First Experimental Evidence for Reversibility of Ammonia Loss from Asparagine
Abstract
:1. Introduction
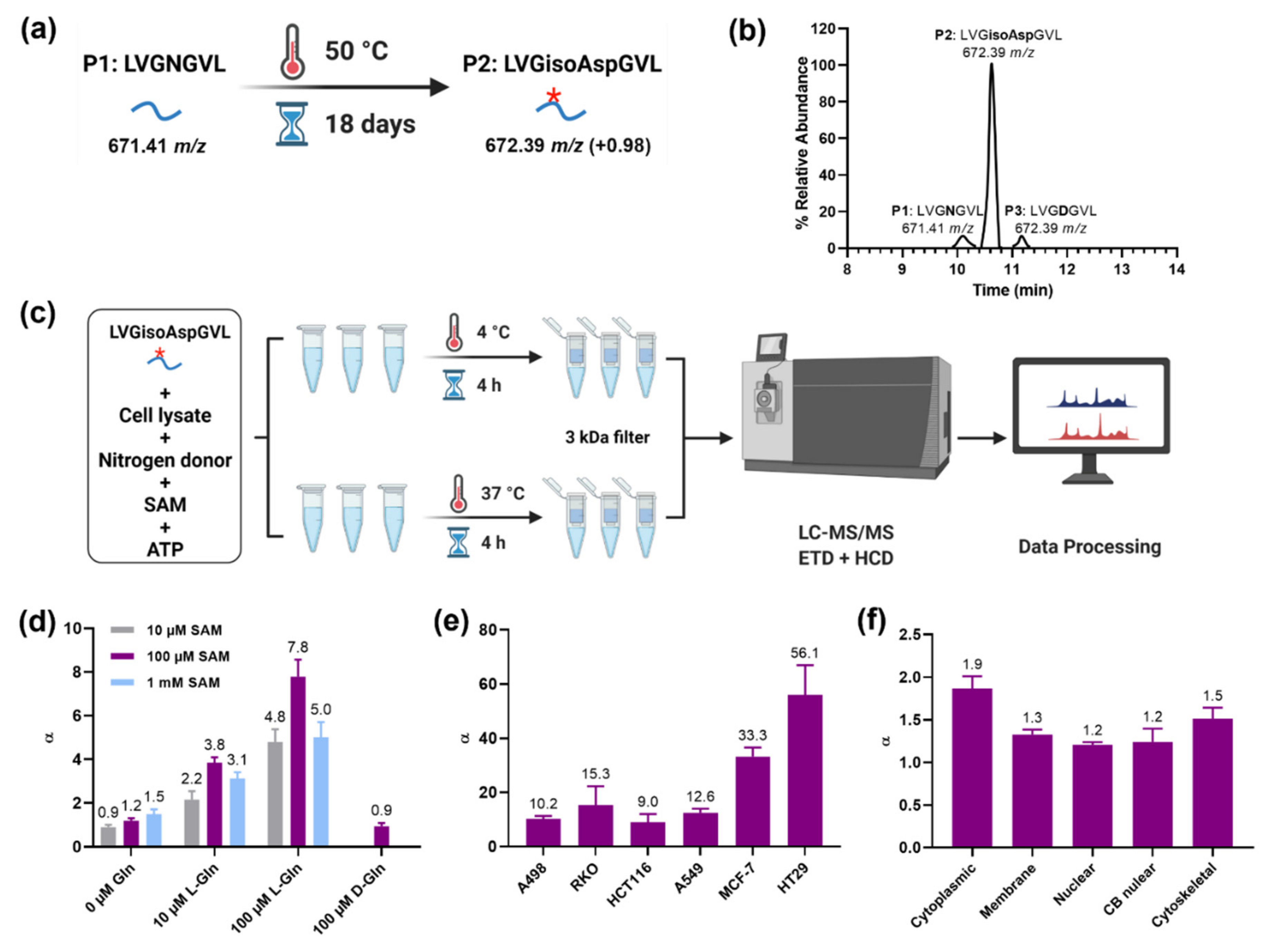
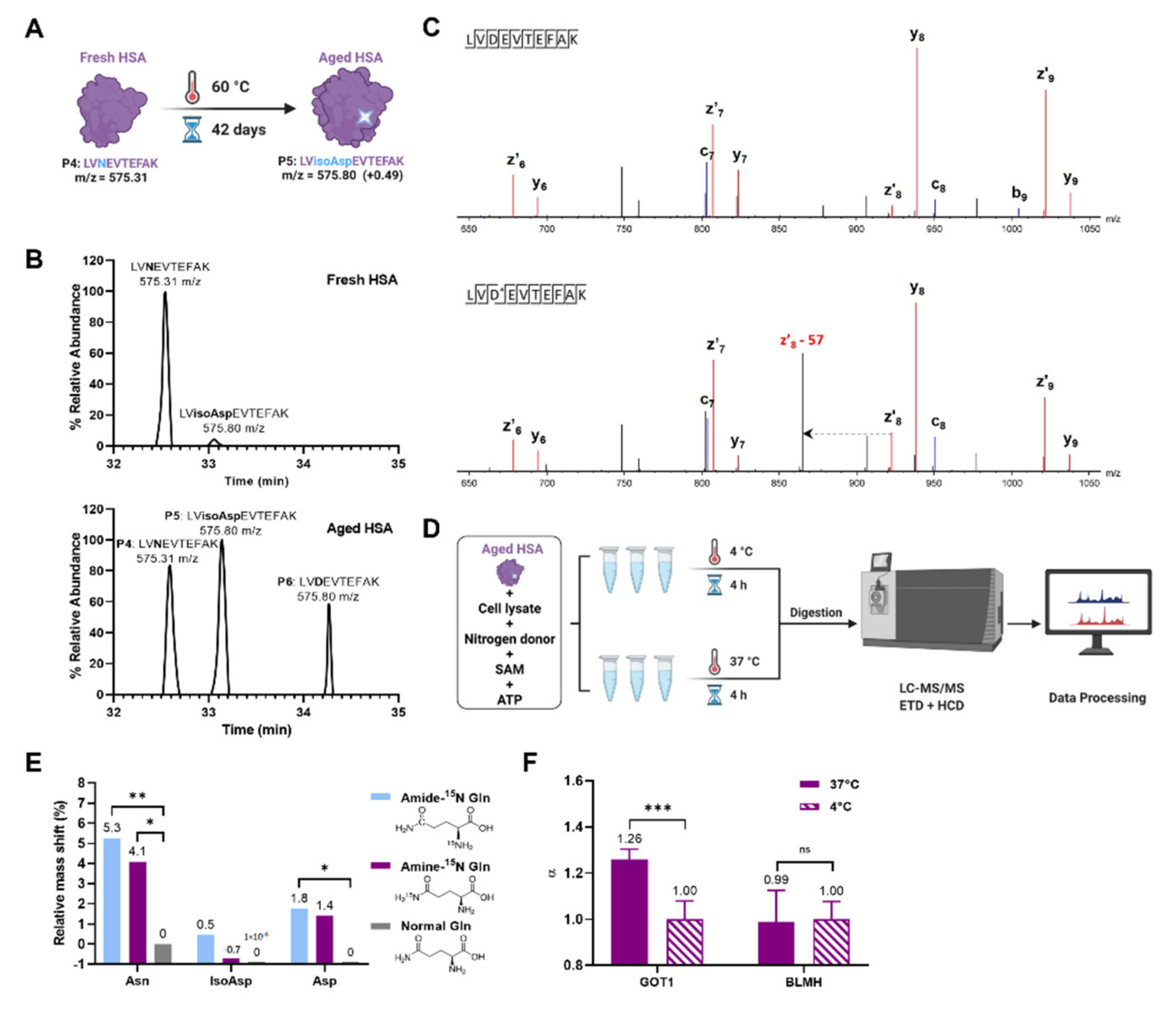
2. Results and Discussion
3. Materials and Methods
3.1. Artificial Aging of the Synthetic Peptide
3.2. PSIAL Activity Experiment on “Aged Peptide”
3.3. PSIAL Activity Experiment in Different Cell Lines
3.4. PSIAL Activity Experiment in Subcellular Fractions
3.5. Thermal Proteome Profiling–Temperature Range (TPP-TR) Experiments
3.6. Artificial Aging of Human Serum Albumin (HSA)
3.7. PSIAL Activity Experiment on “Aged HSA”
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geiger, T.; Clarke, S. Deamidation, Isomerization, and Racemization at Asparaginyl and Aspartyl Residues in Peptides—Succinimide-Linked Reactions That Contribute to Protein-Degradation. J. Biol. Chem. 1987, 262, 785–794. [Google Scholar] [CrossRef]
- Johnson, B.A.; Murray, E.D.; Clarke, S.; Glass, D.B.; Aswad, D.W. Protein Carboxyl Methyltransferase Facilitates Conversion of Atypical L-Isoaspartyl Peptides to Normal L-Aspartyl Peptides. J. Biol. Chem. 1987, 262, 5622–5629. [Google Scholar] [CrossRef]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Wolkow, C.; Wang, R.; Cotter, R.J.; Reardon, I.M.; Zurcherneely, H.A.; Heinrikson, R.L.; Ball, M.J.; et al. Structural Alterations in the Peptide Backbone of Beta-Amyloid Core Protein May Account for Its Deposition and Stability in Alzheimers-Disease. J. Biol. Chem. 1993, 268, 3072–3083. [Google Scholar] [CrossRef]
- Goldberg, A.L. Protein degradation and protection against misfolded or damaged proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef]
- Böhme, L.; Bär, J.W.; Hoffmann, T.; Manhart, S.; Ludwig, H.-H.; Rosche, F.; Demuth, H.-U. Isoaspartate residues dramatically influence substrate recognition and turnover by proteases. Biol. Chem. 2008, 389, 1043–1053. [Google Scholar] [CrossRef]
- Yang, H.; Lyutvinskiy, Y.; Soininen, H.; Zubarev, R.A. Alzheimer’s disease and mild cognitive impairment are associated with elevated levels of isoaspartyl residues in blood plasma proteins. J. Alzheimers Dis. 2011, 27, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, T.; Das, G.; Chatterjee, B.K.; Dhar, J.; Ghosh, S.; Chakrabarti, P. The role of isoaspartate in fibrillation and its prevention by Protein-L-isoaspartyl methyltransferase. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129500. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C.; Meng, Z.; Zwan, M.D.; Chen, X.; Seelow, S.; Lundström, S.L.; Rodin, S.; Teunissen, C.E.; Zubarev, R.A. Testing the link between isoaspartate and Alzheimer’s disease etiology. bioRxiv 2022. [Google Scholar] [CrossRef]
- Johnson, B.A.; Shirokawa, J.M.; Geddes, J.W.; Choi, B.H.; Kim, R.C.; Aswad, D.W. Protein L-Isoaspartyl Methyltransferase in Postmortem Brains of Aged Humans. Neurobiol. Aging 1991, 12, 19–24. [Google Scholar] [CrossRef]
- Robinson, N.E.; Robinson, A.B. Molecular clocks. Proc. Natl. Acad. Sci. USA 2001, 98, 944–949. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, C.M. Protein L-isoaspartyl, D-aspartyl O-methyltransferases: Catalysts for protein repair. Enzymes 2006, 24, 385–433. [Google Scholar] [CrossRef]
- Mishra, P.K.K.; Mahawar, M. PIMT-Mediated Protein Repair: Mechanism and Implications. Biochem. Mosc. 2019, 84, 453–463. [Google Scholar] [CrossRef]
- Hao, P.L.; Ren, Y.; Alpert, A.J.; Sze, S.K. Detection, Evaluation and Minimization of Nonenzymatic Deamidation in Proteomic Sample Preparation. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Chelius, D.; Rehder, D.S.; Bondarenko, P.V. Identification and characterization of deamidation sites in the conserved regions of human Immunoglobulin Gamma antibodies. Anal. Chem. 2005, 77, 6004–6011. [Google Scholar] [CrossRef]
- Gervais, D. Protein deamidation in biopharmaceutical manufacture: Understanding, control and impact. J. Chem. Technol. Biotechnol. 2016, 91, 569–575. [Google Scholar] [CrossRef]
- Vaintraub, I.A.; Kotova, L.V.; Shaha, R. Protein Deamidase from Germinating Wheat Grains. FEBS Lett. 1992, 302, 169–171. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zeng, Y.; Xu, S.; Chen, J.; Shen, G.; Yu, C.; Knipe, D.; Yuan, W.; Peng, J.; Xu, W.; et al. A Viral Deamidase Targets the Helicase Domain of RIG-I to Block RNA-Induced Activation. Cell Host Microbe 2016, 20, 770–784. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, J.; Xu, S.; Li, J.; He, S.; Zeng, Y.; Xie, L.; Xie, N.; Liu, T.; Lee, K.; et al. Species-Specific Deamidation of cGAS by Herpes Simplex Virus UL37 Protein Facilitates Viral Replication. Cell Host Microbe 2018, 24, 234–248.e235. [Google Scholar] [CrossRef] [Green Version]
- Konuklar, F.A.S.; Aviyente, V.; Sen, T.Z.; Bahar, I. Modeling the deamidation of asparagine residues via succinimide intermediates. J. Mol. Model. 2001, 7, 147–160. [Google Scholar] [CrossRef]
- Russell, J.B.; Strobel, H.J. Concentration of Ammonia across Cell-Membranes of Mixed Rumen Bacteria. J. Dairy Sci. 1987, 70, 970–976. [Google Scholar] [CrossRef]
- Wang, J.; Lundström, S.L.; Seelow, S.; Rodin, S.; Meng, Z.; Astorga-Wells, J.; Jia, Q.; Zubarev, R.A. First Immunoassay for Measuring Isoaspartate in Human Serum Albumin. Molecules 2021, 26, 6709. [Google Scholar] [CrossRef] [PubMed]
- Catak, S.; Monard, G.; Aviyente, V.; Ruiz-Lopez, M.F. Deamidation of asparagine residues: Direct hydrolysis versus succinimide-mediated deamidation mechanisms. J. Phys. Chem. A 2009, 113, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Peracchi, A. The Limits of Enzyme Specificity and the Evolution of Metabolism. Trends Biochem. Sci. 2018, 43, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; D’Alterio, C.; Camerlingo, R.; Tirino, V.; Consales, C.; Riccio, A.; Ierano, C.; Cecere, S.C.; Losito, N.S.; Greggi, S.; et al. Identification of a distinct population of CD133+CXCR4+ cancer stem cells in ovarian cancer. Sci. Rep. 2015, 5, 10357. [Google Scholar] [CrossRef] [Green Version]
- Mateus, A.; Maatta, T.A.; Savitski, M.M. Thermal proteome profiling: Unbiased assessment of protein state through heat-induced stability changes. Proteome Sci. 2017, 15, 13. [Google Scholar] [CrossRef] [Green Version]

| Protein ID | Protein Name | Gene Name | |Median △T| | −Log10 (p Value) | Score * |
|---|---|---|---|---|---|
| P17174 | Aspartate aminotransferase, cytoplasmic | GOT1 | 9.8 | 4.6 | 45.6 |
| P36959 | GMP reductase 1 | GMPR | 9.6 | 3.1 | 29.9 |
| Q9UFN0 | Protein NipSnap homolog 3A | NIPSNAP3A | 12.4 | 2.1 | 25.6 |
| E2QRF9 | Geminin | GMNN | 7.7 | 2.6 | 20.3 |
| O94992 | Protein HEXIM1 | HEXIM1 | 7.7 | 2.4 | 19.0 |
| Q13126 | S-methyl-5-thioadenosine phosphorylase | MTAP | 4.3 | 4.2 | 17.9 |
| P41236 | Protein phosphatase inhibitor 2 | PPP1R2 | 10.2 | 1.7 | 17.4 |
| P61956-2 | Small ubiquitin-related modifier 2 | SUMO2 | 8.1 | 2.0 | 16.3 |
| O43852 | Calumenin | CALU | 7.6 | 2.1 | 16.2 |
| P00390-2 | Glutathione reductase, mitochondrial | GSR | 8.6 | 1.8 | 15.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Rodin, S.; Saei, A.A.; Zhang, X.; Zubarev, R.A. First Experimental Evidence for Reversibility of Ammonia Loss from Asparagine. Int. J. Mol. Sci. 2022, 23, 8371. https://doi.org/10.3390/ijms23158371
Wang J, Rodin S, Saei AA, Zhang X, Zubarev RA. First Experimental Evidence for Reversibility of Ammonia Loss from Asparagine. International Journal of Molecular Sciences. 2022; 23(15):8371. https://doi.org/10.3390/ijms23158371
Chicago/Turabian StyleWang, Jijing, Sergey Rodin, Amir Ata Saei, Xuepei Zhang, and Roman A. Zubarev. 2022. "First Experimental Evidence for Reversibility of Ammonia Loss from Asparagine" International Journal of Molecular Sciences 23, no. 15: 8371. https://doi.org/10.3390/ijms23158371






