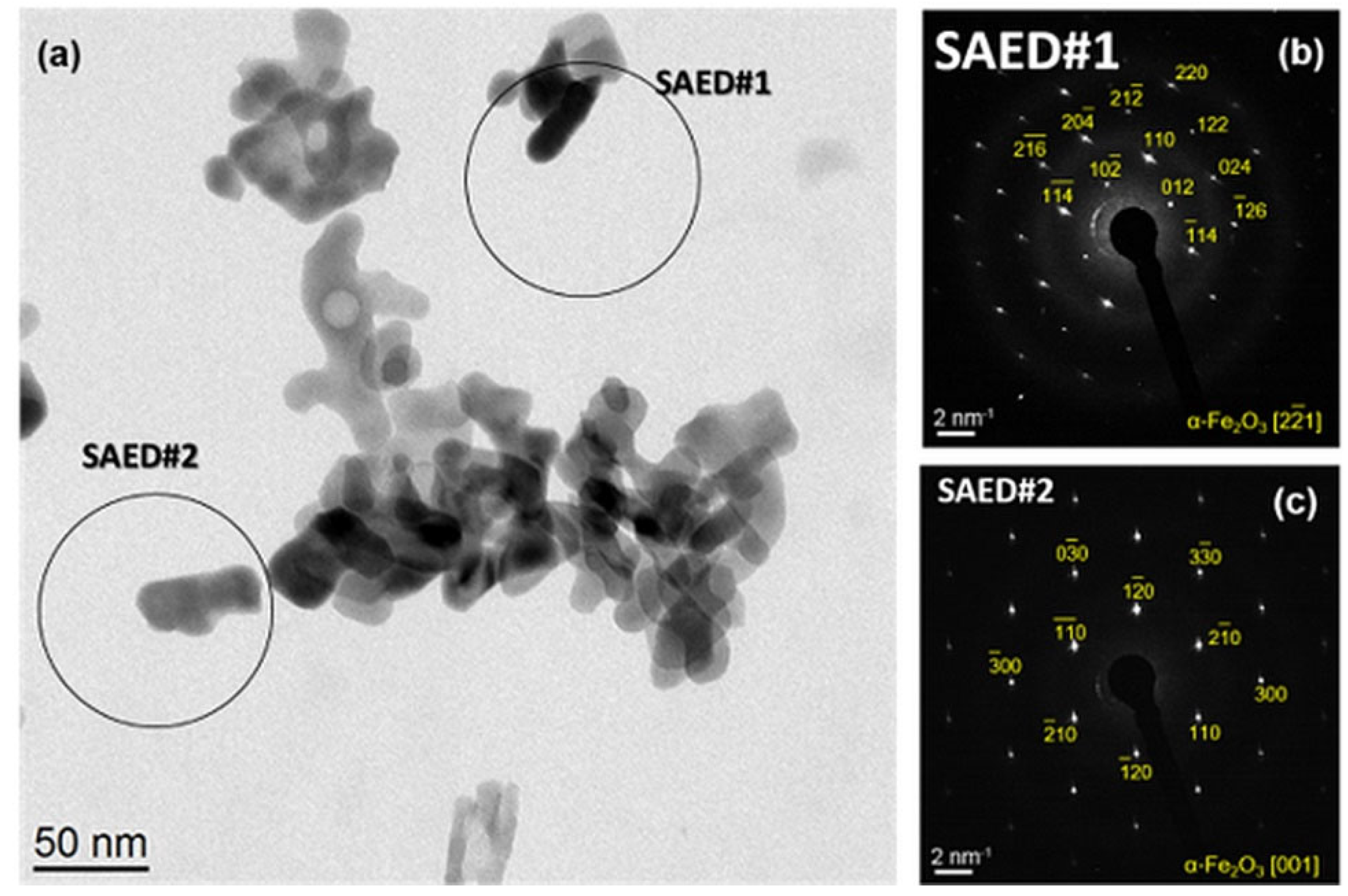
2.2. Size and Morphology of the Calcined NPs
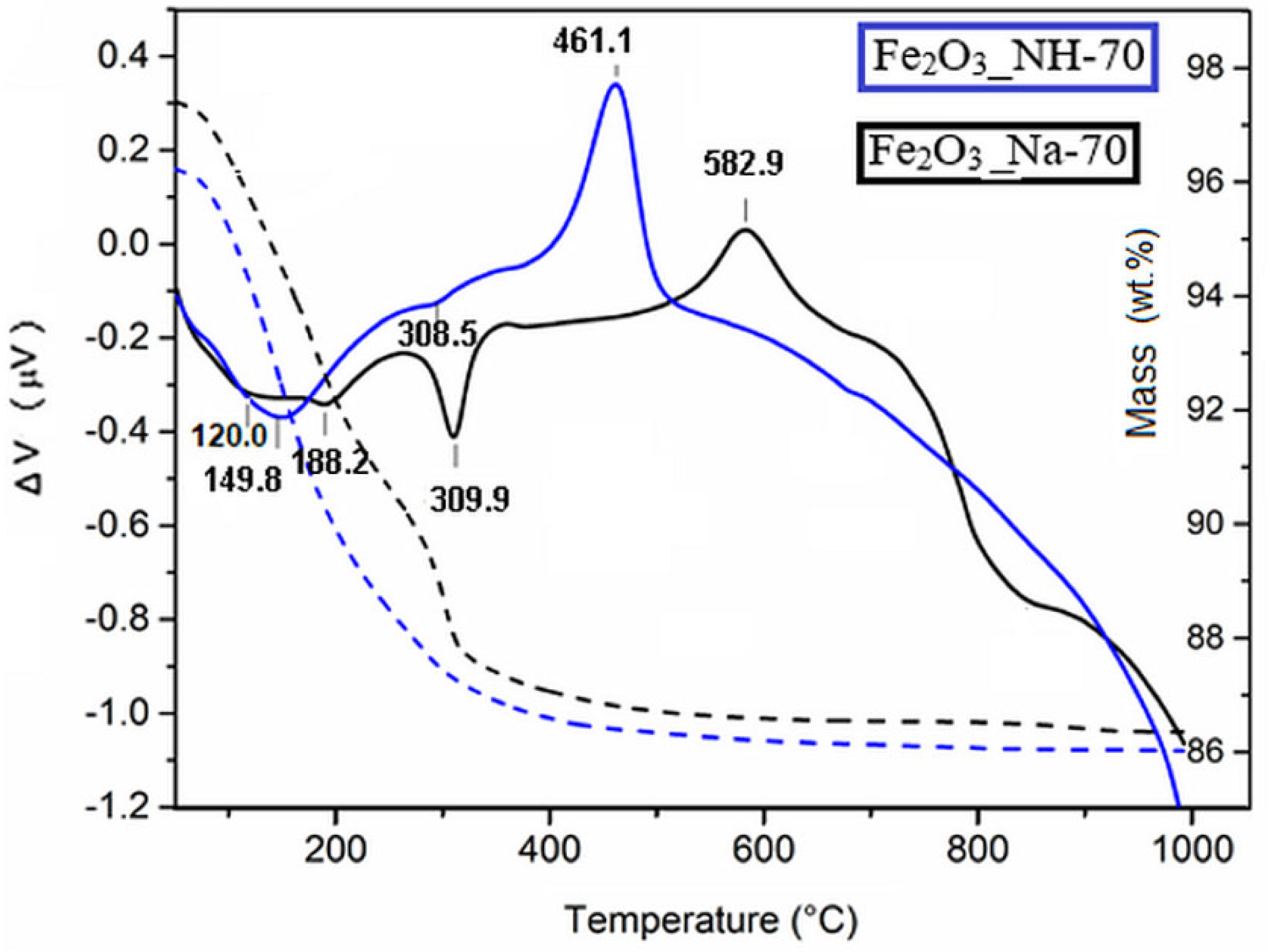
2.3. Thermal Transformation of Precipitates
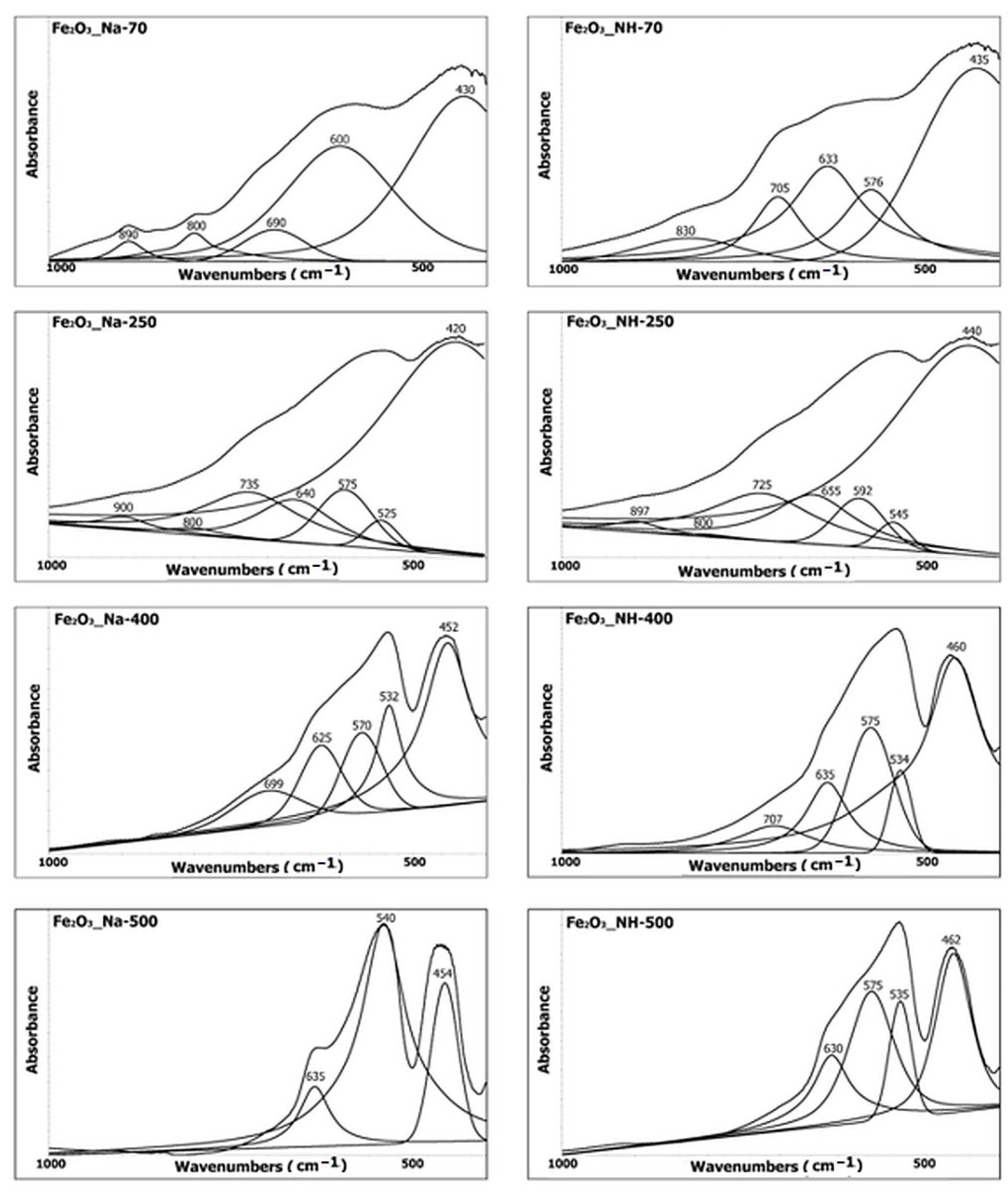
2.4. Infrared Spectroscopy Analysis of NPs
- (a)
- The stretching hydroxyl vibration region
- (b)
- The deformation hydroxyl vibration and lattice vibration in the Fe–O region
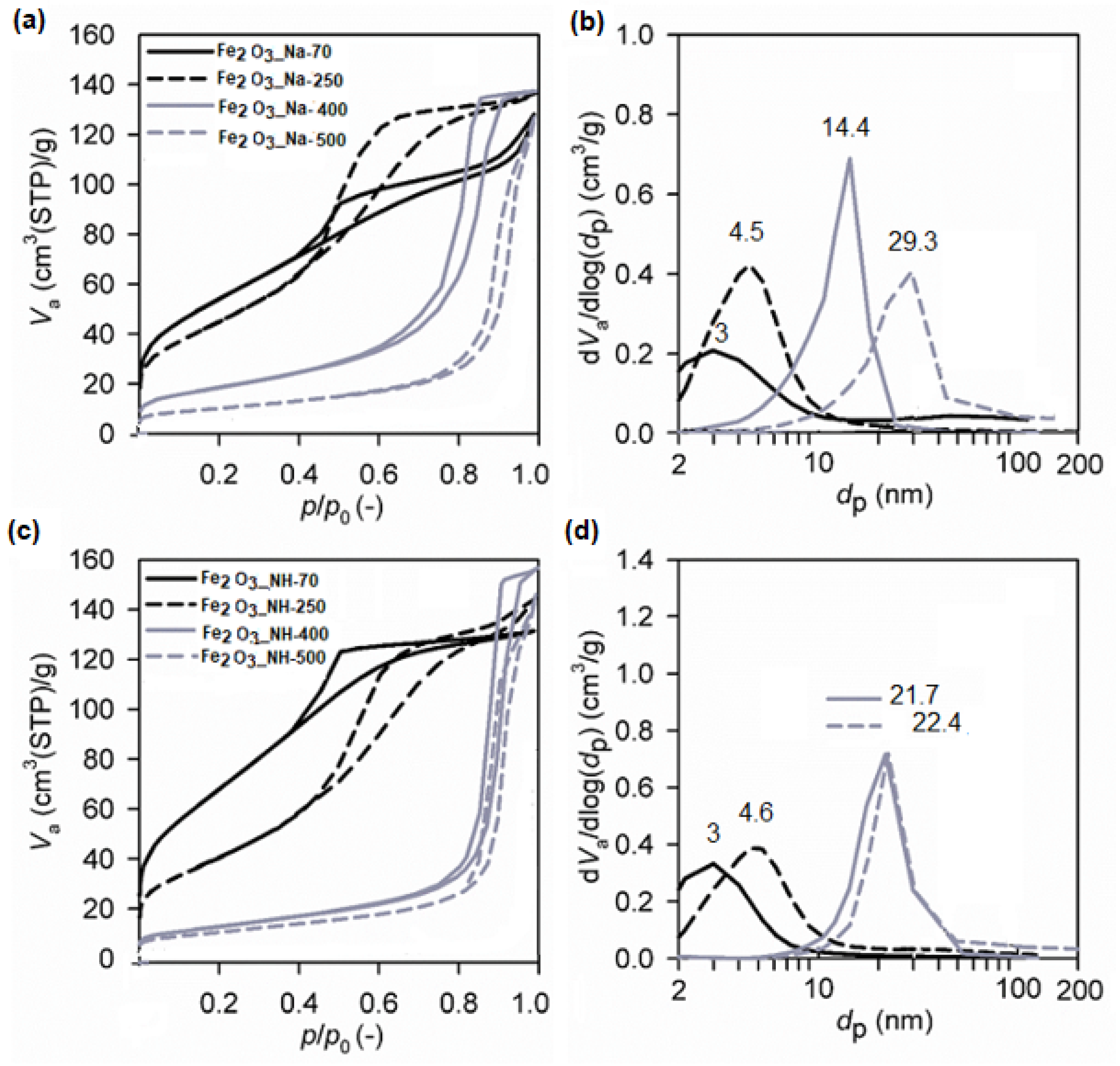
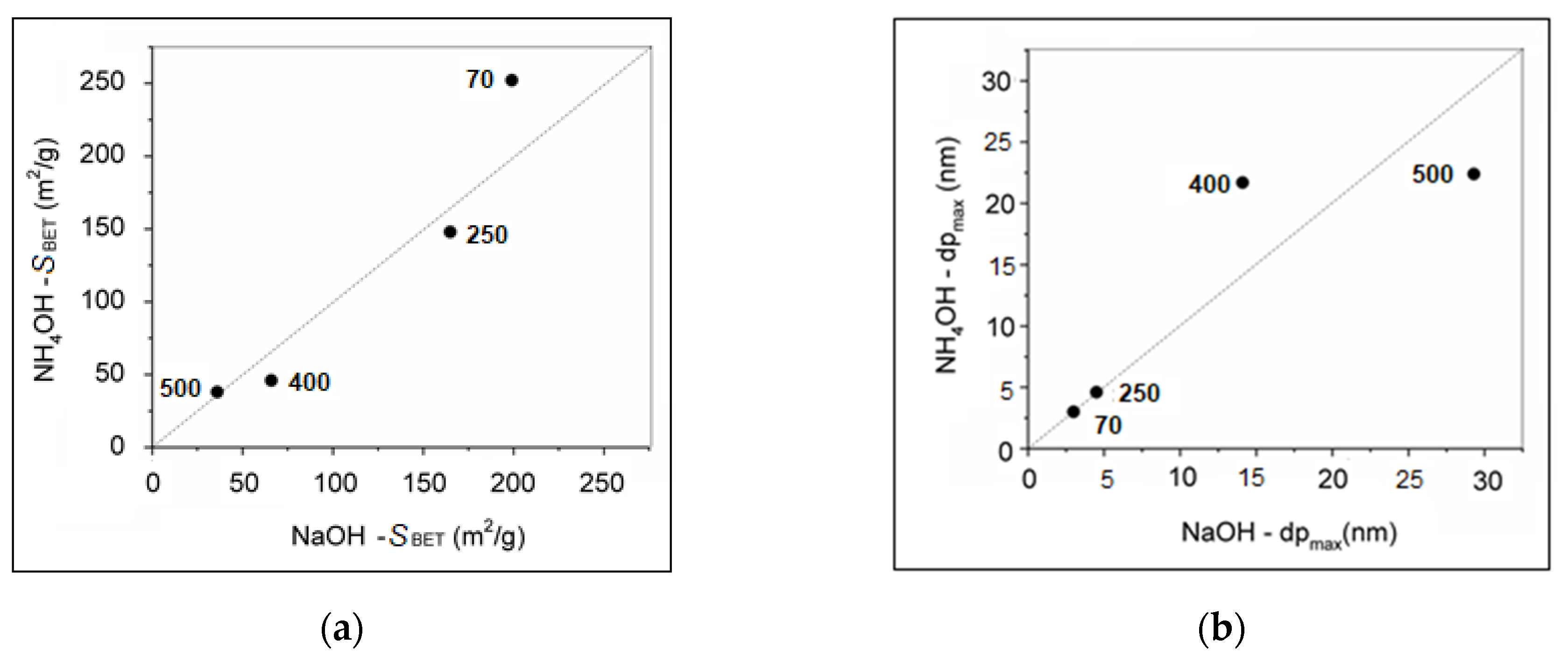
2.5. Textural Properties (SBET, Vnet) of NPs
2.6. Methane Catalytic Oxidation Test
| Sample | SBET (m2/g) | Vnet (cm3/g) | dp (cm3/g) | 1 D (nm) | 2 D (nm) | CH4conv (%) | H2 (vol.%) | CO2 (vol.%) | Ctot (wt.%) | Corg (%) | Cg (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3_Na-70 | 199 | 3 | 3 | 39.4 | 0.009 | 2.8 | 0.63 | 87.9 | 12.1 | ||
| Fe2O3_Na-500 | 36 | 197 | 29.3 | 51.8 ± 15.2 | 49.5 ± 0.8 | 30.7 | 0.021 | 3.1 | 0.46 | 83.6 | 16.4 |
| Fe2O3_NH-70 | 252 | 3 | 3 | 45.7 | 0.004 | 2.5 | 0.75 | 97.9 | 2.1 | ||
| Fe2O3_NH-500 | 38 | 224 | 22.4 | 31.8 ± 4.2 | 37.6 ± 0.3 | 36.4 | 0.006 | 2.4 | 1.4 | 95.8 | 4.2 |
3. Materials and Methods
3.1. Materials and Samples Preparation
3.2. Methane Catalytic Oxidation Experiment
3.3. Methods Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, M.; Chun, D.M. α- Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Valášková, M.; Tokarský, J.; Pavlovský, J.; Prostějovský, T.; Kočí, K. α-Fe2O3 nanoparticles/vermiculite clay material: Structural, optical and photocatalytic properties. Materials 2019, 12, 1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.Z.; Yin, P.; Zhu, X.; OuYang, C.Z.; Xie, Y. Synthesis of hematite (alpha-Fe2O3) nanorods: Diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors. J. Phys. Chem. B 2006, 110, 17806–17812. [Google Scholar] [CrossRef]
- Fang, X.L.; Chen, C.; Jin, M.S.; Kuang, Q.; Xie, Z.X.; Xie, S.Y.; Huang, R.B.; Zeng, L.S. Single-crystal-like hematite colloidal nanocrystal clusters: Synthesis and application in gas sensors, photocatalysis and water treatment. J. Mater. Chem. 2009, 19, 6154–6160. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba, R.; Rao, G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Foster, M.; Ling, Y.C.; Wang, G.M.; Zhai, T.; Tong, Y.X.; Cowan, A.J.; Li, Y. Acid treatment enables suppression of electron-hole recombination in hematite for photoelectrochemical water splitting. Angew. Chem. Int. Edit. 2016, 553, 403–3407. [Google Scholar]
- Choi, Y.; Jeon, D.; Choi, Y.; Kim, D.; Kim, N.; Gu, M.; Bae, S.; Lee, T.; Lee, H.W.; Kim, B.S.; et al. Interface engineering of hematite with nacre-like catalytic multilayers for solar water oxidation. ASC Nano 2019, 13, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.T.; Lin, Y.; Yuan, G.; Wang, D. Forming heterojunctions at the nanoscale for improved photoelectrochemical water splitting by semiconductor materials: Case studies on hematite. Acc. Chem. Res. 2013, 46, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Reli, M.; Ambrožová, N.; Valášková, M.; Edelmannová, M.; Čapek, L.; Schimpf, C.; Motylenko, M.; Rafaja, D.; Kočí, K. Photocatalytic water splitting over CeO2/Fe2O3/Ver photocatalysts. Energy Convers. Manag. 2021, 238, 114156. [Google Scholar] [CrossRef]
- Brown, A.; Hargreaves, J.; Rijniersce, B. A study of the structural and catalytic effects of sulfation on iron oxide catalysts prepared from goethite and ferrihydrite precursors for methane oxidation. Catal. Lett. 1998, 53, 7–13. [Google Scholar] [CrossRef]
- Barbosa, A.L.; Herguido, J.; Santamaria, J. Methane combustion over unsupported iron oxide catalysts. Catal. Today 2001, 64, 43–50. [Google Scholar] [CrossRef]
- He, Y.; Guo, F.; Yang, K.R.; Heinlein, J.A.; Bamonte, S.M.; Fee, J.J.; Hu, S.; Suib, S.L.; Haller, G.L.; Batista, V.S.; et al. In situ identification of reaction intermediates and mechanistic understandings of methane oxidation over hematite: A combined experimental and theoretical study. J. Am. Chem. Soc. 2020, 142, 17119–17130. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.J.; Posner, A.M.; Quirk, J.P. Adsorption of potential determining ions at the ferric oxide-aqueous electrolyte interface. J. Phys. Chem. 1967, 71, 550–558. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Y.; Sun, Y. The Formation of hematite from ferrihydrite using Fe(II) as a catalyst. J. Mol. Catal. Chem. 2005, 226, 135–140. [Google Scholar] [CrossRef]
- Han, L.; Liu, H.; Wei, Y. In situ synthesis of hematite nanoparticles using a low-temperature microemulsion method. Powder Technol. 2011, 207, 42–46. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Tang, J.Y.; Hu, X.Y. Controllable synthesis and magnetic properties of pure hematite and maghemite nanocrystals from a molecular precursor. J. Alloys Compd. 2008, 462, 24–28. [Google Scholar] [CrossRef]
- Fiore, A.M.; Varvaro, G.; Agostinelli, E.; Mangone, A.; De Giglio, E.; Terzano, R.; Allegretta, I.; Dell’Anna, M.M.; Fiore, S.; Mastrorilli, P. Synthesis and use in catalysis of hematite nanoparticles obtained from a polymer supported Fe(III) complex. Eur. J. Inorg. Chem. 2022, 7, e202100943. [Google Scholar] [CrossRef]
- Su, D.; Kim, H.S.; Kim, W.S.; Wang, G. Synthesis of tuneable porous hematites (α-Fe2O3) for gas sensing and lithium storage in lithium ion batteries. Micropor. Mesopor. Mat. 2012, 149, 36–45. [Google Scholar] [CrossRef]
- Supattarasakda, K.; Petcharoen, K.; Permpool, T.; Sirivat, A.; Lerdwijitjarud, W. Control of hematite nanoparticle size and shape by the chemical precipitation method. Powder Technol. 2013, 249, 353–359. [Google Scholar] [CrossRef]
- Matijevicć, E.; Scheiner, P. Ferric hydrous oxide sols: III. Preparation of uniform particles by hydrolysis of Fe(III)-chloride-nitrate, and -perchlorate solutions. J. Colloid Interf. Sci. 1978, 63, 509–524. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gardi, A.; Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Schwertmann, U.; Murad, E. Effect of pH on the formation of goethite and hematite from ferrihydrite. Clays Clay Miner. 1983, 31, 277–284. [Google Scholar] [CrossRef]
- Peterson, K.M.; Heaney, P.J.; Post, J.E.; Eng, P.J. A refined monoclinic structure for a variety of “hydrohematite”. Am. Mineral. 2015, 100, 570–579. [Google Scholar] [CrossRef]
- Ståhl, K.; Nielsen, K.; Jiang, J.; Lebech, B.; Hanson, J.C.; Norby, P.; van Lanschot, J. On the akaganéite crystal structure, phase transformations and possible role in post-excavational corrosion of iron artifacts. Corros. Sci. 2003, 45, 2563–2575. [Google Scholar] [CrossRef]
- Wolska, E. The structure of hydrohematite. Z. Krist. Cryst. Mater. 1981, 154, 69–75. [Google Scholar] [CrossRef]
- Wolska, E.; Schwertmann, U. Nonstoichiometric structures during dehydroxylation of goethite. Z. Krist. Cryst. Mater. 1989, 189, 223–237. [Google Scholar] [CrossRef]
- Dang, M.Z.; Rancourt, D.G.; Dutrizac, J.E.; Lamarche, G.; Provencher, R. Interplay of surface conditions, particle size, stoichiometry, cell parameters, and magnetism in synthetic hematite-like materials. Hyperfine Interact. 1998, 117, 271–319. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Gorte, R.J.; Fornasiero, P. Catalytic oxidation of methane: Pd and beyond. Eur. J. Inorg. Chem. 2018, 2018, 2884–2893. [Google Scholar] [CrossRef]
- Song, C.; Liu, F.; Kang, W.; Zhao, J.; Yang, L.; Guo, C. A novel concept for ultra-low concentration methane treatment based on chemical looping catalytic oxidation. Fuel Process. Technol. 2022, 228, 107159. [Google Scholar] [CrossRef]
- Farrauto, R.J. Low-temperature oxidation of methane. Science 2012, 337, 659–660. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R.; Richards, G.; Carpenter, S. Kinetics of the reduction of hematite (Fe2O3) by methane (CH4) during chemical looping combustion: A global mechanism. Chem. Eng. J. 2013, 232, 478–487. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Peterson, K.M.; Heaney, P.J.; Post, J.E. Evolution in the structure of akaganeite and hematite during hydrothermal growth: An in situ synchrotron X-ray diffraction analysis. Powder Diffr. 2018, 33, 287–297. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. Topotactic transformations of goethite and lepidocrocite into hematite and maghemite. Solid State Sci. 2005, 7, 520–529. [Google Scholar] [CrossRef]
- Zhang, W.J.; Huo, C.F.; Feng, G.; Li, Y.W.; Wang, J.; Jiao, H. Dehydration of goethite to hematite from molecular dynamic simulation. J. Mol. Struct. TEOCHEM 2010, 950, 20–26. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Neumann, S.; Menter, C.; Mahmoud, A.S.; Segets, D.; Rafaja, D. Microstructure characteristics of non-monodisperse quantum dots: On the potential of transmission electron microscopy combined with X-ray diffraction. CrystEngComm 2020, 22, 3644–3655. [Google Scholar] [CrossRef]
- Gialanella, S.; Girardi, F.; Ischia, G.; Lonardelli, I.; Mattarelli, M.; Montagna, M. On the goethite to hematite phase transformation. J. Therm. Anal. Calorim. 2010, 102, 867–873. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Infrared spectroscopy of goethite dehydroxylation. II. Effect of aluminium substitution on the behaviour of hydroxyl units. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 58, 479–491. [Google Scholar] [CrossRef]
- Veneranda, M.; Aramendia, J.; Bellot-Gurlet, L.; Colomban, P.; Castro, K.; Madariaga, J.M. FTIR spectroscopic semi-quantification of iron phases: A new method to evaluate the protection ability index (PAI) of archaeological artefacts corrosion systems. Corros. Sci. 2018, 133, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Serna, C.J.; Rendon, J.L.; Iglesias, J.E. Infrared surface modes in corundum-type microcrystalline oxides. Spectrochim. Acta A Mol. Spectrosc. 1982, 38, 797–802. [Google Scholar] [CrossRef]
- Wolska, E.; Szajda, W. Structural and spectroscopic characteristics of synthetic hydrohematite. J. Mater. Sci. 1985, 20, 4407–4412. [Google Scholar] [CrossRef]
- Burgina, E.B.; Kustova, G.N.; Tsybulya, S.V.; Kryukova, G.N.; Litvak, G.S.; Isupova, L.A.; Sadykov, V.A. Structure of the metastable modification of iron (III) oxide. J. Struct. Chem. 2000, 41, 396–402. [Google Scholar] [CrossRef]
- Lee, E.H. Iron oxide catalysts for dehydrogenation of ethylbenzene in the presence of steam. Catal. Rev. 1974, 8, 285–305. [Google Scholar] [CrossRef]
- Walter, D.; Buxbaum, G.; Laqua, W. The mechanism of the thermal transformation from goethite to hematite. J. Therm. Anal. Calorim. 2001, 63, 733–748. [Google Scholar] [CrossRef]
- Katoh, M.; Orihara, M.; Moriga, T.; Nakabayashi, I.; Sugiyama, S.; Tanaka, S. In situ XRD and in situ IR spectroscopic analyses of structural change of goethite in methane oxidation. J. Solid State Chem. 2001, 156, 225–229. [Google Scholar] [CrossRef]
- Breault, R.W.; Monazam, E.R. Analysis of fixed bed data for the extraction of a rate mechanism for the reaction of hematite with methane. J. Ind. Eng. Chem. 2015, 29, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Gött. Nachr. 1918, 2, 98–100. [Google Scholar]
- Choudhary, V.R.; Patil, V.P.; Jana, P.; Uphade, B.S. Nano-gold supported on Fe2O3: A highly active catalyst for low temperature oxidative destruction of methane green house gas from exhaust/waste gase. Appl. Catal. A Gen. 2008, 350, 186–190. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Till, J.L.; Nowaczyk, N. Authigenic magnetite formation from goethite and hematite and chemical remanent magnetization acquisition. Geophys. J. Int. 2018, 213, 1818–1831. [Google Scholar] [CrossRef]
- Huang, L.; Tang, M.; Fan, M.; Cheng, H. Density functional theory study on the reaction between hematite and methane during chemical looping process. Appl. Energy 2015, 159, 132–144. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valášková, M.; Leštinský, P.; Matějová, L.; Klemencová, K.; Ritz, M.; Schimpf, C.; Motylenko, M.; Rafaja, D.; Bělík, J. Hematites Precipitated in Alkaline Precursors: Comparison of Structural and Textural Properties for Methane Oxidation. Int. J. Mol. Sci. 2022, 23, 8163. https://doi.org/10.3390/ijms23158163
Valášková M, Leštinský P, Matějová L, Klemencová K, Ritz M, Schimpf C, Motylenko M, Rafaja D, Bělík J. Hematites Precipitated in Alkaline Precursors: Comparison of Structural and Textural Properties for Methane Oxidation. International Journal of Molecular Sciences. 2022; 23(15):8163. https://doi.org/10.3390/ijms23158163
Chicago/Turabian StyleValášková, Marta, Pavel Leštinský, Lenka Matějová, Kateřina Klemencová, Michal Ritz, Christian Schimpf, Mykhailo Motylenko, David Rafaja, and Jakub Bělík. 2022. "Hematites Precipitated in Alkaline Precursors: Comparison of Structural and Textural Properties for Methane Oxidation" International Journal of Molecular Sciences 23, no. 15: 8163. https://doi.org/10.3390/ijms23158163
APA StyleValášková, M., Leštinský, P., Matějová, L., Klemencová, K., Ritz, M., Schimpf, C., Motylenko, M., Rafaja, D., & Bělík, J. (2022). Hematites Precipitated in Alkaline Precursors: Comparison of Structural and Textural Properties for Methane Oxidation. International Journal of Molecular Sciences, 23(15), 8163. https://doi.org/10.3390/ijms23158163






