Sogatella furcifera Saliva Mucin-like Protein Is Required for Feeding and Induces Rice Defences
Abstract
:1. Introduction
2. Results
2.1. SFMLP Is Highly Expressed in WBPH Salivary Glands
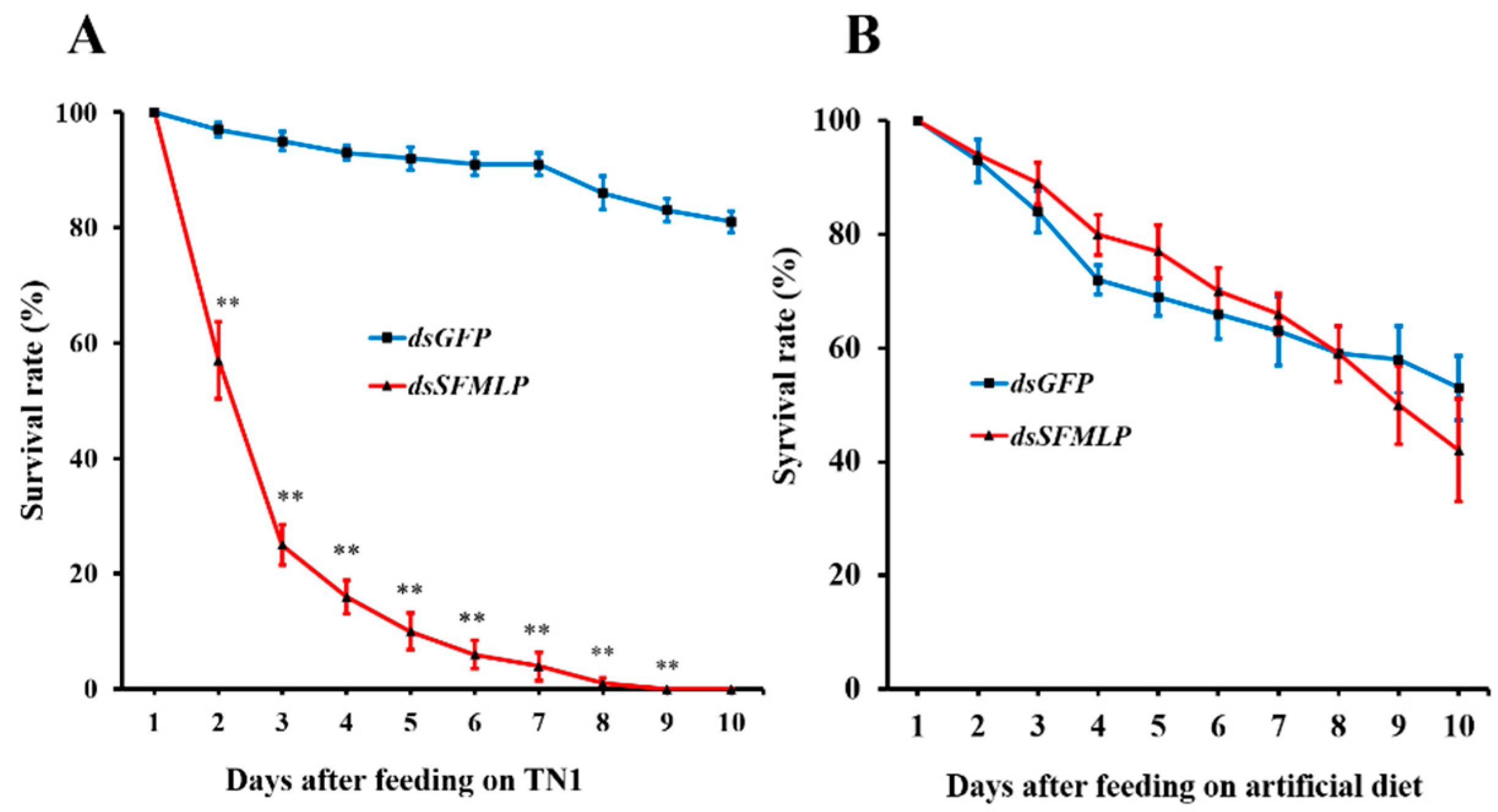
2.2. Bioassay of WBPH Injection with dsSFMLP
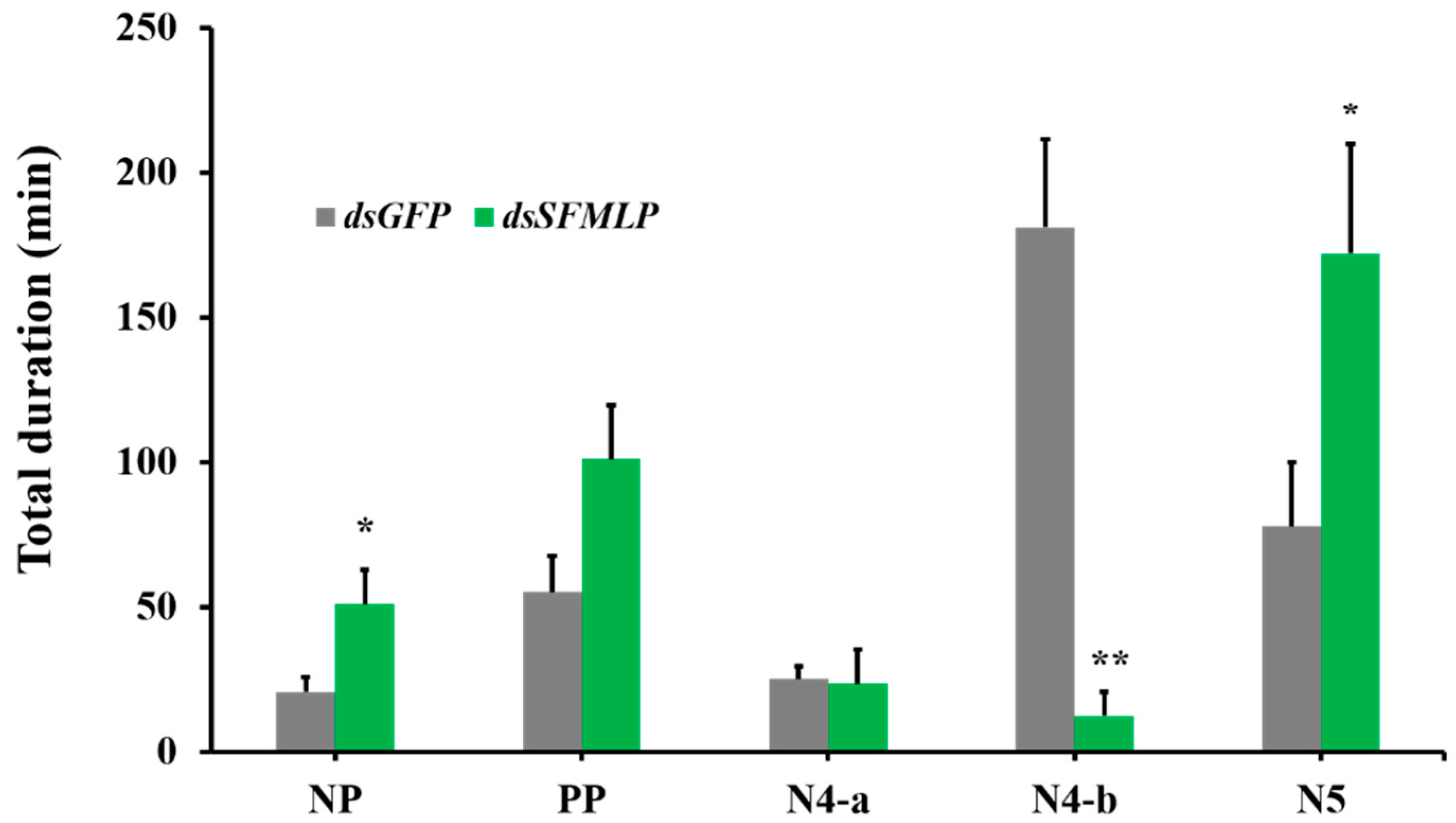
2.3. The Effects of SFMLP Knockdown on Feeding Behavior
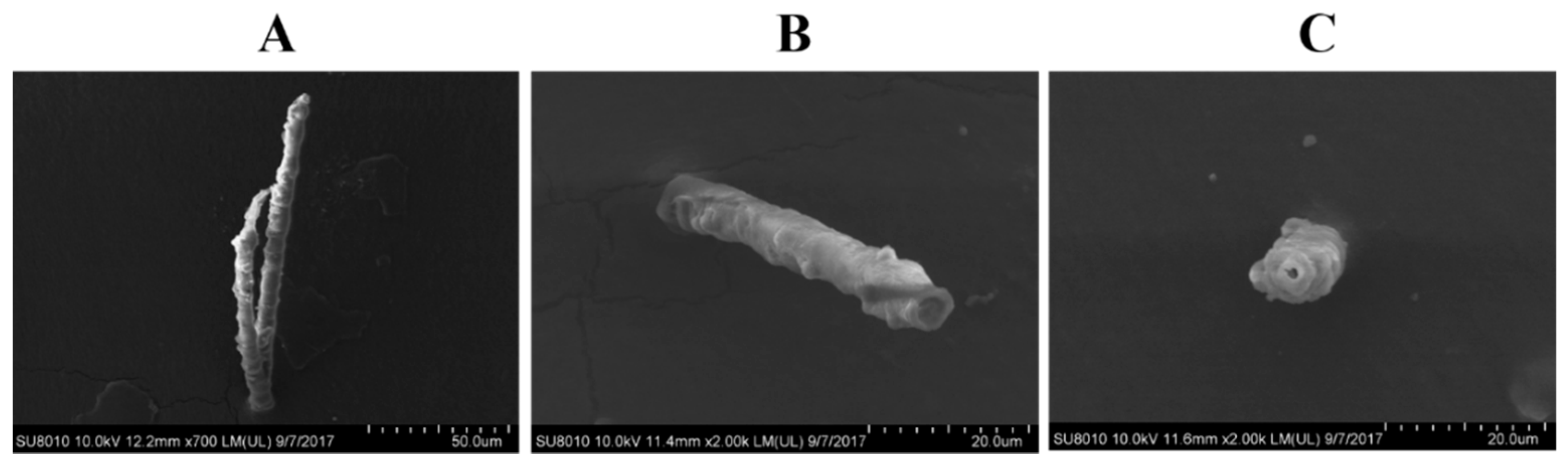
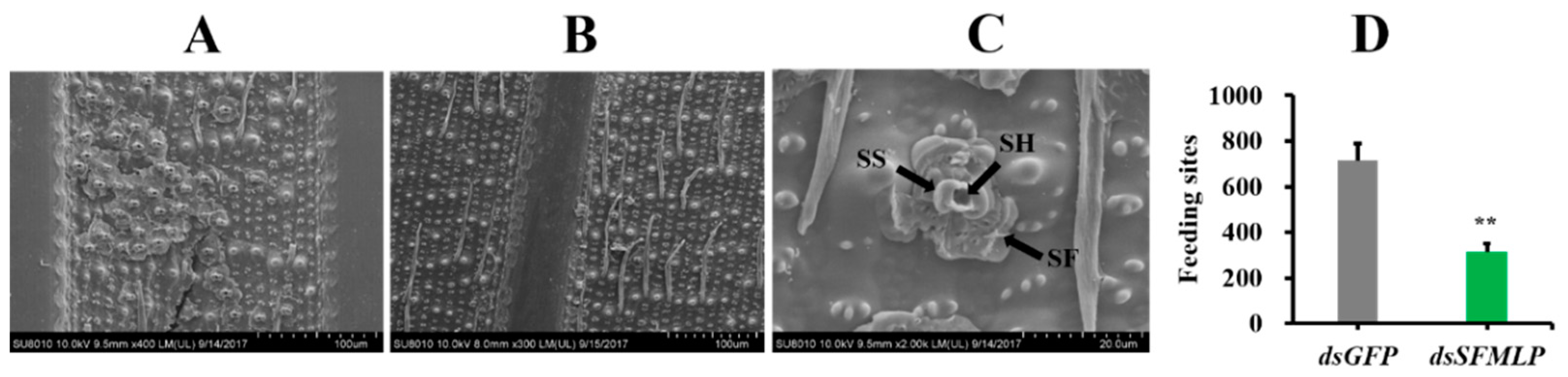
2.4. SFMLP Is Necessary for Salivary Sheath Formation
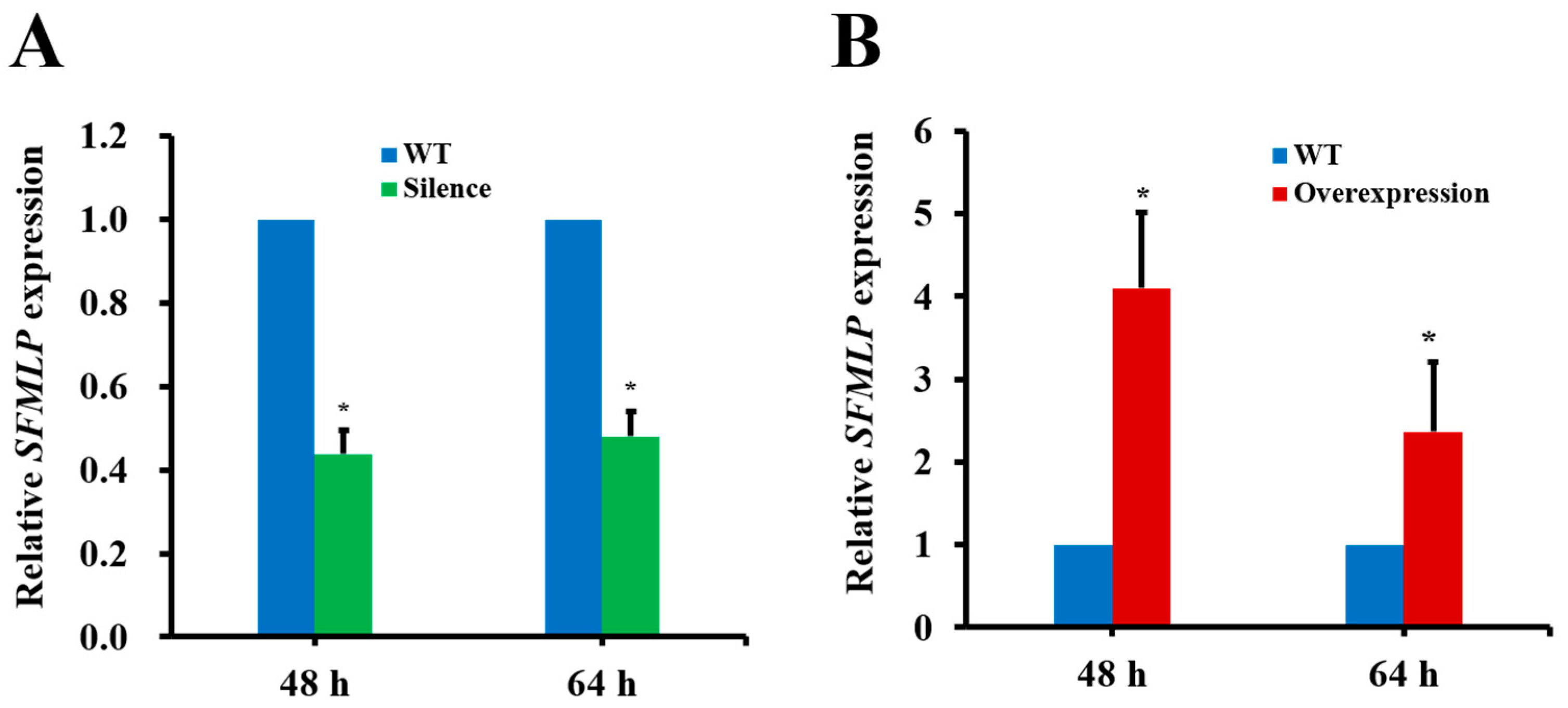
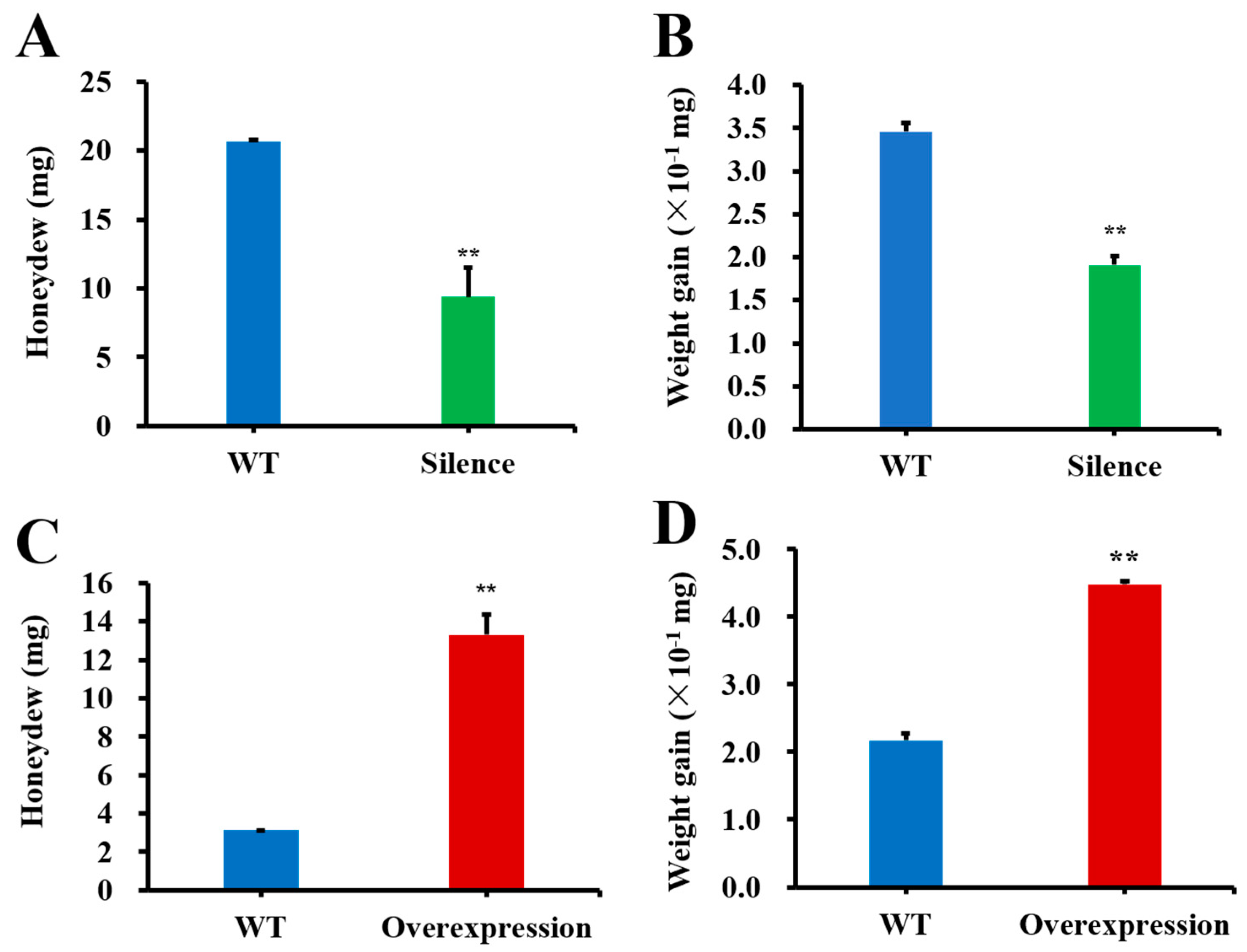
2.5. The Influences of SFMLP Silencing and Overexpression Mediated by Transgenic Rice on WBPH Performance
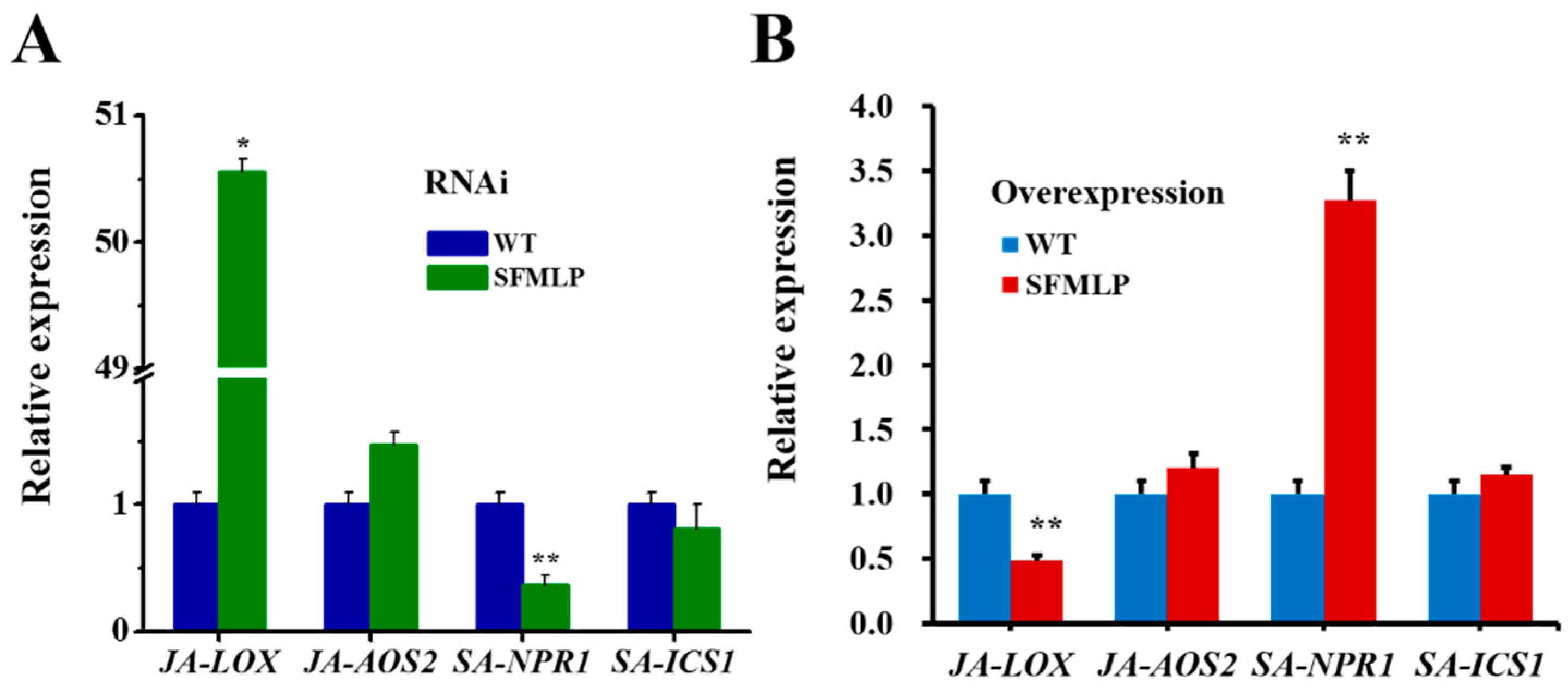
2.6. Transgenic Rice-Mediated Plant Defence Responses
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Insects
4.2. RNAi Experiment
4.3. Bioassay of WBPH Injected with dsRNA
4.4. EPG Recording of WBPH Feeding Behavior
4.5. Observation of Salivary Glands and Salivary Sheaths of WBPH Injected with dsRNA
4.6. Transgenic Rice Plant Development and the WBPH Bioassay
4.7. Analysis of the Relative Expression of Phytohormone-Related Genes
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felton, G.W.; Chung, S.H.; Hernandez, M.G.E.; Louis, J.; Peiffer, M.; Tian, D. Herbivore oral secretions are the first line of protection against plant-induced defences. Annu. Plant Rev. 2014, 47, 37–76. [Google Scholar]
- Miles, P.W. Aphid saliva. Biol. Rev. Camb. Philos. Soc. 1999, 74, 41–85. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Van der Hoorn, R.A.; Terauchi, R.; Kamoun, S. Emerging concepts in effector biology of plant-associated organisms. Mol. Plant Microbe Interact. 2009, 22, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Hao, P.; Liu, C.; Wang, Y.; Chen, R.; Tang, M.; Du, B.; Zhu, L.; He, G. Herbivore-induced callose deposition on the sieve plates of rice: An important mechanism for host resistance. Plant Physiol. 2008, 146, 1810–1820. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.; Ye, W.; Chen, H.; Zeng, J.; Li, H.; Yu, H.; Li, J.; Lou, Y. A salivary endo-β-1,4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol. 2017, 173, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Yu, H.; Jian, Y.; Zeng, J.; Ji, R.; Chen, H.; Lou, Y. A salivary EF-hand calcium-binding protein of the brown planthopper Nilaparvata lugens functions as an effector for defense responses in rice. Sci. Rep. 2017, 7, 40498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.J.; Liu, C.W.; Huang, X.H.; Zhou, X.; Zhuo, J.C.; Zhang, C.X.; Bao, Y.Y. Screening and functional analyses of Nilaparvata lugens salivary proteome. J. Proteome Res. 2016, 15, 1883–1896. [Google Scholar] [CrossRef]
- Shangguan, X.; Zhang, J.; Liu, B.; Zhao, Y.; Wang, H.; Wang, Z.; Guo, J.; Rao, W.; Jing, S.; Guan, W.; et al. A mucin-like protein of planthopper is required for feeding and induces immunity response in plants. Plant Physiol. 2018, 176, 552–565. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.; Fu, J.; Shi, Y.; Li, J.; Jing, M.; Wang, L.; Yang, S.; Tian, T.; Wang, L.; Ju, J.; et al. Vitellogenin from planthopper oral secretion acts as a novel effector to impair plant defenses. New Phytol. 2021, 232, 802–817. [Google Scholar] [CrossRef]
- Tian, T.; Ji, R.; Fu, J.; Li, J.; Wang, L.; Zhang, H.; Yang, S.; Ye, W.; Fang, J.; Zhu-Salzman, K. A salivary calcium-binding protein from Laodelphax striatellus acts as an effector that suppresses defense in rice. Pest Manag. Sci. 2021, 77, 2272–2281. [Google Scholar] [CrossRef]
- Huang, H.J.; Cui, J.R.; Xia, X.; Chen, J.; Ye, Y.X.; Zhang, C.X.; Hong, X.Y. Salivary DNase II from Laodelphax striatellus acts as an effector that suppresses plant defence. New Phytol. 2019, 224, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Atamian, H.S.; Chaudhary, R.; Cin, V.D.; Bao, E.; Girke, T.; Kaloshian, I. In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol. Plant Microbe Interact. 2013, 26, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, J.I.; Prince, D.; Pitino, M.; Maffei, M.E.; Win, J.; Hogenhout, S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010, 6, e1001216. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, D.A.; De Vos, M.; Jander, G. Suppression of plant defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol. Plant Microbe Interact. 2014, 27, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Zhang, Y.; Tong, J.; Ge, P.; Wang, Q.; Zhao, Z.; Zhu-Salzman, K.; Hogenhout, S.A.; Ge, F.; Sun, Y. An aphid-secreted salivary protease activates plant defense in phloem. Curr. Biol. 2020, 30, 4826–4836. [Google Scholar] [CrossRef]
- Mutti, N.S.; Louis, J.; Pappan, L.K.; Pappan, K.; Begum, K.; Chen, M.S.; Park, Y.; Dittmer, N.; Marshall, J.; Reese, J.C.; et al. A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc. Natl. Acad. Sci. USA 2008, 105, 9965–9969. [Google Scholar] [CrossRef] [Green Version]
- Pitino, M.; Hogenhout, S.A. Aphid protein effectors promote aphid colonization in a plant species-specific manner. Mol. Plant. Microbe Interact. 2013, 26, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Walling, L.L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008, 146, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Qi, J.; Ren, N.; Cheng, J.; Erb, M.; Mao, B.; Lou, Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.; Li, F.; Liao, X.; Ali, S.; Hou, M. A virus plays a role in partially suppressing plant defenses induced by the viruliferous vectors. Sci. Rep. 2018, 8, 9027. [Google Scholar] [CrossRef]
- Xu, H.X.; Qian, L.X.; Wang, X.W.; Shao, R.X.; Hong, Y.; Liu, S.S.; Wang, X.W. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, P.J.; Thompson, G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001, 125, 1074–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.J.; Li, W.D.; Huang, F.; Zhang, J.M.; Xu, F.C.; Lu, Y.B. Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J. Chem. Ecol. 2013, 39, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Alon, M.; Malka, O.; Eakteiman, G.; Elbaz, M.; Moyal Ben Zvi, M.; Vainstein, A.; Morin, S. Activation of the phenylpropanoid pathway in Nicotiana tabacum improves the performance of the whitefly Bemisia tabaci via reduced jasmonate signaling. PLoS ONE 2013, 8, e76619. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Valenzuela, J.G.; Pham, V.M.; Garfield, M.K.; Ribeiro, J.M. Toward a catalog for the transcripts and proteins (sialome) from the salivary gland of the malaria vector Anopheles gambiae. J. Exp. Biol. 2002, 205, 2429–2451. [Google Scholar] [CrossRef]
- Thakur, N.; Upadhyay, S.K.; Verma, P.C.; Chandrashekar, K.; Tuli, R.; Singh, P.K. Enhanced whitefly resistance in transgenic tobacco plants expressing double stranded RNA of v-ATPase A gene. PLoS ONE 2014, 9, e87235. [Google Scholar] [CrossRef] [Green Version]
- Abdellatef, E.; Will, T.; Koch, A.; Imani, J.; Vilcinskas, A.; Kogel, K.H. Silencing the expression of the salivary sheath protein causes transgenerational feeding suppression in the aphid Sitobion avenae. Plant Biotechnol. J. 2015, 13, 849–857. [Google Scholar] [CrossRef]
- Pitino, M.; Coleman, A.D.; Maffei, M.E.; Ridout, C.J.; Hogenhout, S.A. Silencing of aphid genes by dsRNA feeding from plants. PLoS ONE 2011, 6, e25709. [Google Scholar] [CrossRef]
- Sun, Y.; Sparks, C.; Jones, H.; Riley, M.; Francis, F.; Du, W.; Xia, L. Silencing an essential gene involved in infestation and digestion in grain aphid through plant-mediated RNA interference generates aphid-resistant wheat plants. Plant Biotechnol. J. 2019, 17, 852–854. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Sun, X.Q.; Zhu-Salzman, K.; Qin, Q.M.; Feng, H.Q.; Kong, X.D.; Zhou, X.G.; Cai, Q.N. Host-induced gene silencing of brown planthopper glutathione S-transferase gene enhances rice resistance to sap-sucking insect pests. J. Pest Sci. 2020, 94, 769–781. [Google Scholar] [CrossRef]
- Zha, W.; Peng, X.; Chen, R.; Du, B.; Zhu, L.; He, G. Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect Nilaparvata lugens. PLoS ONE 2011, 6, e20504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.J.; Liu, C.W.; Xu, H.J.; Bao, Y.Y.; Zhang, C.X. Mucin-like protein, a saliva component involved in brown planthopper virulence and host adaptation. J. Insect Physiol. 2017, 98, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Komatsu, S.; Noda, H.; Matsumoto, Y. Proteome analysis of watery saliva secreted by green rice leafhopper, Nephotettix cincticeps. PLoS ONE 2015, 10, e0123671. [Google Scholar]
- Das, S.; Radtke, A.; Choi, Y.J.; Mendes, A.M.; Valenzuela, J.G.; Dimopoulos, G. Transcriptomic and functional analysis of the Anopheles gambiae salivary gland in relation to blood feeding. BMC Genom. 2010, 11, 566. [Google Scholar] [CrossRef] [Green Version]
- Korayem, A.M.; Fabbri, M.; Takahashi, K.; Scherfer, C.; Lindgren, M.; Schmidt, O.; Ueda, R.; Dushay, M.S.; Theopold, U. A Drosophila salivary gland mucin is also expressed in immune tissues: Evidence for a function in coagulation and the entrapment of bacteria. Insect Biochem. Mol. Biol. 2004, 34, 1297–1304. [Google Scholar] [CrossRef]
- Carolan, J.C.; Fitzroy, C.I.; Ashton, P.D.; Douglas, A.E.; Wilkinson, T.L. The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics 2009, 9, 2457–2467. [Google Scholar] [CrossRef]
- Will, T.; Vilcinskas, A. The structural sheath protein of aphids is required for phloem feeding. Insect Biochem. Mol. Biol. 2015, 57, 34–40. [Google Scholar] [CrossRef]
- Miao, Y.T.; Deng, Y.; Jia, H.K.; Liu, Y.D.; Hou, M.L. Proteomic analysis of watery saliva secreted by white-backed planthopper, Sogatella furcifera. PLoS ONE 2018, 13, e0193831. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhou, H.; Zhao, J.; Hua, H.; He, Y. Identification of the secreted watery saliva proteins of the rice brown planthopper, Nilaparvata lugens (Stål) by transcriptome and Shotgun LC-MS/MS approach. J. Insect Physiol. 2016, 89, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhou, G.; Xiang, C.; Du, M.; Cheng, J.; Liu, S.; Lou, Y. β-Glucosidase treatment and infestation by the rice brown planthopper Nilaparvata lugens elicit similar signaling pathways in rice plants. Chin. Sci. Bull. 2008, 53, 53–57. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Z.; Meng, J.; Zhou, P.; Luo, T.; Zhang, J.; Wu, J.; Lou, Y. Rice phenolamindes reduce the survival of female adults of the white-backed planthopper Sogatella furcifera. Sci. Rep. 2020, 10, 5778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.A.; Shi, X.; Chen, D.; Yang, N.; Yin, C.F.; Yang, J.; Wang, G.F.; Huang, W.K.; Peng, H.; Peng, D.L.; et al. Host-induced silencing of a nematode chitin synthase gene enhances resistance of soybeans to both pathogenic Heterodera glycines and Fusarium oxysporum. Plant Biotechnol. J. 2022, 20, 809–811. [Google Scholar] [CrossRef]
- Coleman, A.D.; Wouters, R.H.; Mugford, S.T.; Hogenhout, S.A. Persistence and transgenerational effect of plant-mediated RNAi in aphids. J. Exp. Bot. 2015, 66, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, W.; Li, P.; Han, Y.; Gong, S.; Yang, L.; Hou, M. EPG recordings reveal differential feeding behaviors in Sogatella furcifera in response to plant virus infection and transmission success. Sci. Rep. 2016, 6, 30240. [Google Scholar] [CrossRef]
- Khan, Z.R.; Saxena, R.C. Electronically recorded waveforms associated with the feeding behavior of Sogatella furcifera (Homoptera: Delphacidae) on susceptible and resistant rice varieties. J. Econ. Entomol. 1984, 77, 1479–1482. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.Y.; Kwon, Y.H.; Jung, J.K.; Kim, G.H. Electrical penetration graphic waveforms in relation to the actual positions of the stylet tips of Nilaparvata lugens in rice tissue. J. Asia-Pac. Entomol. 2009, 12, 89–95. [Google Scholar] [CrossRef]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Excel workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Primers | Primer Sequence (5′-3′) | |
|---|---|---|
| qPCR | ||
| mucin-F | TCATCTTCTGGTGTGCATTC | |
| mucin-R | GGGGGGTGTTGAAAATAGTA | |
| L9-F | CAAGATGAGAGCCGTGTA | |
| L9-R | CGAGTTGGTAACAGTGAC | |
| L10-F | GCGACTTCATCCGTTCCA | |
| L10-R | CACTCTAGCCACTGTTCCTT | |
| SA-NPR1-F | TTTCCGATGGAGGCAAGAG | |
| SA-NPR1-R | GCTGTCATCCGAGCTAAGTGTT | |
| JA-LOX-F | GCATCCCCAACAGCACATC | |
| JA-LOX-R | AATAAAGATTTGGGAGTGACATA | |
| RNAi | ||
| dsmucin-T7-F | TAATACGACTCACTATAGGGGTACTACCCAAAACTCTCCCAAATC | |
| dsmucin-T7-R | TAATACGACTCACTATAGGGGCAAGTGCCTGAAGAACAATGAAG | |
| dsGFP-T7-F | TAATACGACTCACTATAGGGGGAGAAGAACTTTTCACTGG | |
| dsGFP-T7-R | TAATACGACTCACTATAGGGAGTTGAACGGATCCATCTTC | |
| Expression in plant | ||
| Overexpression-Mucin-F | CCG gatatc ATGAGGTGTTTCTCAGTTATCG | |
| Overexpression-Mucin-R | ATA actagt CCAGGCACTGTAACCACCTC | |
| dsmucin-F | GGATCCtctctgctgcatcatgctacgg | |
| dsmucin-R | CTCGAGccataagctccaccagctgc | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yi, J.; Jia, H.; Miao, Y.; Hou, M. Sogatella furcifera Saliva Mucin-like Protein Is Required for Feeding and Induces Rice Defences. Int. J. Mol. Sci. 2022, 23, 8239. https://doi.org/10.3390/ijms23158239
Liu Y, Yi J, Jia H, Miao Y, Hou M. Sogatella furcifera Saliva Mucin-like Protein Is Required for Feeding and Induces Rice Defences. International Journal of Molecular Sciences. 2022; 23(15):8239. https://doi.org/10.3390/ijms23158239
Chicago/Turabian StyleLiu, Yudi, Jinyu Yi, Haokang Jia, Yutong Miao, and Maolin Hou. 2022. "Sogatella furcifera Saliva Mucin-like Protein Is Required for Feeding and Induces Rice Defences" International Journal of Molecular Sciences 23, no. 15: 8239. https://doi.org/10.3390/ijms23158239
APA StyleLiu, Y., Yi, J., Jia, H., Miao, Y., & Hou, M. (2022). Sogatella furcifera Saliva Mucin-like Protein Is Required for Feeding and Induces Rice Defences. International Journal of Molecular Sciences, 23(15), 8239. https://doi.org/10.3390/ijms23158239







