Phenotype Diversity of Macrophages in Osteoarthritis: Implications for Development of Macrophage Modulating Therapies
Abstract
:1. Introduction
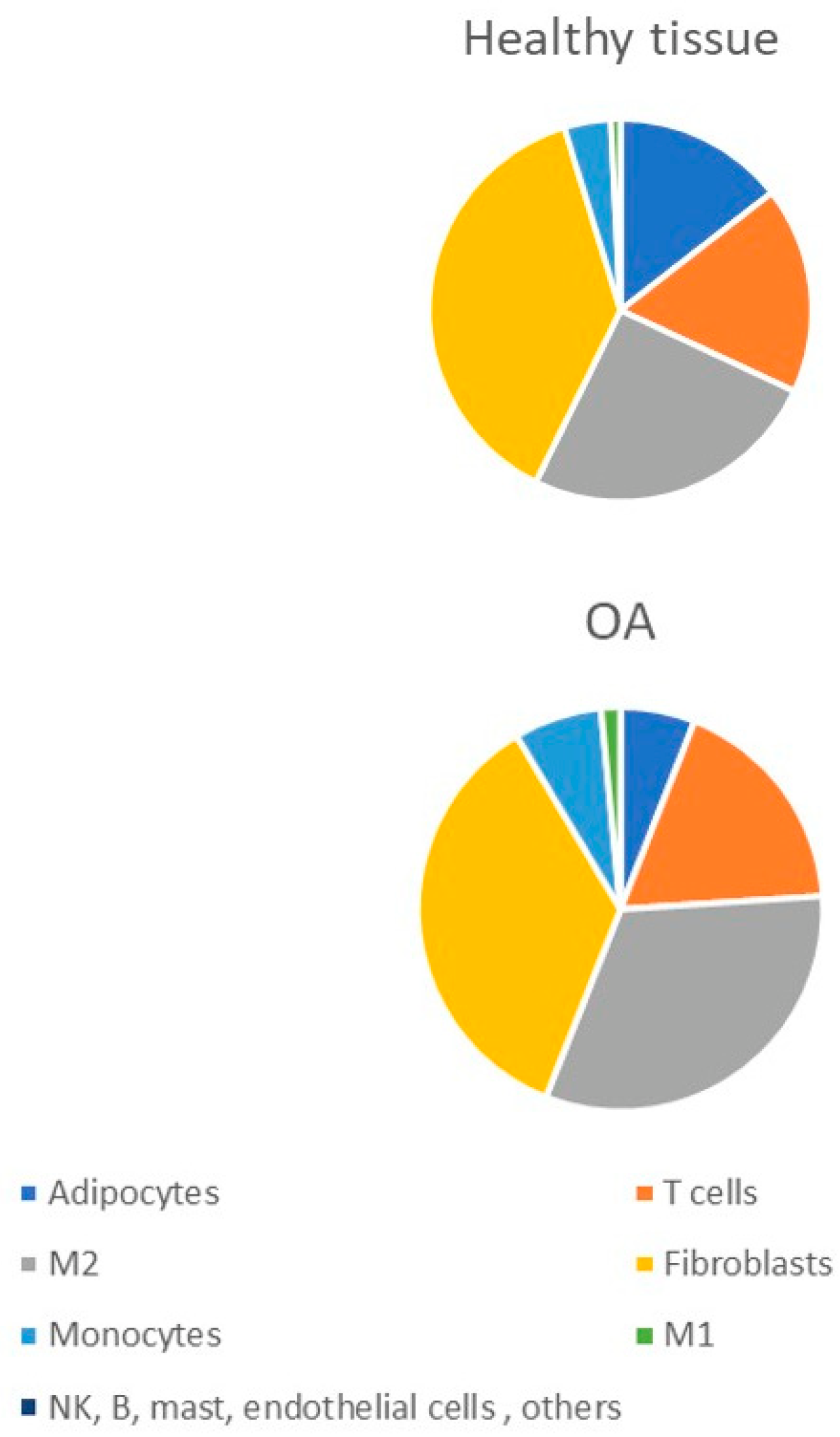
2. Macrophage Infiltration Pattern in OA
3. Functional Diversity of Synovial Macrophages in OA
4. Macrophage Polarization Signals and Modulation of Functional Phenotypes in OA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grässel, S.; Zaucke, F.; Madry, H.J. Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J. Clin. Med. 2021, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Schwartz, T.A.; Helmick, C.G.; Renner, J.B.; Tudor, G.; Koch, G.; Dragomir, A.; Kalsbeek, W.D.; Luta, G.; Jordan, J.M. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Care Res. 2008, 59, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Wenham, C.Y.; Conaghan, P.G. The role of synovitis in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Ayral, X.; Pickering, E.H.; Woodworth, T.G.; Mackillop, N.; Dougados, M. Synovitis: A potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—Results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr. Cartil. 2005, 13, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, A.; Hilkens, C.M.U. Synovial Macrophages in Osteoarthritis: The Key to Understanding Pathogenesis? Front. Immunol. 2021, 12, 678757. [Google Scholar] [CrossRef]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 2, CD005328. [Google Scholar]
- Osani, M.C.; Vaysbrot, E.E.; Zhou, M.; McAlindon, T.E.; Bannuru, R.R. Duration of symptom relief and early trajectory of adverse events for oral NSAIDs in knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res. 2020, 72, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Tamaki, Y.; Takakubo, Y.; Hirayama, T.; Konttinen, Y.T.; Goodman, S.B.; Yamakawa, M.; Takagi, M.J. Expression of Toll-like receptors and their signaling pathways in rheumatoid synovitis. J. Rheumatol. 2011, 38, 810–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, G.; Sandelin, J.; Salem, A.; Nordström, D.C.; Waris, E. Toll-like receptors and their soluble forms differ in the knee and thumb basal osteoarthritic joints. Acta Orthop. 2017, 88, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Liu-Bryan, R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr. Rheumatol. Rep. 2013, 15, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehli, M. Of mice and men: Species variations of Toll-like receptor expression. Trends Immunol. 2002, 23, 375–378. [Google Scholar] [CrossRef]
- Barreto, G.; Manninen, M.; Eklund, K. Osteoarthritis and Toll-Like Receptors: When Innate Immunity Meets Chondrocyte Apoptosis. Biology 2020, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Lepus, C.M.; Raghu, H.; Reber, L.L.; Tsai, M.M.; Wong, H.H.; von Kaeppler, E.; Lingampalli, N.; Bloom, M.S.; Hu, N.; et al. IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis. Elife 2019, 8, e39905. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Valente, J.; Calvo, L.; Vacca, V.; Simeoli, R.; Arévalo, J.C.; Malcangio, M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthr. Cartil. 2018, 26, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takata, K.; Uchida, K.; Mukai, M.; Takano, S.; Aikawa, J.; Iwase, D.; Sekiguchi, H.; Miyagi, M.; Inoue, G.; Takaso, M. Increase in Tryptase and Its Role in the Synovial Membrane of Overweight and Obese Patients with Osteoarthritis of the Knee. Diabetes Metab. Syndr. Obes. 2020, 13, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Mishima, S.; Kashiwakura, J.; Sasaki-Sakamoto, T.; Seki, M.; Saito, S.; Ra, C.; Tokuhashi, Y.; Okayama, Y. Interleukin-17A expression in human synovial mast cells in rheumatoid arthritis and osteoarthritis. Allergol. Int. 2016, 65 (Suppl. S1), 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, T.M.; Scanzello, C.R. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 57–63. [Google Scholar] [PubMed]
- Wu, C.L.; Harasymowicz, N.S.; Klimak, M.A.; Collins, K.H.; Guilak, F. The Role of Macrophages in Osteoarthritis and Cartilage Repair. Osteoarthr. Cartil. 2020, 28, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, C.W.; Xu, H.; Wang, Y.F.; Yung, P.S.H.; Jiang, Y.; Lee, O.K. Phenotypic alteration of macrophages during osteoarthritis: A systematic review. Arthritis Res. Ther. 2021, 23, 110. [Google Scholar] [CrossRef]
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The Role of Synovial Macrophages and Macrophage-Produced Cytokines in Driving Aggrecanases, Matrix Metalloproteinases, and Other Destructive and Inflammatory Responses in Osteoarthritis. Arthritis Res. Ther. 2006, 8, R187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, T.L.; Gomoll, A.H.; Latterman, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Kraus, V.B.; McDaniel, G.; Huebner, J.L.; Stabler, T.V.; Pieper, C.F.; Shipes, S.W.; Petry, N.A.; Low, P.S.; Shen, J.; McNearney, T.A.; et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1613–1621. [Google Scholar] [CrossRef] [Green Version]
- Daghestani, H.N.; Pieper, C.F.; Kraus, V.B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015, 67, 956–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, C.H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.; Gregory, S.; Kraus, V.B. Synovial Cell Cross-Talk with Cartilage Plays a Major Role in the Pathogenesis of Osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, Y.; Li, X.; Deng, Z.; Zheng, M.; Zheng, Q. The Immune Cell Landscape in Different Anatomical Structures of Knee in Osteoarthritis: A Gene Expression-Based Study. BioMed Res. Int. 2020, 2020, 9647072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, L.; Gulko, P.S.; Zhu, J. Computational deconvolution of synovial tissue cellular composition: Presence of adipocytes in synovial tissue decreased during arthritis pathogenesis and progression. Physiol. Genom. 2019, 51, 241–253. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Pan, J.; Xu, S.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- de la Durantaye, M.; Piette, A.B.; van Rooijen, N.; Frenette, J. Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J. Orthop. Res. 2014, 32, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, Z.; Liu, Z.H.; Chen, S.P.; Li, M.; Shahveranov, A.; Ye, D.W.; Tian, Y.K. Interleukin-6: An emerging regulator of pathological pain. Neuroinflammation 2016, 13, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. BioMed Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Yan, Z.P.; Chen, X.Y.; Ni, G.X. Infrapatellar Fat Pad and Knee Osteoarthritis. Aging Dis. 2020, 11, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Aristizabal, A.; Gandhi, R.; Mahomed, N.N.; Marshall, K.W.; Viswanathan, S. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: A cohort study. Arthritis Res. Ther. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.L.; McNeill, J.; Goon, K.; Little, D.; Kimmerling, K.; Huebner, J.; Kraus, V.; Guilak, F. Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice. Arthritis Rheumatol. 2017, 69, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef] [PubMed]
- Timur, U.T.; Caron, M.M.J.; Bastiaansen-Jenniskens, Y.M.; Welting, T.J.M.; van Rhijn, L.W.; van Osch, G.J.V.M.; Emans, P.J. Celecoxib-mediated reduction of prostanoid release in Hoffa's fat pad from donors with cartilage pathology results in an attenuated inflammatory phenotype. Osteoarthr. Cartil. 2018, 26, 697–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Kaeppler, E.P.; Wang, Q.; Raghu, H.; Bloom, M.S.; Wong, H.; Robinson, W.H. Interleukin 4 promotes anti-inflammatory macrophages that clear cartilage debris and inhibits osteoclast development to protect against osteoarthritis. Clin. Immunol. 2021, 229, 108784. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lin, X.; Xu, H.; Sun, W.; Bouta, E.M.; Zuscik, M.J.; Chen, D.; Schwarz, E.M.; Xing, L. Attenuated joint tissue damage associated with improved synovial lymphatic function following treatment with Bortezomib in a mouse model of experimental posttraumatic osteoarthritis. Arthritis Rheumatol. 2019, 71, 244–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambamurthy, N.; Zhou, C.; Nguyen, V.; Smalley, R.; Hankenson, K.D.; Dodge, G.R.; Scanzello, C.R. Deficiency of the pattern-recognition receptor CD14 protects against joint pathology and functional decline in a murine model of osteoarthritis. PLoS ONE 2018, 13, e0206217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Mei, J.; Han, X.; Li, H.; Yang, S.; Wang, M.; Chu, L.; Qiao, H.; Tang, T. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-kappa B/MAPK signaling and protecting chondrocytes. Acta Pharm. Sin. B 2019, 9, 973–985. [Google Scholar] [CrossRef]
- Yarnall, B.W.; Chamberlain, C.S.; Hao, Z.; Muir, P. Proinflammatory polarization of stifle synovial macrophages in dogs with cruciate ligament rupture. Veter Surg. 2019, 48, 1005–1012. [Google Scholar] [CrossRef]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Ngo, Y.; Were, S.R.; Barrett, S.H.; Rodgerson, D.H.; Dahlgren, L.A. Macrophage Activation in the Synovium of Healthy and Osteoarthritic Equine Joints. Front. Vet. Sci. 2020, 7, 568756. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.J.; Leckenby, A.; Reynolds, G.; Spiering, R.; Pratt, A.G.; Rankin, K.S.; Isaacs, J.D.; Haniffa, M.A.; Milling, S.; Hilkens, C.M. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight 2019, 4, e125325. [Google Scholar] [CrossRef] [Green Version]
- van den Bosch, M.H.; Blom, A.B.; Schelbergen, R.F.; Koenders, M.I.; van de Loo, F.A.; van den Berg, W.B.; Vogl, T.; Roth, J.; van der Kraan, P.M.; van Lent, P.L. Alarmin S100A9 Induces Proinflammatory and Catabolic Effects Predominantly in the M1 Macrophages of Human Osteoarthritic Synovium. J. Rheumatol. 2016, 43, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, Z.; He, C.; Gao, F.; Guo, Y.; Qu, F.; Liu, Y. Single-Cell Transcriptome Analysis Profile of Meniscal Tissue Macrophages in Human Osteoarthritis. J. Immunol. Res. 2020, 2020, 8127281. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.; Ding, J.; Cohn, I.S.; Zhang, F.; Wei, K.; Rao, D.A.; Rozo, C.; Sokhi, U.K.; Shanaj, S.; Oliver, D.J.; et al. HBEGF+ macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci. Transl. Med. 2019, 11, eaau8587. [Google Scholar] [CrossRef] [PubMed]
- Culemann, S.; Grüneboom, A.; Nicolás-Ãvila, J.Ã.; Weidner, D.; Lämmle, K.F.; Rothe, T.; Quintana, J.A.; Kirchner, P.; Krljanac, B.; Eberhardt, M.; et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 2019, 572, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining Inflammatory Cell States in Rheumatoid Arthritis Joint Synovial Tissues by Integrating Single-Cell Transcriptomics and Mass Cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; MacDonald, L.; Elmesmari, A.; Finlay, S.; Tolusso, B.; Gigante, M.R.; Petricca, L.; Di Mario, C.; Bui, L.; Perniola, S.; et al. Distinct Synovial Tissue Macrophage Subsets Regulate Inflammation and Remission in Rheumatoid Arthritis. Nat. Med. 2020, 26, 1295–1306. [Google Scholar] [CrossRef]
- Li, G.S.; Cui, L.; Wang, G.D. miR-155-5p regulates macrophage M1 polarization and apoptosis in the synovial fluid of patients with knee osteoarthritis. Exp. Ther. Med. 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Mason, C.; Were, S.R.; Luo, X.; Byron, C.R.; Kalbfleisch, T.S.; MacLeod, J.N.; Dahlgren, L.A. Inflamed synovial fluid induces a homeostatic response in bone marrow mononuclear cells in vitro: Implications for joint therapy. FASEB J. 2020, 34, 4430–4444. [Google Scholar] [CrossRef] [Green Version]
- van Lent, P.L.; Blom, A.B.; Schelbergen, R.F.; Slöetjes, A.; Lafeber, F.P.; Lems, W.F.; Cats, H.; Vogl, T.; Roth, J.; van den Berg, W.B. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012, 64, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ding, L.; Yin, S.; Huang, Z.; Zhang, L.; Mei, W.; Wu, P.; Wang, P.; Pan, K. Relationship between the pyroptosis of fibroblast-like synoviocytes and HMGB1 secretion in knee osteoarthritis. Mol. Med. Rep. 2021, 23, 97. [Google Scholar] [CrossRef] [PubMed]
- Mahon, O.R.; Kelly, D.J.; McCarthy, G.M.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals alter immune cell metabolism and promote M1 macrophage polarization. Osteoarthr. Cartil. 2020, 28, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Denoble, A.E.; Huffman, K.M.; Stabler, T.V.; Kelly, S.J.; Hershfield, M.S.; McDaniel, G.E.; Coleman, R.E.; Kraus, V.B. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl. Acad. Sci. USA 2011, 108, 2088–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, F.; Pufe, T.; Conradi, L.; Varoga, D.; Tsokos, M.; Papendieck, J.; Petersen, W. Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes. J. Pathol. 2002, 198, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.W.; Wang, Y.; Xiao, K.; Xu, H.; Luo, Z.Y.; Li, L.; Pei, F.X.; Kraus, V.B.; Huang, Z.Y. Alpha defensin-1 attenuates surgically induced osteoarthritis in association with promoting M1 to M2 macrophage polarization. Osteoarthr. Cartil. 2021, 29, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.Y.; Stabler, T.; Pei, F.X.; Kraus, V.B. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 2016, 24, 1769–1775. [Google Scholar] [CrossRef] [Green Version]
- Barreto, G.; Senturk, B.; Colombo, L.; Brück, O.; Neidenbach, P.; Salzmann, G.; Zenobi-Wong, M.; Rottmar, M. Lumican is upregulated in osteoarthritis and contributes to TLR4-induced pro-inflammatory activation of cartilage degradation and macrophage polarization. Osteoarthr. Cartil. 2020, 28, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleshner, M.; Crane, C.R. Exosomes, DAMPs and miRNA: Features of Stress Physiology and Immune Homeostasis. Trends Immunol. 2017, 38, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Kuang, L.; Chen, H.; Xie, Y.; Zhang, B.; Ouyang, J.; Wu, J.; Zhou, S.; Chen, L.; Su, N.; et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1beta production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Yan, Y.; Li, R.; Dai, H.; Xu, J. Extracellular vesicles from M1-polarized macrophages promote inflammation in the temporomandibular joint via miR-1246 activation of the Wnt/beta-catenin pathway. Ann. N. Y. Acad. Sci. 2021, 1503, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Rockel, J.S.; Kapoor, M.; Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 2021, 302, 126–146. [Google Scholar] [CrossRef] [PubMed]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019, 570, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Eberly, L.; Richter, D.; Comerci, G.; Ocksrider, J.; Mercer, D.; Mlady, G.; Wascher, D.; Schenck, R. Psychosocial and demographic factors influencing pain scores of patients with knee osteoarthritis. PLoS ONE 2018, 13, e0195075. [Google Scholar] [CrossRef]
- Kaukinen, P.; Podlipská, J.; Guermazi, A.; Niinimäki, J.; Lehenkari, P.; Roemer, F.W.; Nieminen, M.T.; Koski, J.M.; Arokoski, J.P.A.; Saarakkala, S. Associations between MRI-defined structural pathology and generalized and localized knee pain—The Oulu Knee Osteoarthritis study. Osteoarthr. Cartil. 2016, 24, 1565–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riis, R.G.; Gudbergsen, H.; Henriksen, M.; Ballegaard, C.; Bandak, E.; Röttger, D.; Bliddal, H.; Hansen, B.B.; Hangaard, S.; Boesen, M. Synovitis assessed on static and dynamic contrast-enhanced magnetic resonance imaging and its association with pain in knee osteoarthritis: A cross-sectional study. Eur. J. Radiol. 2016, 85, 1099–1108. [Google Scholar] [CrossRef]
- Geraghty, T.; Winter, D.R.; Miller, R.J.; Miller, R.E.; Malfait, A.M. Neuroimmune interactions and osteoarthritis pain: Focus on macrophages. Pain Rep. 2021, 6, e892. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Belmadani, A.; Ishihara, S.; Tran, P.B.; Ren, D.; Miller, R.J.; Malfait, A.M. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheumatol. 2015, 67, 2933–2943. [Google Scholar] [CrossRef]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, Y.; Fujita, M.; Kawasaki, S.; Sanaki, T.; Yoshioka, T.; Higashino, K.; Tofukuji, S.; Yoneda, S.; Takahashi, T.; Koda, K.; et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 2019, 160, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Zarebska, M.J.; Chanalaris, A.; Driscoll, C.; Burleigh, A.; Miller, R.E.; Malfait, A.M.; Stott, B.; Vincent, T.L. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthr. Cartil. 2017, 25, 406–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.C.; Saleh, R.; Achuthan, A.; Fleetwood, A.J.; Förster, I.; Hamilton, J.A.; Cook, A.D. CCL17 blockade as a therapy for osteoarthritis pain and disease. Arthritis Res. Ther. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemen, H.L.; Eijkelkamp, N.; Garza Carbajal, A.; Wang, H.; Mack, M.; Zijlstra, J.; Heijnen, C.J.; Kavelaars, A. Monocytes/macrophages control resolution of transient inflammatory pain. J. Pain 2014, 15, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Xu, X.; Sun, Z.; Yang, Y.X.; Guo, H.; Li, J.; Sun, K.; Wu, R.; Xu, J.; Jiang, Q.; et al. TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca2+/CaMKII/Nrf2 signaling pathway. Cell Death Dis. 2021, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.T.; Castoldi, A.; Andrade-Oliveira, V.; Latancia, M.T.; Terra, F.F.; Correa-Costa, M.; Breda, C.N.S.; Felizardo, R.J.F.; Pereira, W.O.; da Silva, M.B.; et al. The lack of PI3Kgamma favors M1 macrophage polarization and does not prevent kidney diseases progression. Int. Immunopharmacol. 2018, 64, 151–161. [Google Scholar] [CrossRef]
- Festuccia, W.T.; Pouliot, P.; Bakan, I.; Sabatini, D.M.; Laplante, M. Myeloid-specific Rictor deletion induces M1 macrophage polarization and potentiates in vivo pro-inflammatory response to lipopolysaccharide. PLoS ONE 2014, 9, e95432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baschant, U.; Lane, N.E.; Tuckermann, J. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat. Rev. Rheumatol. 2012, 8, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Zhao, L.; Xu, C.; Zhang, L.; Zhao, H. High Molecular Weight Hyaluronan Suppresses Macrophage M1 Polarization and Enhances IL-10 Production in PM2.5-Induced Lung Inflammation. Molecules 2019, 24, 1766. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.C.; Zaki, S.; Ravi, V.; Schiavinato, A.; Smith, M.M.; Little, C.B. The relationship between synovial inflammation, structural pathology, and pain in post-traumatic osteoarthritis: Differential effect of stem cell and hyaluronan treatment. Arthritis Res. Ther. 2020, 22, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kim, H.; Seo, J.; Choi, K.; Lee, Y.; Park, K.; Kim, S.; Mobasheri, A.; Choi, H. TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement. Inflammopharmacology 2020, 28, 1237–1252. [Google Scholar] [CrossRef]
- Osenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiang, E.; Rao, W.; Zhang, Y.Q.; Xiao, C.H.; Li, C.Y.; Han, B.; Wu, D. Intra-articular injection of human umbilical cord mesenchymal stem cells ameliorates monosodium iodoacetate-induced osteoarthritis in rats by inhibiting cartilage degradation and inflammation. Bone Jt. Res. 2021, 10, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.M.; Cheung, W.Y.; Gomez-Aristizábal, A.; Sharma, A.; Nakamura, S.; Chaboureau, A.; Bhatt, S.; Rabani, R.; Kapoor, M.; Foster, P.J.; et al. Iron nanoparticle-labeled murine mesenchymal stromal cells in an osteoarthritic model persists and suggests anti-inflammatory mechanism of action. PLoS ONE 2019, 14, e0214107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Lu, X.; Shen, B.; Zeng, Y. The Therapeutic Potential and Role of meRNA, lncRNA, and circRNA in Osteoarthritis. Curr. Gene Ther. 2019, 19, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, G.; Fu, Q.; Han, B.; Lu, J.; Chen, A.; Zhu, L. circRNA.33186 Contributes to the Pathogenesis of Osteoarthritis by Sponging miR-127-5p. Mol. Ther. 2019, 27, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Wu, Y.; Chen, J.; Xie, Z.; Huang, K.; Wang, G.; Yang, Y.; Ni, W.; Chen, Z.; Shi, P.; et al. CircSERPINE2 protects against osteoarthritis by targeting miR-1271 and ETS-related gene. Ann. Rheum. Dis. 2019, 78, 826–836. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Tang, S.; Nie, X.; Zhou, Z.; Ruan, G.; Han, W.; Zhu, Z.; Ding, C. Decreased miR-214-3p activates NF-kB pathway and aggravates osteoarthritis progression. EBioMedicine 2021, 65, 103283. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, F.; Rong, G.; Tang, Z.; Gui, B. Circular RNA has_circ_0005567 overexpression promotes M2 type macrophage polarization through miR-492/SOCS2 axis to inhibit osteoarthritis progression. Bioengineered 2021, 12, 8920–8930. [Google Scholar] [CrossRef]
- Zhou, F.; Mei, J.; Yang, S.; Han, X.; Li, H.; Yu, Z.; Qiao, H.; Tang, T. Modified ZIF-8 nanoparticles attenuate osteoarthritis by reprogramming the metabolic pathway of synovial macrophages. ACS Appl. Mater. Interfaces 2020, 12, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, A.; Allan, R.; Smith, S.L.; Marreiros, S.S.P.; Steultjens, M. Identification of Clinical Phenotypes in Knee Osteoarthritis: A Systematic Review of the Literature. BMC Musculoskelet. Disord. 2016, 17, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Macrophage Subpopulation | Surface Markers/Gene Expression | Clinical Correlates, Proposed Function | Reference |
|---|---|---|---|
| Synovial macrophages | HLA-DR+, CD14+, CD64+, CD11c+, CD86+, FOLR2+ | iOA and cOA subgroups did not correlate with radiographic measures of disease severity (Kellgren–Lawrence X-ray score), indicators of systemic inflammation, medication | [48] |
| Synovial macrophages with pro-inflammatory phenotype (iOA) | Ki67high, E2F8high, CDT1LK1high, CTRLhigh | ||
| Synovial macrophages with classical phenotype (cOA) | Ki67low, IGFBP5high, HTRA1high, EFEMP1high, SMAD3high, RUNX2low | ||
| Resident macrophages from meniscal tissue | SIGLEC1low, CSF2low, TNFlow | ND | [50] |
| Synovial macrophages specific cluster | CD14+ ITGB5+, ADORA3+, MERTK+ | Functional polarization distinct from both M1/M2 | [51] |
| M1 | CD11c+ C86+ | Increase in M1/M2 ratio in human knee OA was shown to positively correlate with Kellgren–Lawrence grade | [39] |
| M2 | CD206+ CD163+ | ||
| Synovial macrophages with inflammatory phenotype (I-MΦ) | HLA-DRAhigh, CD14high, CD16high, IL1Ahigh, IL1Bhigh, IL6high, TNFhigh, CCL2high CCL3high | Proposed to be the main source of inflammatory cytokines | [29] |
| Synovial macrophages with immunoregulatory phenotype (IR-MΦ) | HLA-DRAhigh, CD14high, CD163high, STAB1high, TNXIPhigh, CD169high | Proposed immunosuppressive function | [29] |
| Resident synovial macrophages | MHCII−, TREM2+, tight junctional proteins | Proposed barrier function | [52] |
| Synovial macrophages SC-M1 | CD11c+ CD38+ IL1B+, NR4A2+, HBEGF+, PLAUR+ IFITM3+ | Expression profile matches mouse monocyte-derived synovial macrophages, proposed pro-inflammatory activity | [53] |
| Synovial macro-phages SC-M2 | CD14+ CD11c+/−CD38− VSIG4+, GPNMB+, MERTK+, NUPR1+, CTSK+, HTRA1+ | Expression profile matches resident synovial macrophages identified in mice | [52,53] |
| Synovial macrophages SC-M3 | CD11c+ CD38+ C1QA+, MARCO+ | Expression profile matches resident synovial macrophages identified in mice | [52,53] |
| Synovial macrophages SC-M4 | CD11c+ CD38+ SPP1+, type I and II IFN induced genes, including IFITM3 and IFI6 | Expression profile matches mouse monocyte-derived synovial macrophages | [52,53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushenkova, N.V.; Nikiforov, N.G.; Shakhpazyan, N.K.; Orekhova, V.A.; Sadykhov, N.K.; Orekhov, A.N. Phenotype Diversity of Macrophages in Osteoarthritis: Implications for Development of Macrophage Modulating Therapies. Int. J. Mol. Sci. 2022, 23, 8381. https://doi.org/10.3390/ijms23158381
Mushenkova NV, Nikiforov NG, Shakhpazyan NK, Orekhova VA, Sadykhov NK, Orekhov AN. Phenotype Diversity of Macrophages in Osteoarthritis: Implications for Development of Macrophage Modulating Therapies. International Journal of Molecular Sciences. 2022; 23(15):8381. https://doi.org/10.3390/ijms23158381
Chicago/Turabian StyleMushenkova, Nataliya V., Nikita G. Nikiforov, Nikolay K. Shakhpazyan, Varvara A. Orekhova, Nikolay K. Sadykhov, and Alexander N. Orekhov. 2022. "Phenotype Diversity of Macrophages in Osteoarthritis: Implications for Development of Macrophage Modulating Therapies" International Journal of Molecular Sciences 23, no. 15: 8381. https://doi.org/10.3390/ijms23158381
APA StyleMushenkova, N. V., Nikiforov, N. G., Shakhpazyan, N. K., Orekhova, V. A., Sadykhov, N. K., & Orekhov, A. N. (2022). Phenotype Diversity of Macrophages in Osteoarthritis: Implications for Development of Macrophage Modulating Therapies. International Journal of Molecular Sciences, 23(15), 8381. https://doi.org/10.3390/ijms23158381







