Multigenerational Exposure to Uranium Changes Sperm Metabolome in Rats
Abstract
:1. Introduction
2. Results
2.1. Effects on Fertility
2.2. Uranium Quantification in Epididymis
2.3. Differences in the NU-Exposed Group in Each Generation
2.4. Identification of Metabolites Common between Generations F0, F1 and F2
2.5. Main Metabolites and Related Pathways in Each Generation
3. Discussion
4. Materials and Methods
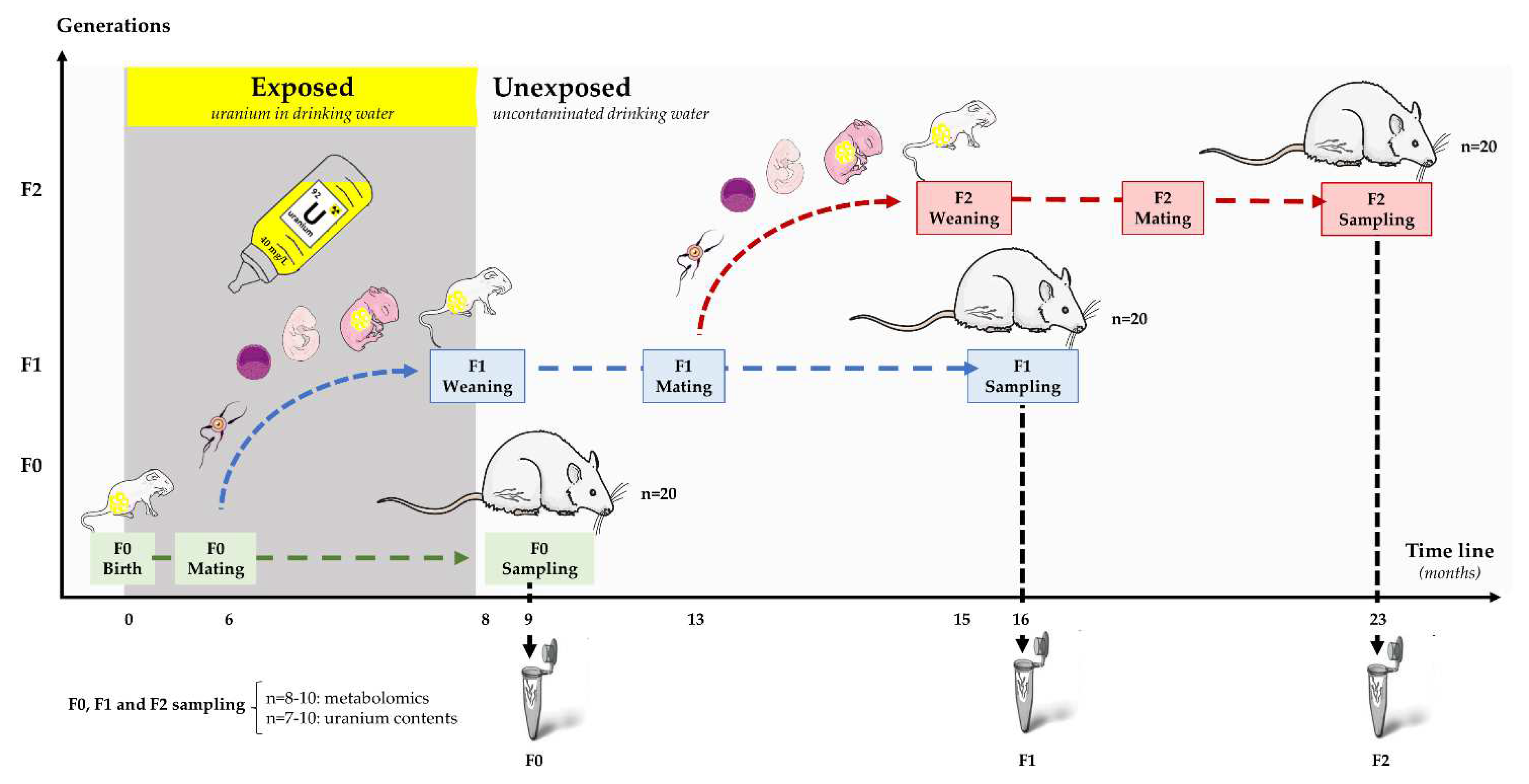
4.1. Experimental Procedure and Sample Collection
4.2. Uranium Content
4.3. Metabolomic Study
4.3.1. Sample Preparation
4.3.2. Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry
4.3.3. Data Pre-Processing
4.4. Statistical Analysis
4.4.1. Uranium Content, Fertility Parameters
4.4.2. LC-MS Data Analysis
4.4.3. Variable Selection and Metabolite Identification
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef]
- Bracke, A.; Peeters, K.; Punjabi, U.; Hoogewijs, D.; Dewilde, S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod. Biomed. Online 2018, 36, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol Pharm. 2017, 51, 56–70. [Google Scholar] [CrossRef]
- Lafuente, R.; Garcia-Blaquez, N.; Jacquemin, B.; Checa, M.A. Outdoor air pollution and sperm quality. Fertil. Steril. 2016, 106, 880–896. [Google Scholar] [CrossRef] [Green Version]
- Carre, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Leandri, R. Does air pollution play a role in infertility? A systematic review. Environ. Health A Glob. Access Sci. Source 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viluksela, M.; Pohjanvirta, R. Multigenerational and Transgenerational Effects of Dioxins. Int. J. Mol. Sci. 2019, 20, 2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.B.; Min, J.Y. Exposure to environmental noise and risk for male infertility: A population-based cohort study. Environ. Pollut 2017, 226, 118–124. [Google Scholar] [CrossRef]
- Wang, S.; Ran, Y.; Lu, B.; Li, J.; Kuang, H.; Gong, L.; Hao, Y. A Review of Uranium-Induced Reproductive Toxicity. Biol. Trace Elem. Res. 2020, 196, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, R.; Xu, L.; Xu, M.; Liu, S. Emerging health risks and underlying toxicological mechanisms of uranium contamination: Lessons from the past two decades. Environ. Int. 2020, 145, 106107. [Google Scholar] [CrossRef]
- UNSCEAR. Sources, effects and Risks of Ionizing Radiation REPORT Annexe D. 2016. Available online: https://www.unscear.org/unscear/en/publications/2016.html (accessed on 24 July 2022).
- Legendre, A.; Elie, C.; Ramambason, C.; Manens, L.; Souidi, M.; Froment, P.; Tack, K. Endocrine effects of lifelong exposure to low-dose depleted uranium on testicular functions in adult rat. Toxicology 2016, 368–369, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Elmhiri, G.; Gloaguen, C.; Grison, S.; Kereselidze, D.; Elie, C.; Tack, K.; Benderitter, M.; Lestaevel, P.; Legendre, A.; Souidi, M. DNA methylation and potential multigenerational epigenetic effects linked to uranium chronic low-dose exposure in gonads of males and females rats. Toxicol. Lett. 2018, 282, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Legendre, A.; Elmhiri, G.; Gloaguen, C.; Magneron, V.; Kereselidze, D.; Saci, N.; Elie, C.; Vaysset, E.; Benadjaoud, M.M.; Tack, K.; et al. Multigenerational exposure to uranium changes morphometric parameters and global DNA methylation in rat sperm. Comptes Rendus Biol. 2019, 342, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gombeau, K.; Bourdineaud, J.P.; Ravanat, J.L.; Armant, O.; Camilleri, V.; Cavalie, I.; Floriani, M.; Adam-Guillermin, C. Epigenetic, histopathological and transcriptomic effects following exposure to depleted uranium in adult zebrafish and their progeny. Aquat. Toxicol. 2017, 184, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Linares, V.; Albina, M.L.; Bellés, M.; Mayayo, E.; Sánchez, D.J.; Domingo, J.L. Combined action of uranium and stress in the rat: II. Effects on male reproduction. Toxicol. Lett. 2005, 158, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, R.; Leng, Y.; Ren, J.; Liu, J.; Ai, G.; Xu, H.; Su, Y.; Cheng, T. The reproductive effects in rats after chronic oral exposure to low-dose depleted uranium. J. Radiat. Res. 2012, 53, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arfsten, D.P.; Still, K.R.; Wilfong, E.R.; Johnson, E.W.; McInturf, S.M.; Eggers, J.S.; Schaeffer, D.J.; Bekkedal, M.Y. Two-generation reproductive toxicity study of implanted depleted uranium (DU) in CD rats. J. Toxicol. Environ. Health A 2009, 72, 410–427. [Google Scholar] [CrossRef]
- ICRP. Publication 103—The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- UNSCEAR. Hereditary Effects of Radiation. 2001. Available online: https://www.unscear.org/docs/publications/2001/UNSCEAR_2001_Report.pdf (accessed on 24 July 2022).
- International Atomic Energy Agency. Low Doses of Ionizing Radiation: Biological Effects and Regulatory Control Invited Papers and Discussions Proceedings of an International Conference; International Atomic Energy Agency (IAEA): Vienna, Austria, 1998. [Google Scholar]
- WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. Available online: https://apps.who.int/iris/handle/10665/44261 (accessed on 24 July 2022).
- Esteves, S.C.; Miyaoka, R.; Agarwal, A. An update on the clinical assessment of the infertile male. [corrected]. Clinics 2011, 66, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panner Selvam, M.K.; Finelli, R.; Baskaran, S.; Agarwal, A. Dysregulation of Key Proteins Associated with Sperm Motility and Fertility Potential in Cancer Patients. Int. J. Mol. Sci. 2020, 21, 6754. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.; Amaral, A.; Rodriguez, M.; Canyellas, N.; Correig, X.; Ballesca, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of endogenous metabolites in human sperm cells using proton nuclear magnetic resonance ((1) H-NMR) spectroscopy and gas chromatography-mass spectrometry (GC-MS). Andrology 2015, 3, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Pastuszak, A.W.; Lamb, D.J. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil. Steril. 2013, 99, 998–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panner Selvam, M.K.; Finelli, R.; Agarwal, A.; Henkel, R. Proteomics and metabolomics—Current and future perspectives in clinical andrology. Andrologia 2021, 53, e13711. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Courant, F.; Antignac, J.-P.; Monteau, F.; Le Bizec, B. Metabolomics as a Potential New Approach for Investigating Human Reproductive Disorders. J. Proteome Res. 2013, 12, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; de Mateo, S.; Estanyol, J.M. Sperm cell proteomics. Proteomics 2009, 9, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hao, X.; Chen, H.; Wang, L.; Chen, A.; Song, X.; Hu, Z.; Su, Y.; Lin, H.; Fan, P. Metabolomic characterization of semen from asthenozoospermic patients using ultra-high-performance liquid chromatography-tandem quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. BMC 2020, 34, e4897. [Google Scholar] [CrossRef]
- Baker, M.A.; Hetherington, L.; Reeves, G.; Muller, J.; Aitken, R.J. The rat sperm proteome characterized via IPG strip prefractionation and LC-MS/MS identification. Proteomics 2008, 8, 2312–2321. [Google Scholar] [CrossRef]
- Baker, M.A.; Hetherington, L.; Weinberg, A.; Velkov, T. Phosphopeptide analysis of rodent epididymal spermatozoa. JoVE 2014, 51546. [Google Scholar] [CrossRef] [Green Version]
- Lestaevel, P.; Grison, S.; Fave, G.; Elie, C.; Dhieux, B.; Martin, J.C.; Tack, K.; Souidi, M. Assessment of the Central Effects of Natural Uranium via Behavioural Performances and the Cerebrospinal Fluid Metabolome. Neural Plast. 2016, 2016, 9740353. [Google Scholar] [CrossRef] [Green Version]
- Grison, S.; Fave, G.; Maillot, M.; Manens, L.; Delissen, O.; Blanchardon, E.; Banzet, N.; Defoort, C.; Bott, R.; Dublineau, I.; et al. Metabolomics identifies a biological response to chronic low-dose natural uranium contamination in urine samples. Metab. Off. J. Metab. Soc. 2013, 9, 1168–1180. [Google Scholar] [CrossRef] [Green Version]
- Goodson, J.M.; Hardt, M.; Hartman, M.L.; Alqaderi, H.; Green, D.; Tavares, M.; Mutawa, A.S.; Ariga, J.; Soparkar, P.; Behbehani, J.; et al. Salivary N1-Methyl-2-Pyridone-5-Carboxamide, a Biomarker for Uranium Uptake, in Kuwaiti Children Exhibiting Exceptional Weight Gain. Front. Endocrinol. 2019, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Grison, S.; Kereselidze, D.; Cohen, D.; Gloaguen, C.; Elie, C.; Lestaevel, P.; Legendre, A.; Manens, L.; Habchi, B.; Benadjaoud, M.A.; et al. Applying a multiscale systems biology approach to study the effect of chronic low-dose exposure to uranium in rat kidneys. Int. J. Radiat Biol. 2019, 95, 737–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciamanna, I.; Serafino, A.; Shapiro, J.A.; Spadafora, C. The active role of spermatozoa in transgenerational inheritance. Proc. Biol. Sci. 2019, 286, 20191263. [Google Scholar] [CrossRef] [Green Version]
- Breton, S.; Nair, A.V.; Battistone, M.A. Epithelial dynamics in the epididymis: Role in the maturation, protection, and storage of spermatozoa. Andrology 2019, 7, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Sukhn, C.; Awwad, J.; Ghantous, A.; Zaatari, G. Associations of semen quality with non-essential heavy metals in blood and seminal fluid: Data from the Environment and Male Infertility (EMI) study in Lebanon. J. Assist. Reprod. Genet. 2018, 35, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, M.A.; Gucer, P.; Centeno, J.A.; Todorov, T.; Squibb, K.S. Semen Uranium Concentrations in Depleted Uranium Exposed Gulf War Veterans: Correlations with Other Body Fluid Matrices. Biol. Trace Elem. Res. 2019, 190, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Bao, S.; Liu, H.; Zhang, Y.; Zhang, B.; Zhou, A.; Chen, J.; Hao, K.; Xia, W.; et al. Association of adverse birth outcomes with prenatal uranium exposure: A population-based cohort study. Environ. Int. 2020, 135, 105391. [Google Scholar] [CrossRef] [PubMed]
- Grison, S.; Elmhiri, G.; Gloaguen, C.; Elie, C.; Kereselidze, D.; Tack, K.; Lestaevel, P.; Legendre, A.; Manens, L.; Benadjaoud, M.A.; et al. Low dose of uranium induces multigenerational epigenetic effects in rat kidney. Int. J. Radiat. Biol. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Brenner, D.J. Should we worry about inherited radiation risks? Lancet Oncol. 2015, 16, 1275–1276. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, M.A.; Skinner, M.K. Developmental origins of epigenetic transgenerational inheritance. Environ. Epigenetics 2016, 2, dvw002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, D.G.; Watkins, P.B.; Reily, M.D. Metabolomics in toxicology: Preclinical and clinical applications. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 120 (Suppl. 1), S146–S170. [Google Scholar] [CrossRef] [Green Version]
- Grison, S.; Fave, G.; Maillot, M.; Manens, L.; Delissen, O.; Blanchardon, E.; Dublineau, I.; Aigueperse, J.; Bohand, S.; Martin, J.C.; et al. Metabolomics reveals dose effects of low-dose chronic exposure to uranium in rats: Identification of candidate biomarkers in urine samples. Metab. Off. J. Metab. Soc. 2016, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; von Bergen, M.; Grunewald, S. Metabolomic profiling reveals correlations between spermiogram parameters and the metabolites present in human spermatozoa and seminal plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoernes, T.P.; Faserl, K.; Juen, M.A.; Kremser, J.; Gasser, C.; Fuchs, E.; Shi, X.; Siewert, A.; Lindner, H.; Kreutz, C.; et al. Translation of non-standard codon nucleotides reveals minimal requirements for codon-anticodon interactions. Nat. Commun. 2018, 9, 4865. [Google Scholar] [CrossRef]
- Esashi, T.; Suzuki, T.; Sahashi, Y. Effect of Inosine and its Related Compounds on the Storage of Chicken Semen. Jpn. Poult. Sci. 1967, 4, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Hampl, R.; Starka, L. Glucocorticoids affect male testicular steroidogenesis. Physiol. Res. 2020, 69, S205–S210. [Google Scholar] [CrossRef] [PubMed]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends Endocrinol. Metab. 2017, 28, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J. Carnitine—Metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef] [PubMed]
- Pekala, J.; Patkowska-Sokola, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochynski, S.; Librowski, T. L-carnitine—Metabolic functions and meaning in humans life. Curr. Drug Metab. 2011, 12, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Modanloo, M.; Shokrzadeh, M. Analyzing Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis: Potential Role of L-carnitine. Iran. J. Kidney Dis. 2019, 13, 74–86. [Google Scholar] [PubMed]
- Mongioi, L.; Calogero, A.E.; Vicari, E.; Condorelli, R.A.; Russo, G.I.; Privitera, S.; Morgia, G.; La Vignera, S. The role of carnitine in male infertility. Andrology 2016, 4, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Santaolaria, M.L.; Meneu, V.; Alonso, E. Dietary arginine slightly and variably affects tissue polyamine levels in male swiss albino mice. J. Nutr. 2002, 132, 3715–3720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.; Mandal, A.; Johansson, H.E.; Orjalo, A.V.; Park, M.H. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2169–2174. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tabor, H. The protective effect of spermine and other polyamines against heat denaturation of deoxyribonucleic acid. Biochemistry 1962, 1, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.M.; Liddell, S.C.; Wadkins, R.M. Effects of Polyamine Binding on the Stability of DNA i-Motif Structures. ACS Omega 2019, 4, 8967–8973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala-Rabanal, M.; Li, D.C.; Dake, G.R.; Kurata, H.T.; Inyushin, M.; Skatchkov, S.N.; Nichols, C.G. Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol. Pharm. 2013, 10, 1450–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliabadi, E.; Karimi, F.; Rasti, M.; Akmali, M.; Esmaeilpour, T. Effects of L-carnitine and Pentoxifylline on the Activity of Lactate Dehydrogenase C4 isozyme and Motility of Testicular Spermatozoa in Mice. J. Reprod. Infertil. 2013, 14, 56–61. [Google Scholar] [PubMed]
- Baptissart, M.; Vega, A.; Martinot, E.; Pommier, A.J.; Houten, S.M.; Marceau, G.; de Haze, A.; Baron, S.; Schoonjans, K.; Lobaccaro, J.M.; et al. Bile acids alter male fertility through G-protein-coupled bile acid receptor 1 signaling pathways in mice. Hepatology 2014, 60, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Holota, H.; Thirouard, L.; Monrose, M.; Garcia, M.; De Haze, A.; Saru, J.P.; Caira, F.; Beaudoin, C.; Volle, D.H. FXRalpha modulates leydig cell endocrine function in mouse. Mol. Cell. Endocrinol. 2020, 518, 110995. [Google Scholar] [CrossRef]
- Ugur, M.R.; Dinh, T.; Hitit, M.; Kaya, A.; Topper, E.; Didion, B.; Memili, E. Amino Acids of Seminal Plasma Associated With Freezability of Bull Sperm. Front. Cell Dev. Biol. 2019, 7, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahnsteiner, F. A comparative study on the composition and importance of free amino acids in semen of gilthead sea bream, Sparus aurata, and perch, Perca fluviatilis. Fish Physiol. Biochem. 2010, 36, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Grison, S.; Habchi, B.; Gloaguen, C.; Kereselidze, D.; Elie, C.; Martin, J.C.; Souidi, M. Early Metabolomic Markers of Acute Low-Dose Exposure to Uranium in Rats. Metabolites 2022, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballou, L.R. Ceramide and Inflammation. In Sphingolipid-Mediated Signal Transduction; Springer: Berlin/Heidelberg, Germany, 1997; pp. 35–51. [Google Scholar]
- Wappelhorst, O.; Kuhn, I.; Heidenreich, H.; Markert, B. Transfer of selected elements from food into human milk. Nutrition 2002, 18, 316–322. [Google Scholar] [CrossRef]
- Paquet, F.; Houpert, P.; Blanchardon, E.; Delissen, O.; Maubert, C.; Dhieux, B.; Moreels, A.M.; Frelon, S.; Gourmelon, P. Accumulation and distribution of uranium in rats after chronic exposure by ingestion. Health Phys. 2006, 90, 139–147. [Google Scholar] [CrossRef]
- Salonen, L. 238U series radionuclides as a source of increased radioactivity in groundwater originating from Finnish bedrock. IAHS Publ. 1994, 222, 71. [Google Scholar]
- WHO. Uranium in Drinking-Water; World Health Organization: Geneva, Switzerland, 2011.
- Souidi, M.; Tissandie, E.; Racine, R.; Ben Soussan, H.; Rouas, C.; Grignard, E.; Dublineau, I.; Gourmelon, P.; Lestaevel, P.; Gueguen, Y. Uranium: Properties and biological effects after internal contamination. Ann. Biol. Clin. 2009, 67, 23–38. [Google Scholar] [CrossRef]
- Draper, J.; Enot, D.P.; Parker, D.; Beckmann, M.; Snowdon, S.; Lin, W.; Zubair, H. Metabolite signal identification in accurate mass metabolomics data with MZedDB, an interactive m/z annotation tool utilising predicted ionisation behaviour ‘rules’. BMC Bioinform. 2009, 10, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Generations | |||
|---|---|---|---|
| Metabolisms | F0 | F1 | F2 |
| Purines | Adenine | ||
| 2′-O-Methylinosine | |||
| Hypoxanthine | |||
| Glycocorticoïdes | Cortisone | ||
| Carnitines | Lysine | ||
| o-acetyl-l-carnitine hydrochloride | o-acetyl-l-carnitine hydrochloride | ||
| Butyryl-l-carnitine | |||
| Polyamines | L-Methionine | ||
| Spermidine | Spermidine | ||
| Spermine | |||
| Arginine | Arginine | ||
| Bile acids | Taurocholic acid | Taurocholic acid | |
| Glycocholic acid | |||
| Chenodeoxycholic acid | |||
| Proline | Trans-4-hydroxy-L-proline | Trans-4-hydroxy-L-proline | |
| Proline | |||
| Tryptophan | Tryptophan | ||
| Anthranilate | |||
| Nicotinate-nicotinamide | Nicotinamide | ||
| Ceramides | PI-Cer(d20:0/18:0) | ||
| 4-hydroxysphing-8(Z)-enine-16:0, ceramide | |||
| Phospholipids | PS(17:1(9Z)/0:0) | ||
| Prostacyclin | 6-ketoprostaglandin F1 alfa | ||
| Microbiotic origin, glucid metabolism | Hippurate | ||
| Unknow origin | L-beta-homothreonine | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grison, S.; Legendre, A.; Svilar, L.; Elie, C.; Kereselidze, D.; Gloaguen, C.; Lestaevel, P.; Martin, J.-C.; Souidi, M. Multigenerational Exposure to Uranium Changes Sperm Metabolome in Rats. Int. J. Mol. Sci. 2022, 23, 8349. https://doi.org/10.3390/ijms23158349
Grison S, Legendre A, Svilar L, Elie C, Kereselidze D, Gloaguen C, Lestaevel P, Martin J-C, Souidi M. Multigenerational Exposure to Uranium Changes Sperm Metabolome in Rats. International Journal of Molecular Sciences. 2022; 23(15):8349. https://doi.org/10.3390/ijms23158349
Chicago/Turabian StyleGrison, Stéphane, Audrey Legendre, Ljubica Svilar, Christelle Elie, Dimitri Kereselidze, Céline Gloaguen, Philippe Lestaevel, Jean-Charles Martin, and Maâmar Souidi. 2022. "Multigenerational Exposure to Uranium Changes Sperm Metabolome in Rats" International Journal of Molecular Sciences 23, no. 15: 8349. https://doi.org/10.3390/ijms23158349
APA StyleGrison, S., Legendre, A., Svilar, L., Elie, C., Kereselidze, D., Gloaguen, C., Lestaevel, P., Martin, J. -C., & Souidi, M. (2022). Multigenerational Exposure to Uranium Changes Sperm Metabolome in Rats. International Journal of Molecular Sciences, 23(15), 8349. https://doi.org/10.3390/ijms23158349






