Malectin Domain Protein Kinase (MDPK) Promotes Rice Resistance to Sheath Blight via IDD12, IDD13, and IDD14
Abstract
:1. Introduction
2. Results
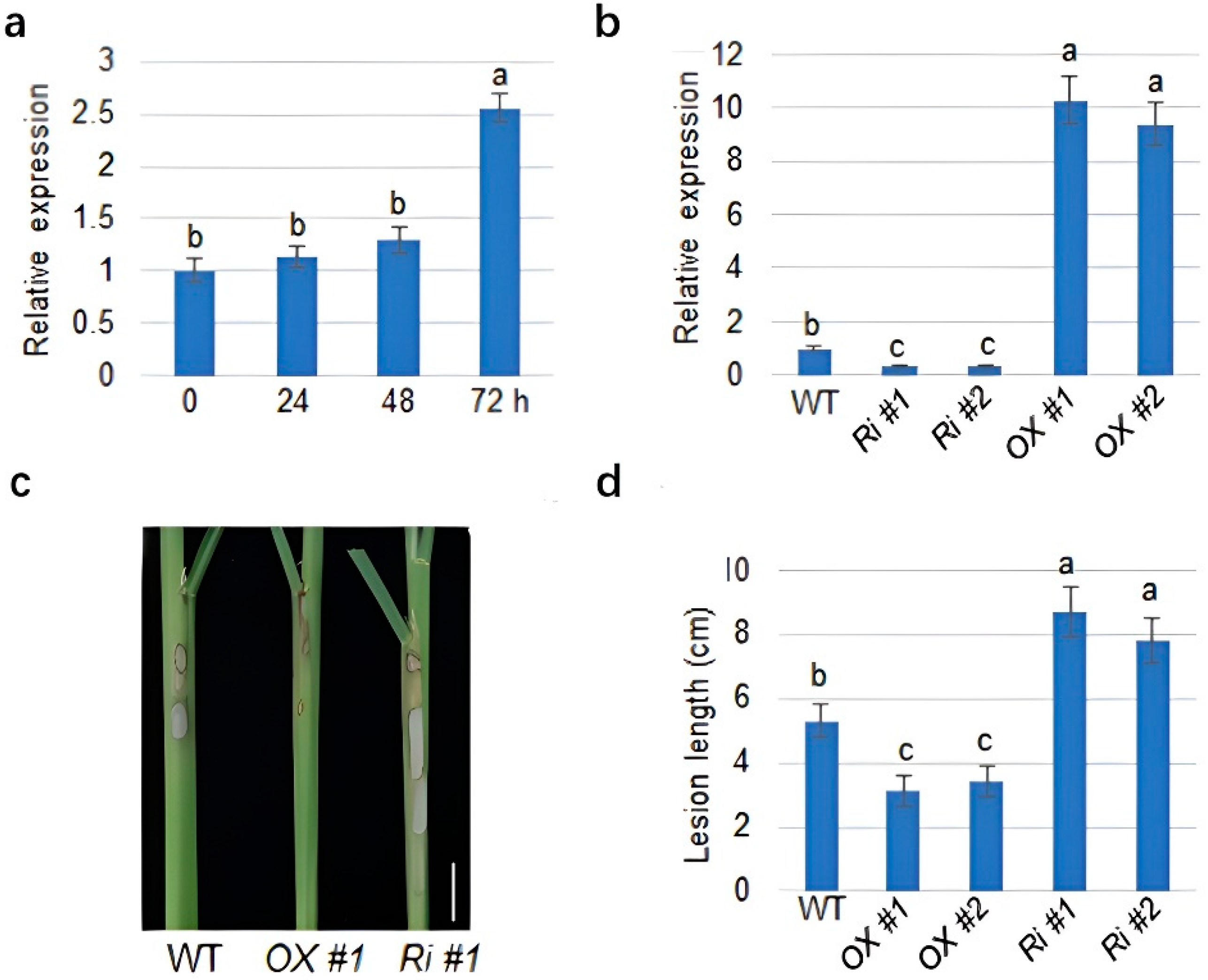
2.1. MDPK Positively Regulates Rice Resistance to ShB
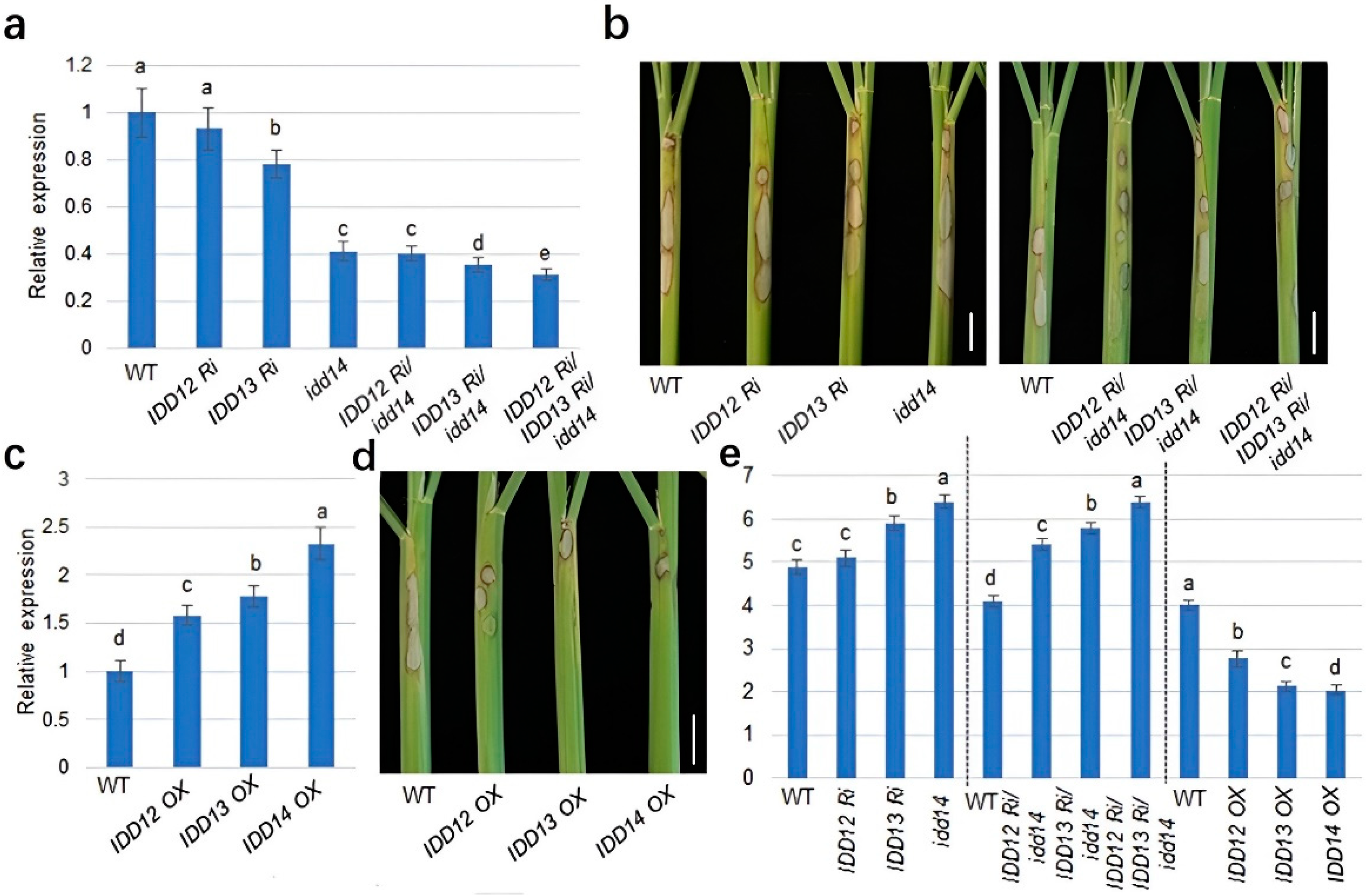
2.2. IDD12, IDD13, and IDD14 Activate MDPK Transcription
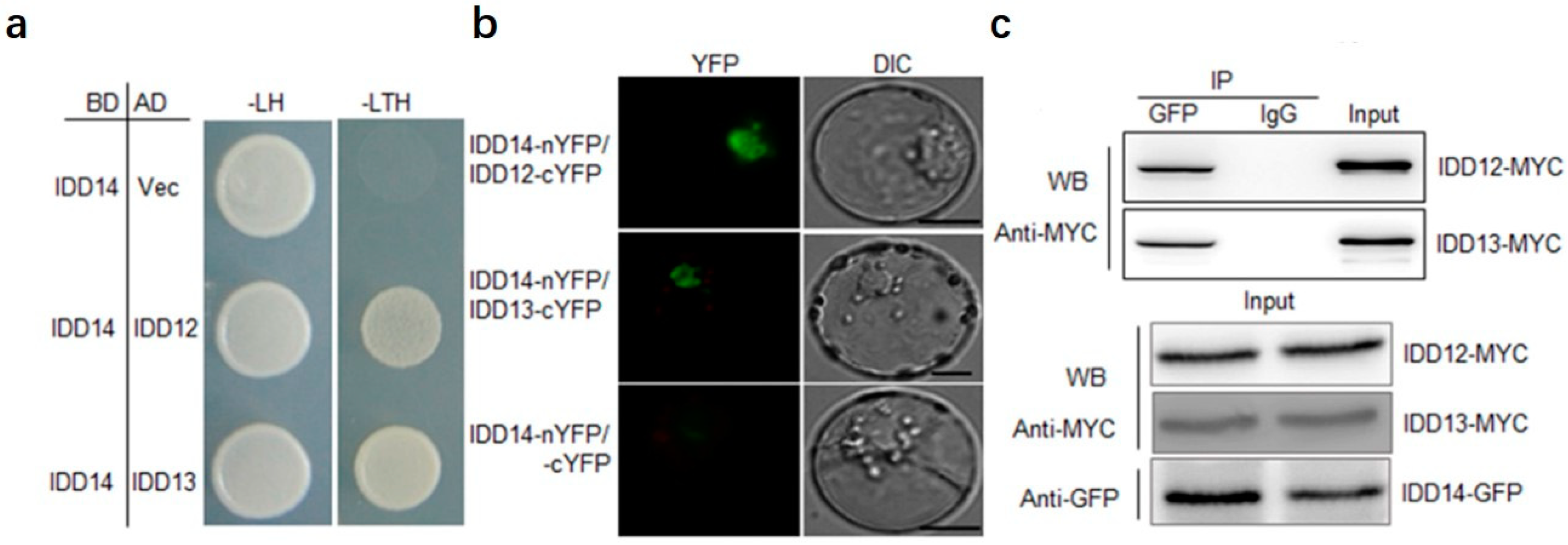
2.3. IDD14 Interacts with IDD12 and IDD13
2.4. IDD12, IDD13, and IDD14 Positively Regulate Rice Defense against ShB
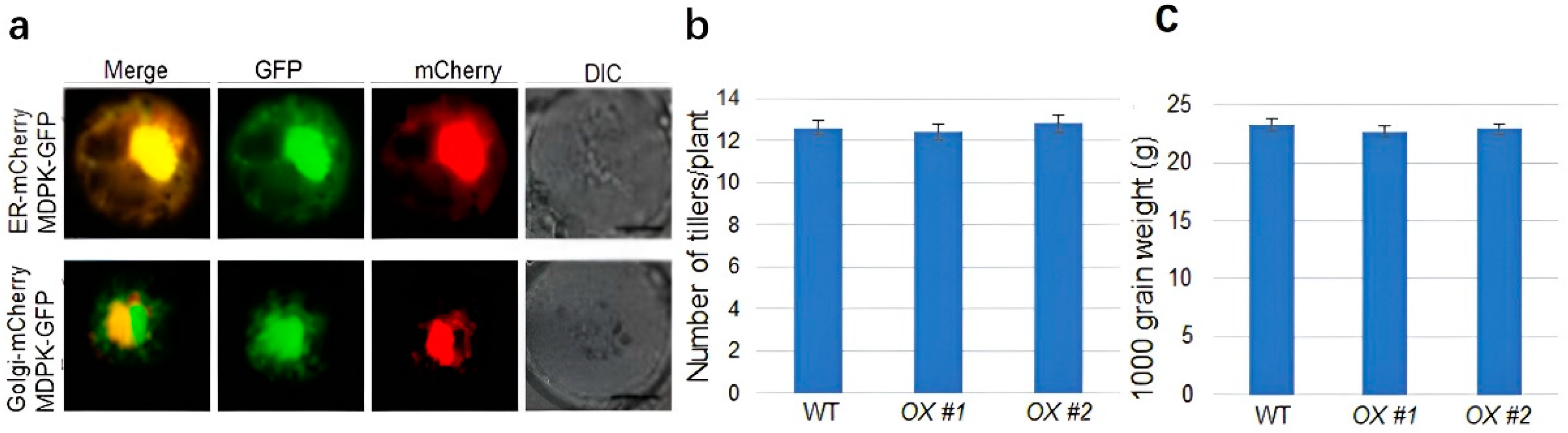
2.5. MDPK Promotes Rice Resistance to ShB without Affecting Yield
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation and R. solani Inoculation
4.2. Plasmid Construction and Rice Transformation
4.3. Real-Time Quantitative PCR (qRT-PCR)
4.4. Yeast One-Hybrid (Y1H) Assay and cDNA Library Construction
4.5. Electrophoretic Mobility Shift Assay (EMSA)
4.6. Transactivation Activity Assay
4.7. Chromatin Immunoprecipitation (ChIP) Assay
4.8. Yeast Two-Hybrid (Y2H) Assay
4.9. Bimolecular Fluorescence Complementation (BiFC) Assay
4.10. Coimmunoprecipitation (Co-IP)
4.11. Subcellular Localization
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takatsuji, H. Development of disease-resistant rice using regulatory components of induced disease resistance. Front. Plant Sci. 2014, 5, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.H.; Pan, X.B.; Chen, Z.X.; Xu, J.Y.; Lu, J.F.; Zhai, W.X.; Zhu, L.H. Mapping quantitative trait loci controlling sheath blight resistance in two rice cultivars (Oryza sativa L.). Theor. Appl. Genet. 2000, 101, 569–573. [Google Scholar] [CrossRef]
- Molla, K.A.; Karmakar, S.; Molla, J.; Bajaj, P.; Varshney, R.K.; Datta, S.K.; Datta, K. Understanding sheath blight resistance in rice: The road behind and the road ahead. Plant Biotechnol. J. 2020, 18, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Taguchi-Shiobara, F.; Ozaki, H.; Sato, H.; Maeda, H.; Kojima, Y.; Ebitani, T.; Yano, M. Mapping and validation of QTLs for rice sheath blight resistance. Breed. Sci. 2013, 63, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouzai, Y.; Kimura, M.; Watanabe, M.; Kusunoki, K.; Osaka, D.; Suzuki, T.; Matsui, H.; Yamamoto, M.; Ichinose, Y.; Toyoda, K.; et al. Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon. New Phytol. 2018, 217, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Lin, B.; Wang, H.; Li, X.; Yang, F.; Ding, X.; Yan, J.; Chu, Z. Natural variation in ZmFBL41 confers banded leaf and sheath blight resistance in maize. Nat. Genet. 2019, 51, 1540–1548. [Google Scholar] [CrossRef]
- Wang, A.; Shu, X.; Jing, X.; Jiao, C.; Chen, L.; Zhang, J.; Ma, L.; Jiang, Y.; Yamamoto, N.; Li, S.; et al. Identification of rice (Oryza sativa L.) genes involved in sheath blight resistance via a genome-wide association study. Plant Biotechnol. J. 2021, 19, 1553–1566. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, H.; Zhou, Y.; Zhao, J.; Lu, S.; Wang, X.; Chen, X.; Yuan, L.; Guan, H.; Wang, G.; et al. Suppressing chlorophyll degradation by silencing OsNYC3 improves rice resistance to Rhizoctonia solani, the causal agent of sheath blight. Plant Biotechnol. J. 2021, 20, 335–349. [Google Scholar] [CrossRef]
- Molla, K.A.; Karmakar, S.; Chanda, P.K.; Sarkar, S.N.; Datta, S.K.; Datta, K. Tissue-specific expression of Arabidopsis NPR1 gene in rice for sheath blight resistance without compromising phenotypic cost. Plant Sci. 2016, 250, 105–114. [Google Scholar] [CrossRef]
- Sadumpati, V.; Kalambur, M.; Vudem, D.R.; Kirti, P.B.; Khareedu, V.R. Transgenic indica rice lines, expressing Brassica juncea Nonexpressor of pathogenesis-related genes 1 (BjNPR1), exhibit enhanced resistance to major pathogens. J. Biotechnol. 2013, 166, 114–121. [Google Scholar] [CrossRef]
- Zhu, G.; Liang, E.; Lan, X.; Li, Q.; Qian, J.; Tao, H.; Zhang, M.; Xiao, N.; Zuo, S.; Chen, J.; et al. ZmPGIP3 Gene Encodes a Polygalacturonase-Inhibiting Protein that Enhances Resistance to Sheath Blight in Rice. Phytopathology 2019, 109, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Xue, C.Y.; Song, H.D.; Gao, Y.; Feng, L.; Li, Y.; Xuan, Y.H. Tissue-specific activation of DOF11 promotes rice resistance to sheath blight disease and increases grain weight via activation of SWEET14. Plant Biotechnol. J. 2021, 19, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Srivastava, S.; Singh, P.C.; Mishra, A.K.; Chakrabarty, D. Functional characterization of tau class glutathione-S-transferase in rice to provide tolerance against sheath blight disease. 3 Biotech 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.J.; Chu, J.; Kumar, V.; Yuan, P.; Li, Z.M.; Mei, Q.; Xuan, Y.H. Protein Phosphatase 2A Catalytic Subunit PP2A-1 Enhances Rice Resistance to Sheath Blight Disease. Front. Genome Ed. 2021, 3, 632136. [Google Scholar] [CrossRef]
- Li, N.; Wei, S.; Chen, J.; Yang, F.; Kong, L.; Chen, C.; Ding, X.; Chu, Z. OsASR2 regulates the expression of a defence-related gene, Os2H16, by targeting the GT-1 cis-element. Plant Biotechnol. J. 2018, 16, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Anuratha, C.S.; Datta, K.; Potrykus, I.; Muthukrishnan, S.; Datta, S.K. Genetic Engineering of Rice for Resistance to Sheath Blight. Bio/Technology 1995, 13, 686–691. [Google Scholar] [CrossRef]
- Peng, X.; Hu, Y.; Tang, X.; Zhou, P.; Deng, X.; Wang, H.; Guo, Z. Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta 2012, 236, 1485–1498. [Google Scholar] [CrossRef]
- Helliwell, E.E.; Wang, Q.; Yang, Y. Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol. J. 2013, 11, 33–42. [Google Scholar] [CrossRef]
- Sun, Q.; Li, T.Y.; Li, D.D.; Wang, Z.Y.; Li, S.; Li, D.P.; Han, X.; Liu, J.M.; Xuan, Y.H. Overexpression of Loose Plant Architecture 1 increases planting density and resistance to sheath blight disease via activation of PIN-FORMED 1a in rice. Plant Biotechnol. J. 2019, 17, 855–857. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Li, D.D.; Chu, J.; Yuan, D.P.; Li, S.; Zhong, L.J.; Han, X.; Xuan, Y.H. Indeterminate Domain Proteins Regulate Rice Defense to Sheath Blight Disease. Rice 2020, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.M.; Mei, Q.; Xue, C.Y.; Wang, Z.Y.; Li, D.P.; Zhang, Y.X.; Xuan, Y.H. Mutation of G-protein gamma subunit DEP1 increases planting density and resistance to sheath blight disease in rice. Plant Biotechnol. J. 2021, 19, 418–420. [Google Scholar]
- Yuan, P.; Zhang, C.; Wang, Z.Y.; Zhu, X.F.; Xuan, Y.H. RAVL1 Activates Brassinosteroids and Ethylene Signaling to Modulate Response to Sheath Blight Disease in Rice. Phytopathology 2018, 108, 1104–1113. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Yang, S.; Guo, X.; Wang, S.; Xuan, Y. RAVL1 Activates IDD3 to Negatively Regulate Rice Resistance to Sheath Blight Disease. Rice Sci. 2021, 28, 146–155. [Google Scholar]
- Chu, J.; Xu, H.; Dong, H.; Xuan, Y.H. Loose Plant Architecture 1-Interacting Kinesin-like Protein KLP Promotes Rice Resistance to Sheath Blight Disease. Rice 2021, 14, 60. [Google Scholar] [CrossRef]
- Colasanti, J.; Tremblay, R.; Wong AY, M.; Coneva, V.; Kozaki, A.; Mable, B.K. The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger prote.ein family in higher plants. BMC Genom. 2006, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Yoshida, H.; Yano, K.; Kinoshita, S.; Kawai, K.; Koketsu, E.; Hattori, M.; Takehara, S.; Huang, J.; Hirano, K.; et al. OsIDD2, a zinc finger and INDETERMINATE DOMAIN protein, regulates secondary cell wall formation. J. Integr. Plant Biol. 2018, 60, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Feng, Z.; Meng, Y.; Bian, L.; Xie, H.; Mysore, K.S.; Liang, J. SLENDER RICE1 and Oryza sativa INDETERMINATE DOMAIN2 regulating OsmiR396 are involved in stem elongation. Plant Physiol. 2020, 182, 2213–2227. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, J.; Jing, Y.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The Arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013, 9, e1003759. [Google Scholar] [CrossRef] [Green Version]
- Dou, M.; Cheng, S.; Zhao, B.; Xuan, Y.; Shao, M. The indeterminate domain protein ROC1 regulates chilling tolerance via activation of DREB1B/CBF1 in rice. Int. J. Mol. Sci. 2016, 17, 233. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Li, L.; Zhang, S.; Shen, J.; Li, S.; Hu, S.; Peng, Q.; Xiao, J.; Wu, C. Suppressor of rid1 (SID1) shares common targets with RID1 on florigen genes to initiate floral transition in rice. PLoS Genet. 2017, 13, e1006642. [Google Scholar] [CrossRef]
- Rajaraman, J.; Douchkov, D.; Hensel, G.; Stefanato, F.L.; Gordon, A.; Ereful, N.; Caldararu, O.F.; Petrescu, A.J.; Kumlehn, J.; Boyd, L.A.; et al. An LRR/Malectin Receptor-Like Kinase Mediates Resistance to Non-adapted and Adapted Powdery Mildew Fungi in Barley and Wheat. Front. Plant Sci. 2016, 7, 1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Huang, Y.; Kong, L.; Yu, X.; Feng, B.; Liu, D.; Zhao, B.; Mendes, G.C.; Yuan, P.; Ge, D.; et al. The malectin-like receptor-like kinase LETUM1 modulates NLR protein SUMM2 activation via MEKK2 scaffolding. Nat. Plants 2020, 6, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Trigo, S.; Gray, J.E.; Smith, L.M. Conserved Roles of CrRLK1L Receptor-Like Kinases in Cell Expansion and Reproduction from Algae to Angiosperms. Front. Plant Sci. 2016, 7, 1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franck, C.M.; Westermann, J.; Boisson-Dernier, A. Plant Malectin-Like Receptor Kinases: From Cell Wall Integrity to Immunity and Beyond. Annu. Rev. Plant Biol. 2018, 69, 301–328. [Google Scholar] [CrossRef]
- Galindo-Trigo, S.; Grand, T.M.; Voigt, C.A.; Smith, L.M. A malectin domain kinesin functions in pollen and seed development in Arabidopsis. J. Exp. Bot. 2020, 71, 1828–1841. [Google Scholar] [CrossRef]
- Yang, H.; Wang, D.; Guo, L.; Pan, H.; Yvon, R.; Garman, S.; Wu, H.M.; Cheung, A.Y. Malectin/Malectin-like domain-containing proteins: A repertoire of cell surface molecules with broad functional potential. Cell Surf. 2021, 7, 100056. [Google Scholar] [CrossRef]
- Feng, H.; Qiu, T.; Yin, C.; Zhao, X.; Xu, G.; Qi, L.; Zhang, Y.; Peng, Y.; Zhao, W. The Rice Malectin Regulates Plant Cell Death and Disease Resistance by Participating in Glycoprotein Quality Control. Int. J. Mol. Sci. 2022, 23, 5819. [Google Scholar] [CrossRef]
- Xu, W.; Yang, R.; Li, M.; Xing, Z.; Yang, W.; Chen, G.; Guo, H.; Gong, X.; Du, Z.; Zhang, Z.; et al. Transcriptome phase distribution analysis reveals diurnal regulated biological processes and key pathways in rice flag leaves and seedling leaves. PLoS ONE 2011, 6, e17613. [Google Scholar] [CrossRef] [Green Version]
- De Yuan, P.; Xu, X.F.; Hong, W.J.; Wang, S.T.; Jia, X.T.; Liu, Y.; Li, S.; Li, Z.M.; Sun, Q.; Mei, Q.; et al. Transcriptome analysis of rice leaves in response to Rhizoctonia solani infection and reveals a novel regulatory mechanism. Plant Biotechnol. Rep. 2020, 14, 559–573. [Google Scholar] [CrossRef]
- Gutjahr, C.; Banba, M.; Croset, V.; An, K.; Miyao, A.; An, G.; Hirochika, H.; Imaizumi-Anraku, H.; Paszkowski, U. Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 2008, 20, 2989–3005. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.; Liu, W.; Wang, G.L. Balancing Immunity and Yield in Crop Plants. Trends Plant Sci. 2017, 22, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Fu, Y.W.; Li, T.M.; Xuan, Y.H. Ac/Ds-Induced Receptor-like Kinase Genes Deletion Provides Broad-Spectrum Resistance to Bacterial Blight in Rice. Int. J. Mol. Sci. 2022, 23, 4561. [Google Scholar] [CrossRef] [PubMed]
- Il Je, B.; Piao, H.L.; Park, S.J.; Park, S.H.; Kim, C.M.; Xuan, Y.H.; Park, S.H.; Huang, J.; Do Choi, Y.; An, G.; et al. RAV-Like1 Maintains Brassinosteroid Homeostasis via the Coordinated Activation of BRI1 and Biosynthetic Genes in Rice. Plant Cell 2010, 22, 1777–1791. [Google Scholar]
- Yamaguchi, M.; Ohtani, M.; Mitsuda, N.; Kubo, M.; Ohme-Takagi, M.; Fukuda, H.; Demura, T. VND-INTERACTING2, a NAC Domain Transcription Factor, Negatively Regulates Xylem Vessel Formation in Arabidopsis. Plant Cell 2010, 22, 1249–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, Y.H.; Priatama, R.A.; Huang, J.; Je, B.I.; Liu, J.M.; Park, S.J.; Piao, H.L.; Son, D.Y.; Lee, J.J.; Park, S.H.; et al. Indeterminate domain 10 regulates ammonium-mediated gene expression in rice roots. New Phytol. 2013, 197, 791–804. [Google Scholar] [CrossRef]
- Yoo, S.-D.; Cho, Y.-H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Xue, C.; Mei, Q.; Xuan, Y. Malectin Domain Protein Kinase (MDPK) Promotes Rice Resistance to Sheath Blight via IDD12, IDD13, and IDD14. Int. J. Mol. Sci. 2022, 23, 8214. https://doi.org/10.3390/ijms23158214
Cui Z, Xue C, Mei Q, Xuan Y. Malectin Domain Protein Kinase (MDPK) Promotes Rice Resistance to Sheath Blight via IDD12, IDD13, and IDD14. International Journal of Molecular Sciences. 2022; 23(15):8214. https://doi.org/10.3390/ijms23158214
Chicago/Turabian StyleCui, Zhibo, Caiyun Xue, Qiong Mei, and Yuanhu Xuan. 2022. "Malectin Domain Protein Kinase (MDPK) Promotes Rice Resistance to Sheath Blight via IDD12, IDD13, and IDD14" International Journal of Molecular Sciences 23, no. 15: 8214. https://doi.org/10.3390/ijms23158214
APA StyleCui, Z., Xue, C., Mei, Q., & Xuan, Y. (2022). Malectin Domain Protein Kinase (MDPK) Promotes Rice Resistance to Sheath Blight via IDD12, IDD13, and IDD14. International Journal of Molecular Sciences, 23(15), 8214. https://doi.org/10.3390/ijms23158214





