Influence of Polydatin on the Tumor Microenvironment In Vitro: Studies with a Colon Cancer Cell Model
Abstract
:1. Introduction
2. Results
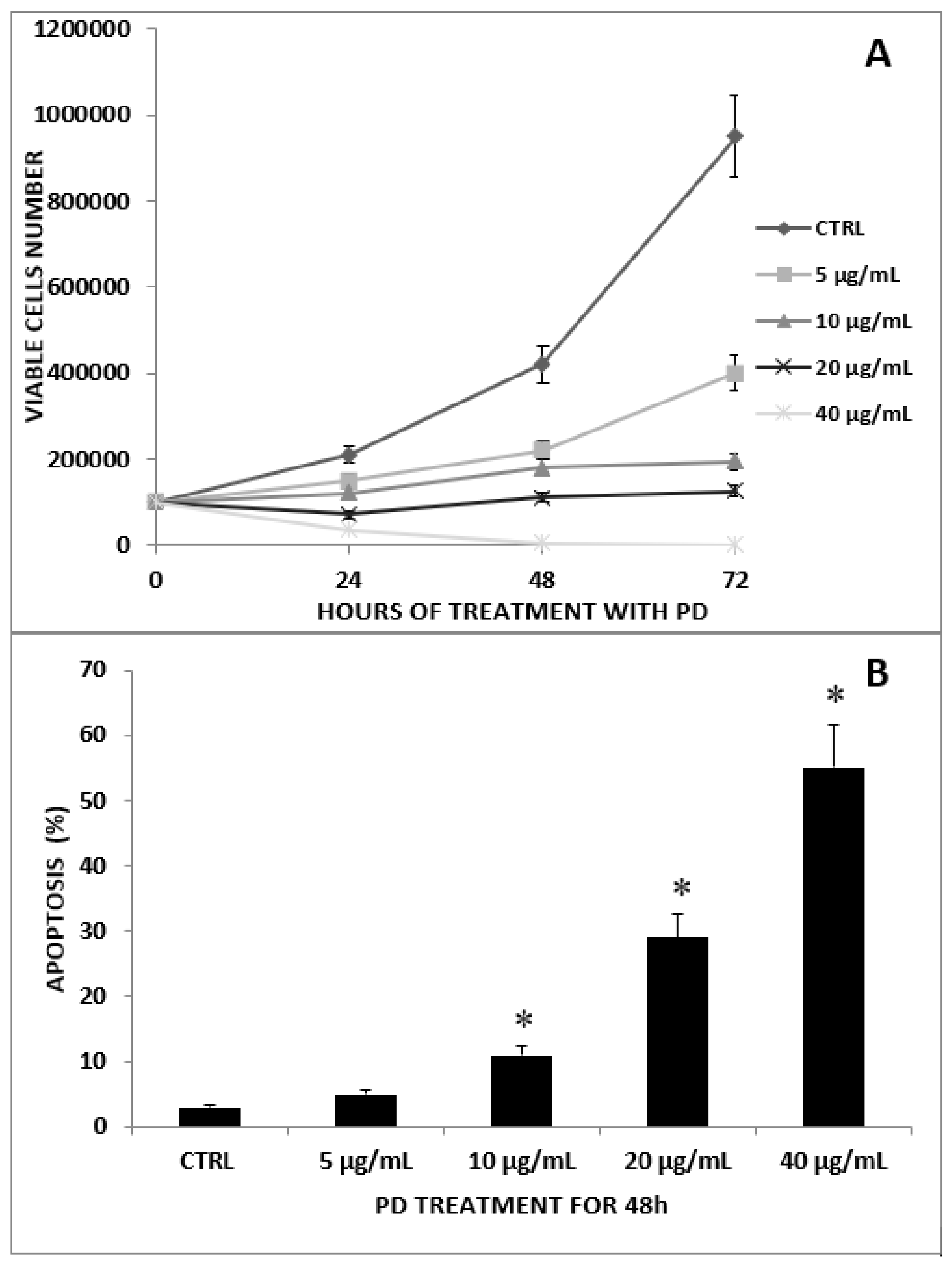
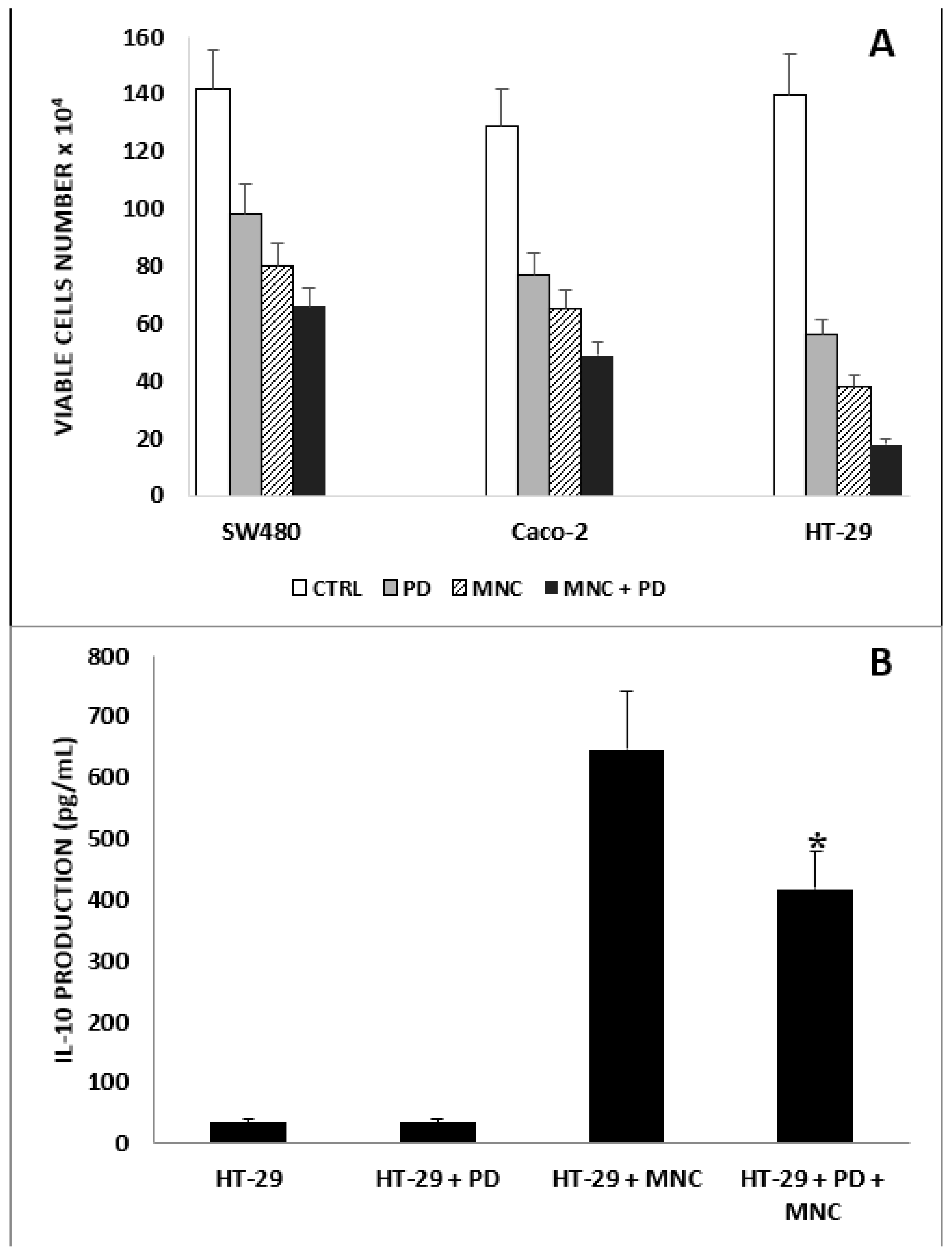
2.1. Antiproliferative Effect of PD on CRC and CRC/MNC Coculture
2.2. Effect of PD and LPS on HT-29 Cell Migration
2.3. Anti-Inflammatory Activity of PD on HT-29 Cells Treated with LPS
2.4. The Effect of PD on CAM Expression of HUVECs
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Reagents
4.3. Determination of Cell Growth and Apoptosis Induction
4.4. Wound-Healing Assay
4.5. CRC and MNC Coculture: Cell Growth and IL-10 Evaluation in Supernatants
4.6. Cell Treatments: CAM Evaluation and Inflammatory Cytokine Production
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, X.; Wu, C. The Role of the Tumor Microenvironment and Treatment Strategies in Colorectal Cancer. Front. Immunol. 2021, 12, 792691. [Google Scholar] [CrossRef] [PubMed]
- Crotti, S.; Piccoli, M.; Rizzolio, F.; Giordano, A.; Nitti, D.; Agostini, M. Extracellular Matrix and Colorectal Cancer: How Surrounding Microenvironment Affects Cancer Cell Behavior? J. Cell. Physiol. 2017, 232, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Chang, C.C.; Wang, C.S.; Lin, K.H. Association between Inflammation and Function of Cell Adhesion Molecules Influence on Gastrointestinal Cancer Development. Cells 2021, 10, 67. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Malekghasemi, S.; Majidi, J.; Baghbanzadeh, A.; Abdolalizadeh, J.; Baradaran, B.; Aghebati-Maleki, L. Tumor-Associated Macrophages: Protumoral Macrophages in Inflammatory Tumor Microenvironment. Adv. Pharm. Bull. 2020, 10, 556–565. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [Green Version]
- Herbeuval, J.P.; Lelievre, E.; Lambert, C.; Dy, M.; Genin, C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J. Immunol. 2004, 172, 4630–4636. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Wu, X.; Wu, Y.; Wang, X. Interaction between Treg cells and tumor-associated macrophages in the tumor microenvironment of epithelial ovarian cancer. Oncol. Rep. 2016, 36, 3472–3478. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, L.; Xu, C.; Wang, Y.; Wang, Z.; Chen, M.; Jiang, Z.; Pan, J.; Yang, C.; Li, X.; et al. Cross-talk between the gut microbiota and monocyte-like macrophages mediates an inflammatory response to promote colitis-associated tumourigenesis. Gut 2021, 70, 1495–1506. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, X.; Liu, K.; Shi, Y.; Wang, C.; Li, Y.; Zhang, T.; He, Y.; Xiang, M.; Zhao, R. Exosomal miR-200c-3p negatively regulates the migraion and invasion of lipopolysaccharide (LPS)-stimulated colorectal cancer (CRC). BMC Mol. Cell Biol. 2020, 21, 48. [Google Scholar] [CrossRef]
- Cario, E.; Rosenberg, I.M.; Brandwein, S.L.; Beck, P.L.; Reinecker, H.C.; Podolsky, D.K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 2000, 164, 966–972. [Google Scholar] [CrossRef]
- Tam, J.S.Y.; Coller, J.K.; Hughes, P.A.; Prestidge, C.A.; Bowen, J.M. Toll-like receptor 4 (TLR4) antagonists as potential therapeutics for intestinal inflammation. Indian J. Gastroenterol. 2021, 40, 5–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Tsuno, N.H.; Asakage, M.; Yamada, J.; Yoneyama, S.; Okaji, Y.; Sasaki, S.; Kitayama, J.; Osada, T.; Takahashi, K.; et al. Apoptosis induction by p38 MAPK inhibitor in human colon cancer cells. Hepatogastroenterology 2008, 55, 930–935. [Google Scholar]
- Allouche, R.; Hafeez, Z.; Papier, F.; Dary-Mourot, A.; Genay, M.; Miclo, L. In Vitro Anti-Inflammatory Activity of Peptides Obtained by Tryptic Shaving of Surface Proteins of Streptococcus thermophilus LMD-9. Foods 2022, 11, 1157. [Google Scholar] [CrossRef]
- Francavilla, C.; Maddaluno, L.; Cavallaro, U. The functional role of cell adhesion molecules in tumor angiogenesis. Semin. Cancer Biol. 2009, 19, 298–309. [Google Scholar] [CrossRef]
- Simiantonaki, N.; Jayasinghe, C.; Kirkpatrick, C.J. Effect of pro-inflammatory stimuli on tumor cell-mediated induction of endothelial cell adhesion molecules in vitro. Exp. Mol. Pathol. 2002, 73, 46–53. [Google Scholar] [CrossRef]
- Muz, B.; Abdelghafer, A.; Markovic, M.; Yavner, J.; Melam, A.; Salama, N.N.; Azab, A.K. Targeting E-selectin to Tackle Cancer Using Uproleselan. Cancers 2021, 13, 335. [Google Scholar] [CrossRef]
- Tedder, T.F.; Steeber, D.A.; Chen, A.; Engel, P. The selectins: Vascular adhesion molecules. FASEB J. 1995, 9, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Läubli, H.; Spanaus, K.S.; Borsig, L. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood 2009, 114, 4583–4591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Ye, P.; Wu, H.; Jiang, Y.; Xiao, X.; Song, D.; Xu, N.; Ma, X.; Zeng, J.; Guo, Y. Old dog, new tricks: Polydatin as a multitarget agent for current diseases. Phytother. Res. 2022, 36, 214–230. [Google Scholar] [CrossRef]
- Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Cottarelli, A.; Falchetti, R.; Ravagnan, G.; Bonmassar, E. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J. Exp. Clin. Cancer Res. 2006, 25, 189–193. [Google Scholar]
- Falchetti, R.; Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Ravagnan, G. Effects of resveratrol on human immune cell function. Life Sci. 2001, 70, 81–96. [Google Scholar] [CrossRef]
- De Maria, S.; Scognamiglio, I.; Lombardi, A.; Amodio, N.; Caraglia, M.; Cartenì, M.; Ravagnan, G.; Stiuso, P. Polydatin, a natural precursor of resveratrol, induces cell cycle arrest and differentiation of human colorectal Caco-2 cell. J. Transl. Med. 2013, 11, 264. [Google Scholar] [CrossRef] [Green Version]
- Lanzilli, G.; Cottarelli, A.; Nicotera, G.; Guida, S.; Ravagnan, G.; Fuggetta, M.P. Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation 2012, 35, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Ravagnan, G.; De Filippis, A.; Cartenì, M.; De Maria, S.; Cozza, V.; Petrazzuolo, M.; Tufano, M.A.; Donnarumma, G. Polydatin, a natural precursor of resveratrol, induces β-defensin production and reduces inflammatory response. Inflammation 2013, 36, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Fuggetta, M.P.; Mattivi, F. The immunomodulating activities of resveratrol glucosides in humans. Recent Pat. Food Nutr. Agric. 2011, 3, 81–90. [Google Scholar] [CrossRef]
- Jin, Y.; Zhan, X.; Zhang, B.; Chen, Y.; Liu, C.; Yu, L. Polydatin Exerts an Antitumor Effect through Regulating the miR-382/PD-L1 Axis in Colorectal Cancer. Cancer Biother. Radiopharm. 2020, 35, 83–91. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X.; et al. Resveratrol suppresses colon cancer growth by targeting the AKT/STAT3 signaling pathway. Int. J. Mol. Med. 2019, 43, 630–640. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Najafi, M. Resveratrol for targeting the tumor microenvironment and its interactions with cancer cells. Int. Immunopharmacol. 2021, 98, 107895. [Google Scholar] [CrossRef]
- Chen, L.; Musa, A.E. Boosting immune system against cancer by resveratrol. Phytother. Res. 2021, 35, 5514–5526. [Google Scholar] [CrossRef]
- Ham, J.S.; Park, H.Y.; Ryu, K.J.; Ko, Y.H.; Kim, W.S.; Kim, S.J. Elevated serum interleukin-10 level and M2 macrophage infiltration are associated with poor survival in angioimmunoblastic T-cell lymphoma. Oncotarget 2017, 8, 76231–76240. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, P.; Zhu, J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine 2015, 76, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.W.; Zhu, H.Z.; Zhu, Y.Q.; Feng, M.H.; Qi, J.; Chen, Z.F. Foxp3 expression in CD4+CD25+Foxp3+ regulatory T cells promotes development of colorectal cancer by inhibiting tumor immunity. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 677–682. [Google Scholar] [CrossRef]
- Cardoso, A.P.; Pinto, M.L.; Pinto, A.T.; Pinto, M.T.; Monteiro, C.; Oliveira, M.I.; Santos, S.G.; Relvas, J.B.; Seruca, R.; Mantovani, A.; et al. Matrix metalloproteases as maestros for the dual role of LPS- and IL-10-stimulated macrophages in cancer cell behaviour. BMC Cancer 2015, 15, 456. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ma, X.; Li, N.; Liu, H.; Dong, Q.; Zhang, J.; Yang, C.; Liu, Y.; Liang, Q.; Zhang, S.; et al. Resveratrol inhibits Hexokinases II mediated glycolysis in non-small cell lung cancer via targeting Akt signaling pathway. Exp. Cell Res. 2016, 349, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Jing, Y.Y.; Yan, F.; Han, Z.P.; Lai, F.B.; Zeng, J.X.; Yu, G.F.; Fan, Q.M.; Li, R.; Zhao, Q.D.; et al. LPS-induced CXCR4-dependent migratory properties and a mesenchymal-like phenotype of colorectal cancer cells. Cell Adhes. Migr. 2017, 11, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.H.; Xie, D.D.; Xu, S.; Xia, M.Z.; Zhang, Z.Q.; Geng, H.; Chen, L.; Wang, D.M.; Wei, W.; Yu, D.X.; et al. Total glucosides of paeony inhibits lipopolysaccharide-induced proliferation, migration and invasion in androgen insensitive prostate cancer cells. PLoS ONE 2017, 12, e0182584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.S.; Liu, Y.; Wang, X.W.; Xu, B.; Lin, Y.L.; Song, Y.; Dong, Y.; Liu, J.L.; Wang, X.J.; Liu, S.; et al. LPS Enhances the Chemosensitivity of Oxaliplatin in HT29 Cells via GSDMD-Mediated Pyroptosis. Cancer Manag. Res. 2020, 12, 10397–10409. [Google Scholar] [CrossRef]
- Hsu, R.Y.; Chan, C.H.; Spicer, J.D.; Rousseau, M.C.; Giannias, B.; Rousseau, S.; Ferri, L.E. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011, 71, 1989–1998. [Google Scholar] [CrossRef] [Green Version]
- Sonoda, H.; Kitamura, C.; Kano, K.; Anzai, H.; Nagai, Y.; Abe, S.; Yokoyama, Y.; Ishii, H.; Kishikawa, J.; Murono, K.; et al. Changes in Lysophospholipid Components in Ulcerative Colitis and Colitis-Associated Cancer. Anticancer Res. 2022, 42, 2461–2468. [Google Scholar] [CrossRef]
- Lee, S.K.; Il Kim, T.; Kim, Y.K.; Choi, C.H.; Yang, K.M.; Chae, B.; Kim, W.H. Cellular differentiation-induced attenuation of LPS response in HT-29 cells is related to the down-regulation of TLR4 expression. Biochem. Biophys. Res. Commun. 2005, 337, 457–463. [Google Scholar] [CrossRef]
- Kim, C.; Le, D.; Lee, M. Diterpenoids Isolated from Podocarpus macrophyllus Inhibited the Inflammatory Mediators in LPS-Induced HT-29 and RAW 264.7 Cells. Molecules 2021, 26, 4326. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: Differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef]
- Schwager, J.; Richard, N.; Widmer, F.; Raederstorff, D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement. Altern. Med. 2017, 17, 309. [Google Scholar] [CrossRef] [Green Version]
- Bergman, M.; Levin, G.S.; Bessler, H.; Djaldetti, M.; Salman, H. Resveratrol affects the cross talk between immune and colon cancer cells. Biomed. Pharm. 2013, 67, 43–47. [Google Scholar] [CrossRef]
- Deng, Y.H.; Alex, D.; Huang, H.Q.; Wang, N.; Yu, N.; Wang, Y.T.; Leung, G.P.; Lee, S.M. Inhibition of TNF-α-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4′-trimethoxystilbene. Phytother. Res. 2011, 25, 451–457. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, Y.; Dai, X.; Xu, S.; Li, T.; Zhang, Q.; Zhao, K.S.; Chen, Z. Polydatin ameliorates injury to the small intestine induced by hemorrhagic shock via SIRT3 activation-mediated mitochondrial protection. Expert Opin. Ther. Targets 2016, 20, 645–652. [Google Scholar] [CrossRef]
- Wang, X.; Buechler, N.L.; Yoza, B.K.; McCall, C.E.; Vachharajani, V.T. Resveratrol attenuates microvascular inflammation in sepsis via SIRT-1-Induced modulation of adhesion molecules in ob/ob mice. Obesity 2015, 23, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- Sferrazza, G.; Corti, M.; Andreola, F.; Giovannini, D.; Nicotera, G.; Zonfrillo, M.; Serra, M.; Tengattini, S.; Calleri, E.; Brusotti, G.; et al. Bioassay-Guided Isolation of Nigracin, Responsible for the Tissue Repair Properties of Drypetes Klainei Stem Bark. Front. Pharmacol. 2020, 10, 1541. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gregorio, A.; Krasnowska, E.K.; Zonfrillo, M.; Ravagnan, G.; Bordignon, V.; Bonmassar, E.; Fuggetta, M.P. Influence of Polydatin on the Tumor Microenvironment In Vitro: Studies with a Colon Cancer Cell Model. Int. J. Mol. Sci. 2022, 23, 8442. https://doi.org/10.3390/ijms23158442
De Gregorio A, Krasnowska EK, Zonfrillo M, Ravagnan G, Bordignon V, Bonmassar E, Fuggetta MP. Influence of Polydatin on the Tumor Microenvironment In Vitro: Studies with a Colon Cancer Cell Model. International Journal of Molecular Sciences. 2022; 23(15):8442. https://doi.org/10.3390/ijms23158442
Chicago/Turabian StyleDe Gregorio, Alex, Ewa Krystyna Krasnowska, Manuela Zonfrillo, Giampietro Ravagnan, Valentina Bordignon, Enzo Bonmassar, and Maria Pia Fuggetta. 2022. "Influence of Polydatin on the Tumor Microenvironment In Vitro: Studies with a Colon Cancer Cell Model" International Journal of Molecular Sciences 23, no. 15: 8442. https://doi.org/10.3390/ijms23158442





