Telocytes and Macrophages in the Gut: From Morphology to Function, Do the Two Cell Types Interact with Each Other? Which Helps Which?
Abstract
:1. Introduction
2. The Gut Telocytes
2.1. Morphological Features and Immunohistochemical Properties
2.2. Locations in the Gut Wall
2.3. Cell-Interactions and Roles
3. The Intestinal (Resident) Macrophages (MCs)
3.1. Morphological Features and Location
3.2. The Ontogenesis
3.3. Cell-Interactions and Functions
3.4. The Recruitment, the Niches and the Longevity
4. Do TCs and Intestinal MCs Interact with Each Other? Which Helps Which?
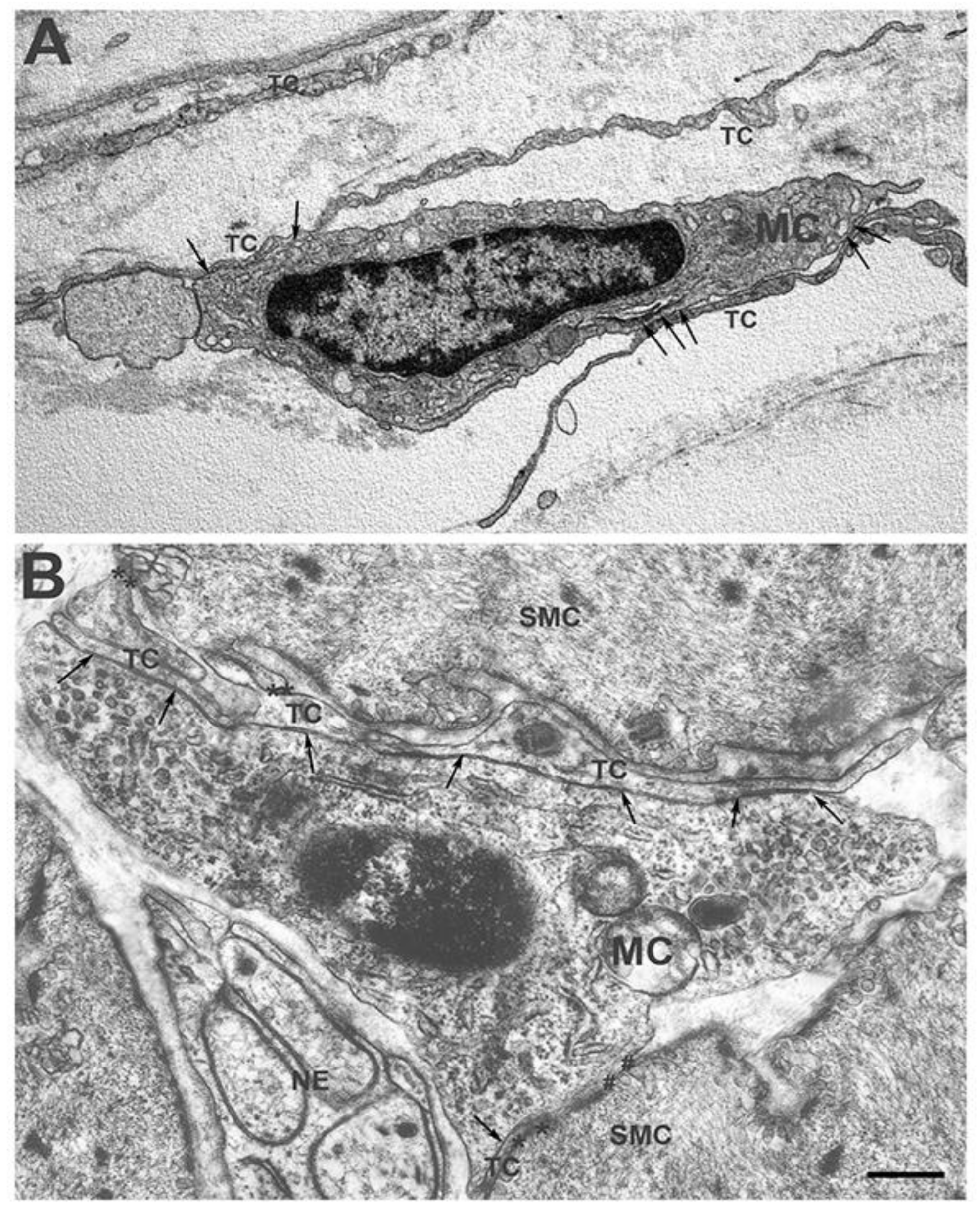
4.1. The Gut TCs Interact with the MCs
4.2. Which Helps Which?
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furness, J.B. The Enteric Nervous System and Neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Koscsó, B.; Rajani, G.M.; Stevanovic, K.; Berres, M.L.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Li, X.M.; Mucida, D.; et al. Crosstalk between Muscularis Macrophages and Enteric Neurons Regulates Gastrointestinal Motility. Cell 2014, 158, 1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Smidt, I.; Boeckx, B.; Dierckx de Casterlé, I.; et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell 2018, 175, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannucchi, M.G. The Telocytes: Ten Years after Their Introduction in the Scientific Literature. an Update on Their Morphology, Distribution, and Potential Roles in the Gut. Int. J. Mol. Sci. 2020, 21, 4478. [Google Scholar] [CrossRef]
- Popescu, L.M.; Faussone-Pellegrini, M.S. TELOCYTES—A Case of Serendipity: The Winding Way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell. Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Faussone-Pellegrini, M.S.; Popescu, L.M. Telocytes. Biomol. Concepts 2011, 2, 481–489. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Faussone-Pellegrini, M.S. The Telocyte Subtypes. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 913, pp. 115–126. [Google Scholar] [CrossRef]
- Lavin, Y.; Mortha, A.; Rahman, A.; Merad, M. Regulation of Macrophage Development and Function in Peripheral Tissues. Nat. Rev. Immunol. 2015, 15, 733–744. [Google Scholar] [CrossRef] [Green Version]
- De Schepper, S.; Stakenborg, N.; Matteoli, G.; Verheijden, S.; Boeckxstaens, G.E. Muscularis Macrophages: Key Players in Intestinal Homeostasis and Disease. Cell. Immunol. 2018, 330, 142–150. [Google Scholar] [CrossRef]
- Kurahashi, M.; Nakano, Y.; Hennig, G.W.; Ward, S.M.; Sanders, K.M. Platelet-Derived Growth Factor Receptor α-Positive Cells in the Tunica Muscularis of Human Colon. J. Cell. Mol. Med. 2012, 16, 1397–1404. [Google Scholar] [CrossRef]
- Kurahashi, M.; Nakano, Y.; Peri, L.E.; Townsend, J.B.; Ward, S.M.; Sanders, K.M. Novel Population of Subepithelial Platelet-Derived Growth Factor Receptor α-Positive Cells in the Mouse and Human Colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G823–G834. [Google Scholar] [CrossRef] [Green Version]
- Grover, M.; Bernard, C.E.; Pasricha, P.J.; Parkman, H.P.; Abell, T.L.; Nguyen, L.A.; Snape, W.; Shen, K.R.; Sarr, M.; Swain, J.; et al. Platelet-Derived Growth Factor Receptor α (PDGFRα)-Expressing “Fibroblast-like Cells” in Diabetic and Idiopathic Gastroparesis of Humans. Neurogastroenterol. Motil. 2012, 24, 844–852. [Google Scholar] [CrossRef] [Green Version]
- Vannucchi, M.G.; Traini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini, M.S. Telocytes Express PDGFRα in the Human Gastrointestinal Tract. J. Cell. Mol. Med. 2013, 17, 1099–1108. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Traini, C. Interstitial Cells of Cajal and Telocytes in the Gut: Twins, Related or Simply Neighbor Cells? Biomol. Concepts 2016, 7, 93–102. [Google Scholar] [CrossRef]
- Traserra, S.; Villarte, S.; Traini, C.; Palacin, S.; Vergara, P.; Vannucchi, M.G.; Jimenez, M. The Asymmetric Innervation of the Circular and Longitudinal Muscle of the Mouse Colon Differently Modulates Myogenic Slow Phasic Contractions. Neurogastroenterol. Motil. 2020, 32, e13778. [Google Scholar] [CrossRef]
- Cretoiu, D.; Roatesi, S.; Bica, I.; Plesca, C.; Stefan, A.; Bajenaru, O.; Condrat, C.E.; Cretoiu, S.M. Simulation and Modeling of Telocytes Behavior in Signaling and Intercellular Communication Processes. Int. J. Mol. Sci. 2020, 21, 2615. [Google Scholar] [CrossRef] [Green Version]
- Milia, A.F.; Ruffo, M.; Manetti, M.; Rosa, I.; Conte, D.; Fazi, M.; Messerini, L.; Ibba-Manneschi, L. Telocytes in Crohn’s Disease. J. Cell. Mol. Med. 2013, 17, 1525–1536. [Google Scholar] [CrossRef]
- Manetti, M.; Rosa, I.; Messerini, L.; Ibba-Manneschi, L. Telocytes Are Reduced during Fibrotic Remodelling of the Colonic Wall in Ulcerative Colitis. J. Cell. Mol. Med. 2015, 19, 62–73. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Bani, D.; Faussone-Pellegrini, M.-S. Telocytes Contribute as Cell Progenitors and Differentiation Inductors in Tissue Regeneration. Curr. Stem Cell Res. Ther. 2016, 11, 383–389. [Google Scholar] [CrossRef]
- Greicius, G.; Kabiri, Z.; Sigmundsson, K.; Liang, C.; Bunte, R.; Singh, M.K.; Virshup, D.M. PDGFRα+ Pericryptal Stromal Cells Are the Critical Source of Wnts and RSPO3 for Murine Intestinal Stem Cells in Vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E3173–E3181. [Google Scholar] [CrossRef] [Green Version]
- Kaestner, K.H. The Intestinal Stem Cell Niche: A Central Role for Foxl1-Expressing Subepithelial Telocytes. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, L.; Lindahl, P.; Heath, J.K.; Betsholtz, C. Abnormal Gastrointestinal Development in PDGF-A and PDGFR-α Deficient Mice Implicates a Novel Mesenchymal Structure with Putative Instructive Properties in Villus Morphogenesis. Development 2000, 127, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Shoshkes-Carmel, M.; Wang, Y.J.; Wangensteen, K.J.; Tóth, B.; Kondo, A.; Massassa, E.E.; Itzkovitz, S.; Kaestner, K.H. Subepithelial Telocytes Are an Important Source of Wnts That Supports Intestinal Crypts. Nature 2018, 557, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Kaestner, K.H. Emerging Diverse Roles of Telocytes. Development 2019, 146, dev175018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faussone-Pellegrini, M.S.; Gherghiceanu, M. Telocyte’s Contacts. Semin. Cell Dev. Biol. 2016, 55, 3–8. [Google Scholar] [CrossRef]
- Cretoiu, S.M.; Popescu, L.M. Telocytes Revisited. Biomol. Concepts 2014, 5, 353–369. [Google Scholar] [CrossRef]
- Traini, C.; Fausssone-Pellegrini, M.S.; Guasti, D.; Del Popolo, G.; Frizzi, J.; Serni, S.; Vannucchi, M.G. Adaptive Changes of Telocytes in the Urinary Bladder of Patients Affected by Neurogenic Detrusor Overactivity. J. Cell. Mol. Med. 2018, 22, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Traini, C.; Mischopoulou, M.; Gibbons, S.J.; Ligresti, G.; Faussone-Pellegrini, M.S.; Sha, L.; Farrugia, G.; Vannucchi, M.G.; Cipriani, G. Muscularis Macrophages Establish Cell-to-Cell Contacts with Telocytes/PDGFRα-Positive Cells and Smooth Muscle Cells in the Human and Mouse Gastrointestinal Tract. Neurogastroenterol. Motil. 2021, 33, e13993. [Google Scholar] [CrossRef]
- Sanders, K.M.; Kito, Y.; Hwang, S.J.; Ward, S.M. Regulation of Gastrointestinal Smooth Muscle Function by Interstitial Cells. Physiology 2016, 31, 316–326. [Google Scholar] [CrossRef]
- Lu, C.; Huang, X.; Lu, H.L.; Liu, S.H.; Zang, J.Y.; Li, Y.J.; Chen, J.; Xu, W.X. Different Distributions of Interstitial Cells of Cajal and Platelet-Derived Growth Factor Receptor-α Positive Cells in Colonic Smooth Muscle Cell/Interstitial Cell of Cajal/Plateletderived Growth Factor Receptor-α Positive Cell Syncytium in Mice. World J. Gastroenterol. 2018, 24, 4989–5004. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial Cells: Regulators of Smooth Muscle Function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef]
- Blair, P.J.; Rhee, P.L.; Sanders, K.M.; Ward, S.M. The Significance of Interstitial Cells in Neurogastroenterology. J. Neurogastroenterol. Motil. 2014, 20, 294–317. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; An, T.; Tarique, I.; Vistro, W.A.; Liu, Y.; Wang, Z.; Zhang, H.; Shi, Y.H.; Haseeb, A.; et al. Telocytes as a Novel Structural Component in the Muscle Layers of the Goat Rumen. Cell Transplant. 2019, 28, 955–966. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Popescu, L.M. Cardiac Telocytes—Their Junctions and Functional Implications. Cell Tissue Res. 2012, 348, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Rusu, M.C.; Cretoiu, D.; Vrapciu, A.D.; Hostiuc, S.; Dermengiu, D.; Manoiu, V.S.; Cretoiu, S.M.; Mirancea, N. Telocytes of the Human Adult Trigeminal Ganglion. Cell Biol. Toxicol. 2016, 32, 199–207. [Google Scholar] [CrossRef]
- Mikkelsen, H.B. Interstitial Cells of Cajal, Macrophages and Mast Cells in the Gut Musculature: Morphology, Distribution, Spatial and Possible Functional Interactions. J. Cell. Mol. Med. 2010, 14, 818–832. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, H.B.; Larsen, J.O.; Froh, P.; Nguyen, T.H. Quantitative Assessment of Macrophages in the Muscularis Externa of Mouse Intestines. Anat. Rec. 2011, 294, 1557–1565. [Google Scholar] [CrossRef]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2773. [Google Scholar] [CrossRef]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-Immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.J.; Powley, T.L. Macrophages Associated with the Intrinsic and Extrinsic Autonomic Innervation of the Rat Gastrointestinal Tract. Auton. Neurosci. Basic Clin. 2012, 169, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Dora, D.; Ferenczi, S.; Stavely, R.; Toth, V.E.; Varga, Z.V.; Kovacs, T.; Bodi, I.; Hotta, R.; Kovacs, K.J.; Goldstein, A.M.; et al. Evidence of a Myenteric Plexus Barrier and Its Macrophage-Dependent Degradation During Murine Colitis: Implications in Enteric Neuroinflammation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1617–1641. [Google Scholar] [CrossRef]
- Viola, M.F.; Boeckxstaens, G. Intestinal Resident Macrophages: Multitaskers of the Gut. Neurogastroenterol. Motil. 2020, 32, e13843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meroni, E.; Stakenborg, N.; Viola, M.F.; Boeckxstaens, G.E. Intestinal Macrophages and Their Interaction with the Enteric Nervous System in Health and Inflammatory Bowel Disease. Acta Physiol. 2019, 225, e13163. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Ganz, J.; Bayrer, J.; Becker, L.; Bogunovic, M.; Rao, M. Advances in Enteric Neurobiology: The “Brain” in the Gut in Health and Disease. J. Neurosci. 2018, 38, 9346–9354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujko, A.; Atlasy, N.; Landsverk, O.J.B.; Richter, L.; Yaqub, S.; Horneland, R.; Øyen, O.; Aandahl, E.M.; Aabakken, L.; Stunnenberg, H.G.; et al. Transcriptional and Functional Profiling Defines Human Small Intestinal Macrophage Subsets. J. Exp. Med. 2018, 215, 441–458. [Google Scholar] [CrossRef] [Green Version]
- Bain, C.C.; Mowat, A.M. Macrophages in Intestinal Homeostasis and Inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [Green Version]
- Man, A.L.; Gicheva, N.; Regoli, M.; Rowley, G.; De Cunto, G.; Wellner, N.; Bassity, E.; Gulisano, M.; Bertelli, E.; Nicoletti, C. CX 3 CR1 + Cell–Mediated Salmonella Exclusion Protects the Intestinal Mucosa during the Initial Stage of Infection. J. Immunol. 2017, 198, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3+ Regulatory T Cells in the Lamina Propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef]
- Sehgal, A.; Donaldson, D.S.; Pridans, C.; Sauter, K.A.; Hume, D.A.; Mabbott, N.A. The Role of CSF1R-Dependent Macrophages in Control of the Intestinal Stem-Cell Niche. Nat. Commun. 2018, 9, 1272. [Google Scholar] [CrossRef] [Green Version]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef]
- Zigmond, E.; Bernshtein, B.; Friedlander, G.; Walker, C.R.; Yona, S.; Kim, K.W.; Brenner, O.; Krauthgamer, R.; Varol, C.; Müller, W.; et al. Macrophage-Restricted Interleukin-10 Receptor Deficiency, but Not IL-10 Deficiency, Causes Severe Spontaneous Colitis. Immunity 2014, 40, 720–733. [Google Scholar] [CrossRef] [Green Version]
- Shouval, D.S.; Biswas, A.; Goettel, J.A.; McCann, K.; Conaway, E.; Redhu, N.S.; Mascanfroni, I.D.; AlAdham, Z.; Lavoie, S.; Ibourk, M.; et al. Interleukin-10 Receptor Signaling in Innate Immune Cells Regulates Mucosal Immune Tolerance and Anti-Inflammatory Macrophage Function. Immunity 2014, 40, 706–719. [Google Scholar] [CrossRef] [Green Version]
- Krause, P.; Morris, V.; Greenbaum, J.A.; Park, Y.; Bjoerheden, U.; Mikulski, Z.; Muffley, T.; Shui, J.W.; Kim, G.; Cheroutre, H.; et al. IL-10-Producing Intestinal Macrophages Prevent Excessive Antibacterial Innate Immunity by Limiting IL-23 Synthesis. Nat. Commun. 2015, 6, 7055. [Google Scholar] [CrossRef]
- Sauter, K.A.; Pridans, C.; Sehgal, A.; Tsai, Y.T.; Bradford, B.M.; Raza, S.; Moffat, L.; Gow, D.J.; Beard, P.M.; Mabbott, N.A.; et al. Pleiotropic Effects of Extended Blockade of CSF1R Signaling in Adult Mice. J. Leukoc. Biol. 2014, 96, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Mortha, A.; Chudnovskiy, A.; Hashimoto, D.; Bogunovic, M.; Spencer, S.P.; Belkaid, Y.; Merad, M. Microbiota-Dependent Crosstalk between Macrophages and ILC3 Promotes Intestinal Homeostasis. Science 2014, 343, 1249288. [Google Scholar] [CrossRef] [Green Version]
- Egea, L.; McAllister, C.S.; Lakhdari, O.; Minev, I.; Shenouda, S.; Kagnoff, M.F. GM-CSF Produced by Nonhematopoietic Cells Is Required for Early Epithelial Cell Proliferation and Repair of Injured Colonic Mucosa. J. Immunol. 2013, 190, 1702–1713. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, N.; Schenten, D.; Nish, S.A.; Walther, Z.; Gagliani, N.; Flavell, R.A.; Reizis, B.; Shen, Z.; Fox, J.G.; Iwasaki, A.; et al. MyD88 Signalling in Colonic Mononuclear Phagocytes Drives Colitis in IL-10-Deficient Mice. Nat. Commun. 2012, 3, 1120. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.; Micci, M.A.; Leser, J.; Shin, C.; Tang, S.C.; Fu, Y.Y.; Liu, L.; Li, Q.; Saha, M.; Li, C.; et al. Adult Enteric Nervous System in Health Is Maintained by a Dynamic Balance between Neuronal Apoptosis and Neurogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E3709–E3718. [Google Scholar] [CrossRef] [Green Version]
- Margolis, K.G.; Gershon, M.D.; Bogunovic, M. Cellular Organization of Neuroimmune Interactions in the Gastrointestinal Tract. Trends Immunol. 2016, 37, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Becker, L.; Nguyen, L.; Gill, J.; Kulkarni, S.; Pasricha, P.J.; Habtezion, A. Age-Dependent Shift in Macrophage Polarisation Causes Inflammation-Mediated Degeneration of Enteric Nervous System. Gut 2018, 67, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, G.; Terhaar, M.L.; Eisenman, S.T.; Ji, S.; Linden, D.R.; Wright, A.M.; Sha, L.; Ordog, T.; Szurszewski, J.H.; Gibbons, S.J.; et al. Muscularis Propria Macrophages Alter the Proportion of Nitrergic but Not Cholinergic Gastric Myenteric Neurons. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 689–691. [Google Scholar] [CrossRef] [Green Version]
- Viola, M.F.; Boeckxstaens, G. Muscularis Macrophages: Trained Guardians of Enteric Neurons. Cell Res. 2022, 32, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Scott, C.L. Does Niche Competition Determine the Origin of Tissue-Resident Macrophages? Nat. Rev. Immunol. 2017, 17, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Avetisyan, M.; Rood, J.E.; Lopez, S.H.; Sengupta, R.; Wright-Jin, E.; Dougherty, J.D.; Behrens, E.M.; Heuckeroth, R.O. Muscularis Macrophage Development in the Absence of an Enteric Nervous System. Proc. Natl. Acad. Sci. USA 2018, 115, 4696–4701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, M.; Farrugia, G.; Lurken, M.S.; Bernard, C.E.; Faussonepellegrini, M.S.; Smyrk, T.C.; Parkman, H.P.; Abell, T.L.; Snape, W.J.; Hasler, W.L.; et al. Cellular Changes in Diabetic and Idiopathic Gastroparesis. Gastroenterology 2011, 140, 1575–1585. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannucchi, M.G. Telocytes and Macrophages in the Gut: From Morphology to Function, Do the Two Cell Types Interact with Each Other? Which Helps Which? Int. J. Mol. Sci. 2022, 23, 8435. https://doi.org/10.3390/ijms23158435
Vannucchi MG. Telocytes and Macrophages in the Gut: From Morphology to Function, Do the Two Cell Types Interact with Each Other? Which Helps Which? International Journal of Molecular Sciences. 2022; 23(15):8435. https://doi.org/10.3390/ijms23158435
Chicago/Turabian StyleVannucchi, Maria Giuliana. 2022. "Telocytes and Macrophages in the Gut: From Morphology to Function, Do the Two Cell Types Interact with Each Other? Which Helps Which?" International Journal of Molecular Sciences 23, no. 15: 8435. https://doi.org/10.3390/ijms23158435





