Targeting Fatty Acid-Binding Protein 4 Improves Pathologic Features of Aortic Stenosis
Abstract
:1. Introduction
2. Results
2.1. Clinical Data in AS Patients
2.2. FABP4 Is Expressed in the Human Aortic Valve
2.3. FABP4 Expression Is Increased in AS Valves with Higher Levels in Men
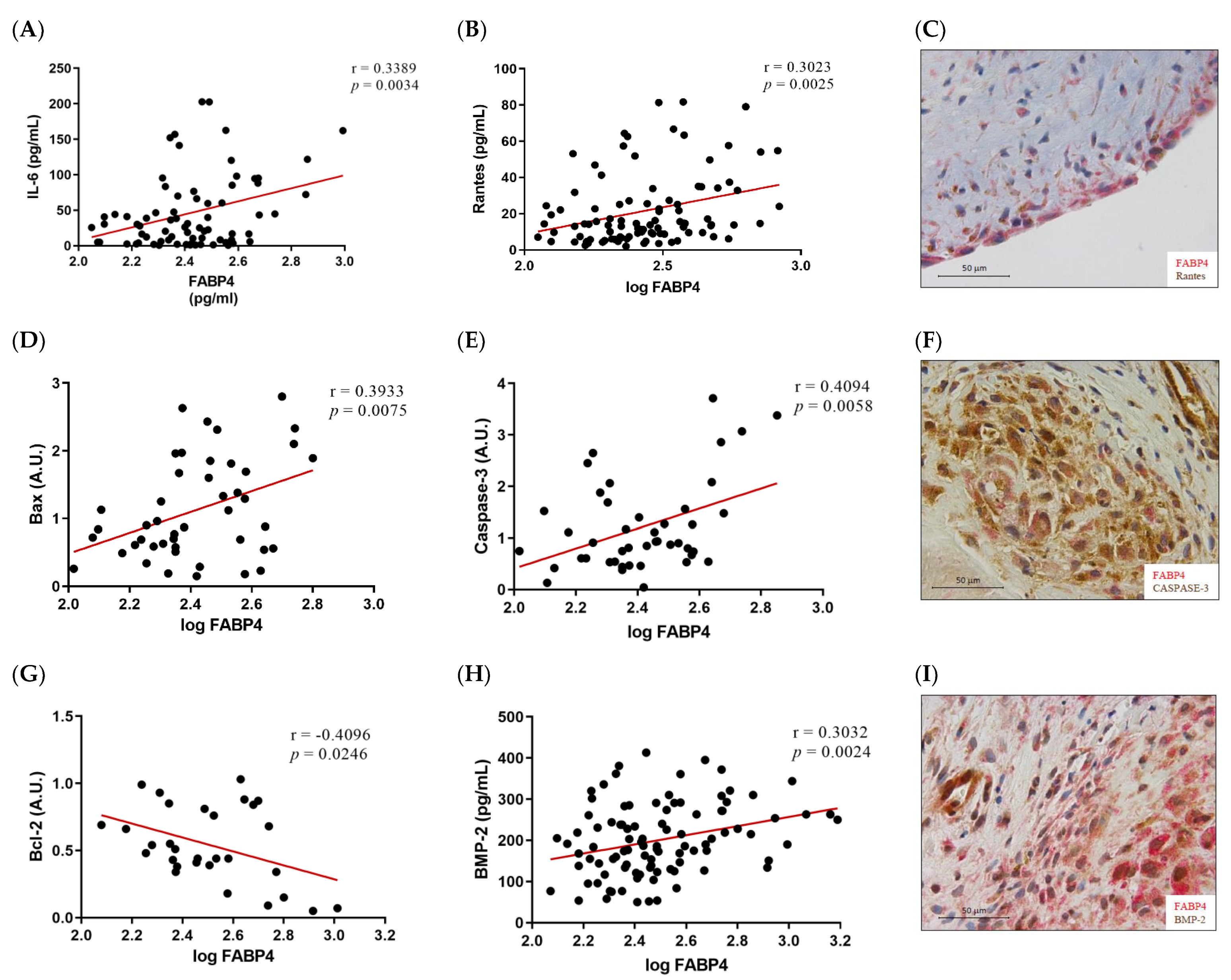
2.4. FABP4 Expression Correlates with Inflammatory, Apoptotic and Osteogenic Markers in AS
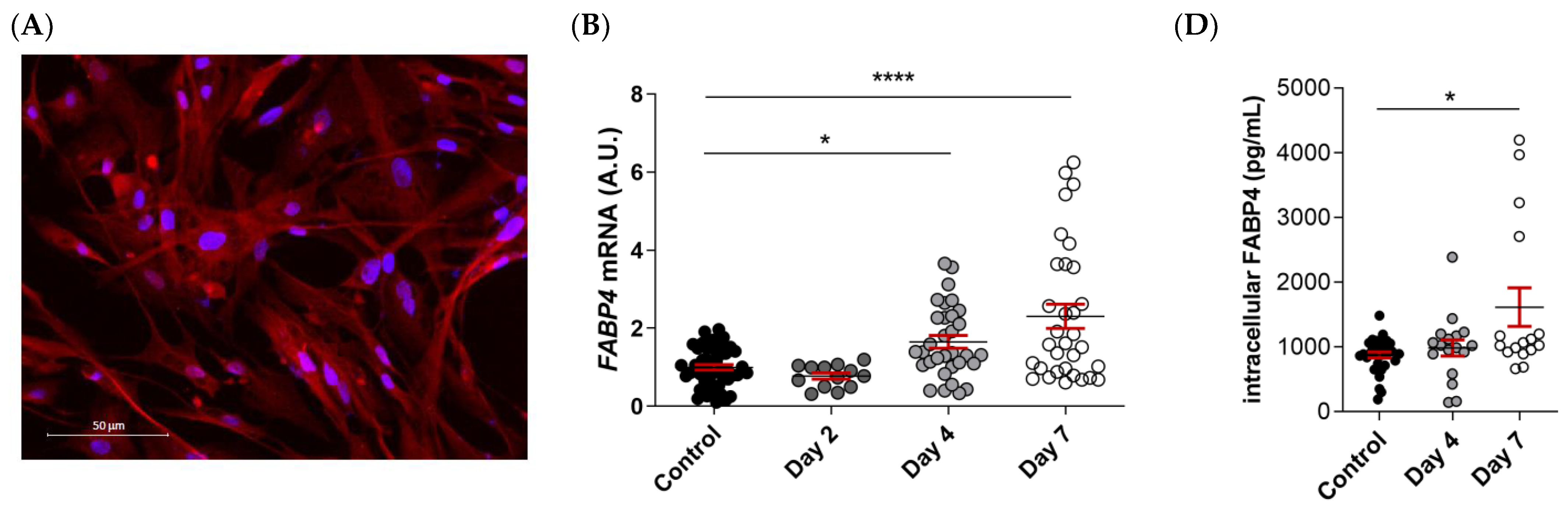
2.5. FABP4 Expression Is Upregulated in Male VICs during Osteogenesis
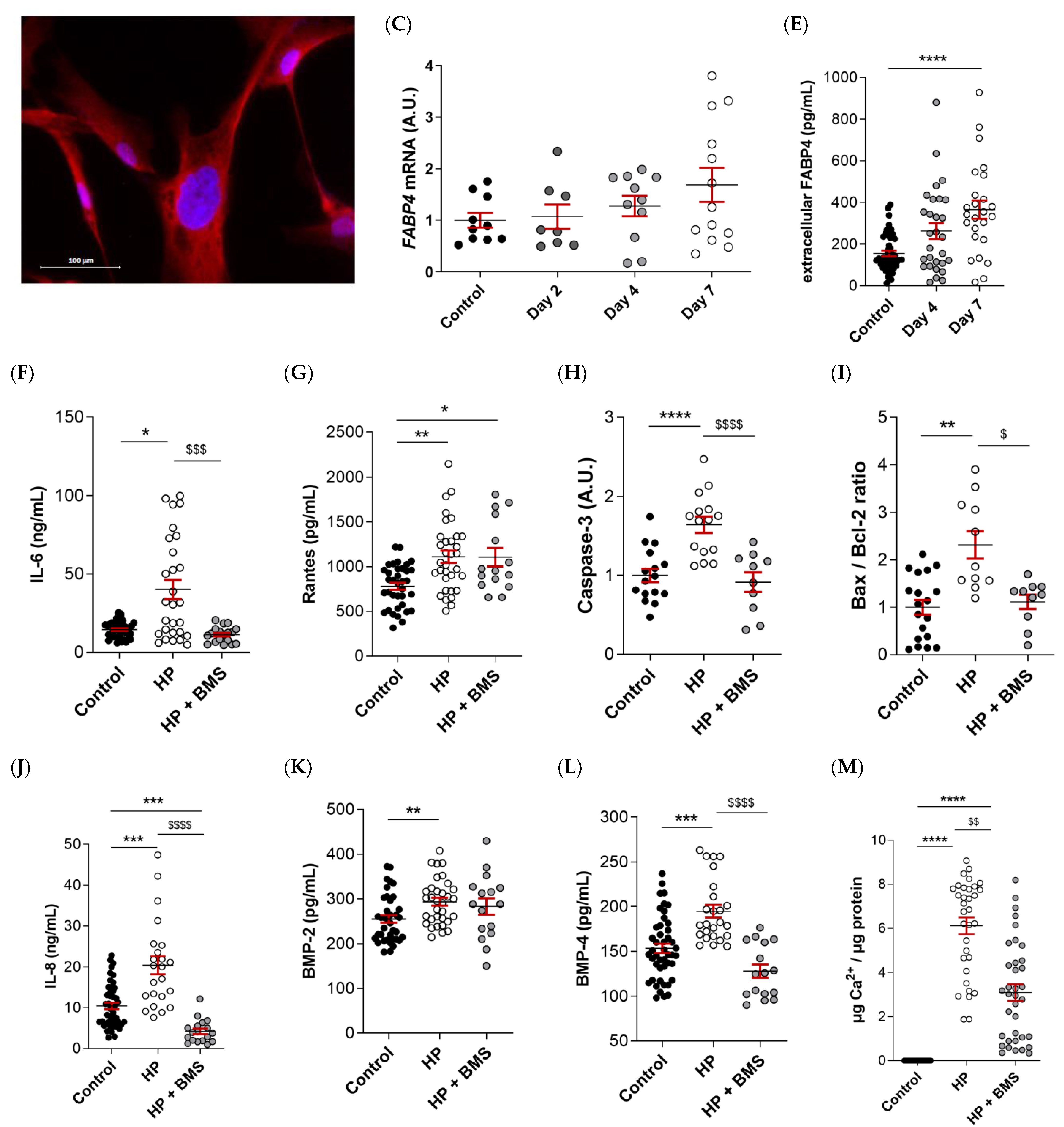
2.6. Pharmacological Inhibition of FABP4 Reduces Inflammation, Apoptosis, and Calcification in VICs
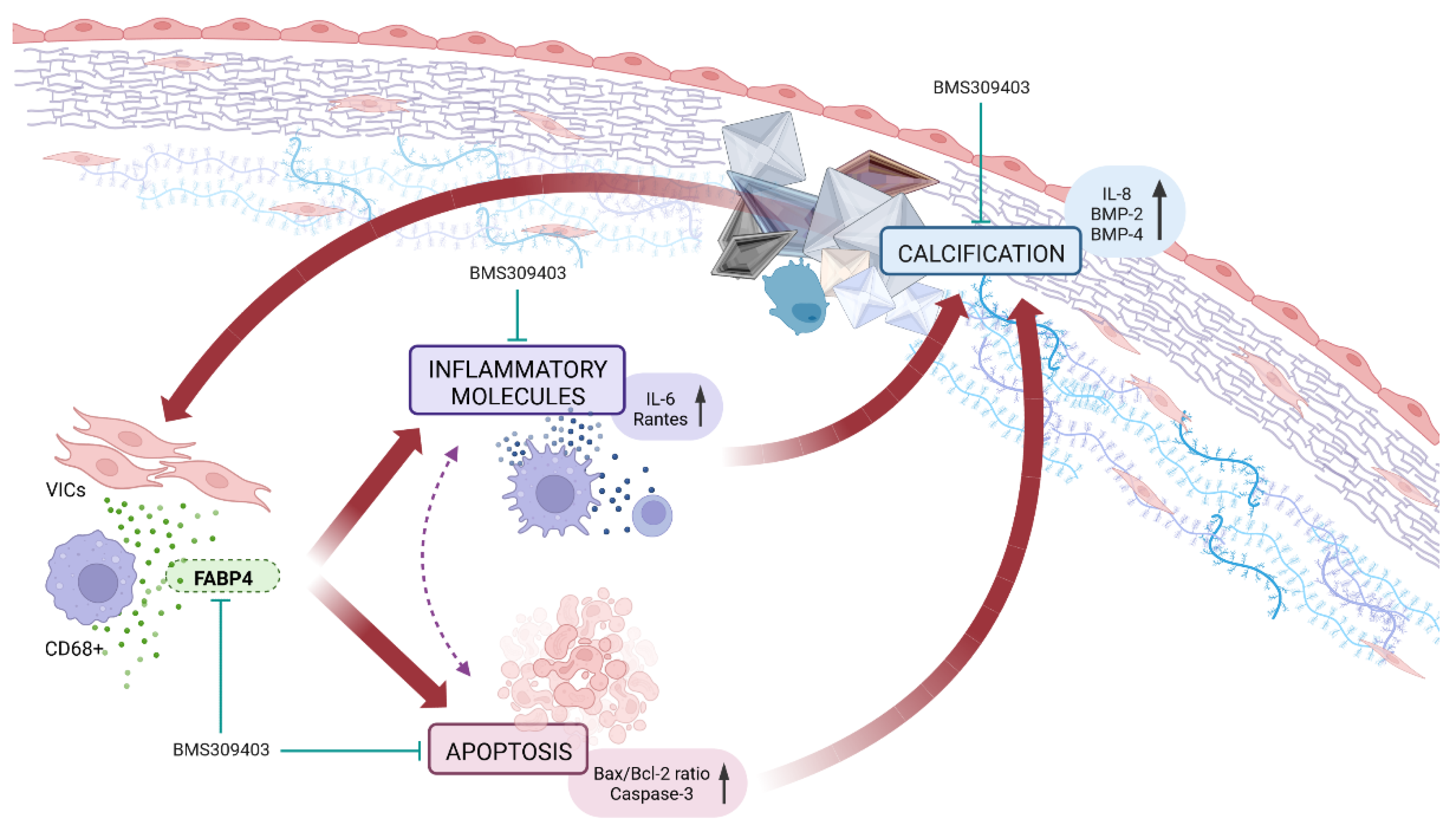
3. Discussion
4. Materials and Methods
4.1. Clinical Cohort
4.2. Histology and Immunohistochemistry Evaluation
4.3. Cell Isolation and Culture
4.4. Immunofluorescence
4.5. Western Blot Analysis
4.6. ELISA
4.7. Quantitative Reverse Transcription PCR
4.8. Calcium Content Determination
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andell, P.; Li, X.; Martinsson, A.; Andersson, C.; Stagmo, M.; Zöller, B.; Sundquist, K.; Smith, J.G. Epidemiology of Valvular Heart Disease in a Swedish Nationwide Hospital-Based Register Study. Heart 2017, 103, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Goody, P.R.; Hosen, M.R.; Christmann, D.; Niepmann, S.T.; Zietzer, A.; Adam, M.; Bönner, F.; Zimmer, S.; Nickenig, G.; Jansen, F. Aortic Valve Stenosis: From Basic Mechanisms to Novel Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, R.; Aspelund, T.; Harris, T.B.; Gudnason, V. The Prevalence of Aortic Stenosis in the Elderly in Iceland and Predictions for the Coming Decades: The AGES-Reykjavík Study. Int. J. Cardiol. 2014, 176, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, B.; Vahanian, A. The 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: A New Template for Heart Teams and Their Patients. Cardiovasc. Res. 2022, 118, E11–E13. [Google Scholar] [CrossRef]
- Yetkin, E.; Waltenberger, J. Molecular and Cellular Mechanisms of Aortic Stenosis. Int. J. Cardiol. 2009, 135, 4–13. [Google Scholar] [CrossRef]
- Bienjonetti-Boudreau, D.; Fleury, M.A.; Voisine, M.; Paquin, A.; Chouinard, I.; Tailleur, M.; Duval, R.; Magnan, P.O.; Beaudoin, J.; Salaun, E.; et al. Impact of Sex on the Management and Outcome of Aortic Stenosis Patients. Eur. Heart J. 2021, 42, 2683–2691. [Google Scholar] [CrossRef]
- Desjardin, J.T.; Chikwe, J.; Hahn, R.T.; Hung, J.W.; Delling, F.N. Sex Differences and Similarities in Valvular Heart Disease. Circ. Res. 2022, 130, 455–473. [Google Scholar] [CrossRef]
- Matilla, L.; Garaikoetxea, M.; Arrieta, V.; García-Peña, A.; Fernández-Celis, A.; Navarro, A.; Gainza, A.; Álvarez, V.; Sádaba, R.; Jover, E.; et al. Sex-Differences in Aortic Stenosis: Mechanistic Insights and Clinical Implications. Front. Cardiovasc. Med. 2022, 9, 299. [Google Scholar] [CrossRef]
- Matilla, L.; Jover, E.; Garaikoetxea, M.; Martín-Nuñez, E.; Arrieta, V.; García-Peña, A.; Navarro, A.; Fernández-Celis, A.; Gainza, A.; Álvarez, V.; et al. Sex-Related Signaling of Aldosterone/Mineralocorticoid Receptor Pathway in Calcific Aortic Stenosis. Hypertension 2022, 79, 1724–1737. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, X.; Xu, A.; Hoo, R.L.C. A-FABP in Metabolic Diseases and the Therapeutic Implications: An Update. Int. J. Mol. Sci. 2021, 22, 9386. [Google Scholar] [CrossRef]
- Furuhashi, M.; Saitoh, S.; Shimamoto, K.; Miura, T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin. Med. Insights. Cardiol. 2015, 8, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Agardh, H.E.; Folkersen, L.; Ekstrand, J.; Marcus, D.; Swedenborg, J.; Hedin, U.; Gabrielsen, A.; Paulsson-Berne, G. Expression of Fatty Acid-Binding Protein 4/AP2 Is Correlated with Plaque Instability in Carotid Atherosclerosis. J. Intern. Med. 2011, 269, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, M.; Fuseya, T.; Murata, M.; Hoshina, K.; Ishimura, S.; Mita, T.; Watanabe, Y.; Omori, A.; Matsumoto, M.; Sugaya, T.; et al. Local Production of Fatty Acid-Binding Protein 4 in Epicardial/Perivascular Fat and Macrophages Is Linked to Coronary Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Han, B.; Li, H.; Tao, Y.; Wu, W. FABP4 Knockdown Suppresses Inflammation, Apoptosis and Extracellular Matrix Degradation in IL-1β-Induced Chondrocytes by Activating PPARγ to Regulate the NF-ΚB Signaling Pathway. Mol. Med. Rep. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Sun, F.; Chen, G.; Yang, Y.; Lei, M. Fatty Acid-Binding Protein 4 Silencing Protects against Lipopolysaccharide-Induced Cardiomyocyte Hypertrophy and Apoptosis by Inhibiting the Toll-like Receptor 4-Nuclear Factor-ΚB Pathway. J. Int. Med. Res. 2021, 49, 0300060521998233. [Google Scholar] [CrossRef]
- Furuhashi, M.; Tuncman, G.; Görgün, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of Diabetes and Atherosclerosis by Inhibiting Fatty-Acid-Binding Protein AP2. Nature 2007, 447, 959–965. [Google Scholar] [CrossRef] [Green Version]
- López-Canoa, J.N.; Couselo-Seijas, M.; Baluja, A.; González-Melchor, L.; Rozados, A.; Llorente-Cortés, V.; De Gonzalo-Calvo, D.; Guerra, J.M.; Vilades, D.; Leta, R.; et al. Sex-Related Differences of Fatty Acid-Binding Protein 4 and Leptin Levels in Atrial Fibrillation. Europace 2021, 23, 682–690. [Google Scholar] [CrossRef]
- Yeung, D.C.Y.; Xu, A.; Cheung, C.W.S.; Wat, N.M.S.; Yau, M.H.; Fong, C.H.Y.; Chau, M.T.; Lam, K.S.L. Serum Adipocyte Fatty Acid-Binding Protein Levels Were Independently Associated with Carotid Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1796–1802. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.P.; Hsu, C.C.; Hung, W.C.; Yu, T.H.; Wu, C.C.; Tsai, I.T.; Tang, W.H.; Chung, F.M.; Houng, J.Y.; Lee, Y.J.; et al. Plasma Fatty Acid-Binding Protein 4 (FABP4) Level Is Associated with Abnormal QTc Interval in Patients with Stable Angina and Chronic Kidney Disease. BMC Cardiovasc. Disord. 2019, 19, 153. [Google Scholar] [CrossRef]
- Śadaba, J.R.; Martínez-Martínez, E.; Arrieta, V.; Álvarez, V.; Fernández-Celis, A.; Ibarrola, J.; Melero, A.; Rossignol, P.; Cachofeiro, V.; López-Andrés, N. Role for Galectin-3 in Calcific Aortic Valve Stenosis. J. Am. Heart Assoc. 2016, 5, e004360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, M.; Hotamisligil, G.S. Fatty Acid-Binding Proteins: Role in Metabolic Diseases and Potential as Drug Targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.Q.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty Acid Binding Protein 4 Is a Target of VEGF and a Regulator of Cell Proliferation in Endothelial Cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuseya, T.; Furuhashi, M.; Matsumoto, M.; Watanabe, Y.; Hoshina, K.; Mita, T.; Ishimura, S.; Tanaka, M.; Miura, T. Ectopic Fatty Acid-Binding Protein 4 Expression in the Vascular Endothelium Is Involved in Neointima Formation After Vascular Injury. J. Am. Heart Assoc. 2017, 6, e006377. [Google Scholar] [CrossRef]
- Schlottmann, I.; Ehrhart-Bornstein, M.; Wabitsch, M.; Bornstein, S.R.; Lamounier-Zepter, V. Calcium-Dependent Release of Adipocyte Fatty Acid Binding Protein from Human Adipocytes. Int. J. Obes. 2014, 38, 1221–1227. [Google Scholar] [CrossRef]
- Kralisch, S.; Klöting, N.; Ebert, T.; Kern, M.; Hoffmann, A.; Krause, K.; Jessnitzer, B.; Lossner, U.; Sommerer, I.; Stumvoll, M.; et al. Circulating Adipocyte Fatty Acid-Binding Protein Induces Insulin Resistance in Mice in Vivo. Obesity 2015, 23, 1007–1013. [Google Scholar] [CrossRef]
- Yao, F.; Li, Z.; Ehara, T.; Yang, L.; Wang, D.; Feng, L.; Zhang, Y.; Wang, K.; Shi, Y.; Duan, H.; et al. Fatty Acid-Binding Protein 4 Mediates Apoptosis via Endoplasmic Reticulum Stress in Mesangial Cells of Diabetic Nephropathy. Mol. Cell. Endocrinol. 2015, 411, 232–242. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, Z.; Gao, Y.; Deng, L.; Wang, M.; Chen, Y.; Li, J.; Cheng, B. FABP4 Inhibitors Suppress Inflammation and Oxidative Stress in Murine and Cell Models of Acute Lung Injury. Biochem. Biophys. Res. Commun. 2018, 496, 1115–1121. [Google Scholar] [CrossRef]
- Wang, B.; Xu, J.; Ren, Q.; Cheng, L.; Guo, F.; Liang, Y.; Yang, L.; Tan, Z.; Fu, P.; Ma, L. Fatty Acid-Binding Protein 4 Is a Therapeutic Target for Septic Acute Kidney Injury by Regulating Inflammatory Response and Cell Apoptosis. Cell Death Dis. 2022, 13, 333. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Shi, J.; Han, Q.; He, L.; Tang, W.; Zhang, A. Serum Fatty Acid Binding Protein 4 Levels Are Associated with Abdominal Aortic Calcification in Peritoneal Dialysis Patients. Ren. Fail. 2021, 43, 1539–1548. [Google Scholar] [CrossRef]
- Bagheri, R.; Qasim, A.N.; Mehta, N.N.; Terembula, K.; Kapoor, S.; Braunstein, S.; Schutta, M.; Iqbal, N.; Lehrke, M.; Reilly, M.P. Relation of Plasma Fatty Acid Binding Proteins 4 and 5 with the Metabolic Syndrome, Inflammation and Coronary Calcium in Patients with Type-2 Diabetes Mellitus. Am. J. Cardiol. 2010, 106, 1118–1123. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, K.; Chen, L.; Usawachintachit, M.; Hamamoto, S.; Kang, M.; Sugino, T.; Unno, R.; Tzou, D.T.; Sherer, B.A.; Okada, A.; et al. Fatty Acid-Binding Protein 4 Downregulation Drives Calcification in the Development of Kidney Stone Disease. Kidney Int. 2020, 97, 1042–1056. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical Factors Associated with Calcific Aortic Valve Disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef] [Green Version]
- Surgical Pathology of the Aortic Valve: A Morphologic Study on 912 Surgically Excised Valves—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/1291412/ (accessed on 28 June 2022).
- Chaudhary, A.; Singla, S.K.; Tandon, C. In Vitro Evaluation of Terminalia Arjuna on Calcium Phosphate and Calcium Oxalate Crystallization. Indian J. Pharm. Sci. 2010, 72, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, M.; Mita, T.; Moniwa, N.; Hoshina, K.; Ishimura, S.; Fuseya, T.; Watanabe, Y.; Yoshida, H.; Shimamoto, K.; Miura, T. Angiotensin II Receptor Blockers Decrease Serum Concentration of Fatty Acid-Binding Protein 4 in Patients with Hypertension. Hypertens. Res. 2015, 38, 252–259. [Google Scholar] [CrossRef]
- Karpisek, M.; Stejskal, D.; Kotolova, H.; Kollar, P.; Janoutova, G.; Ochmanova, R.; Cizek, L.; Horakova, D.; Yahia, R.B.; Lichnovska, R.; et al. Treatment with Atorvastatin Reduces Serum Adipocyte-Fatty Acid Binding Protein Value in Patients with Hyperlipidaemia. Eur. J. Clin. Investig. 2007, 37, 637–642. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hiramitsu, S.; Mita, T.; Fuseya, T.; Ishimura, S.; Omori, A.; Matsumoto, M.; Watanabe, Y.; Hoshina, K.; Tanaka, M.; et al. Reduction of Serum FABP4 Level by Sitagliptin, a DPP-4 Inhibitor, in Patients with Type 2 Diabetes Mellitus. J. Lipid Res. 2015, 56, 2372–2380. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garaikoetxea, M.; Martín-Núñez, E.; Navarro, A.; Matilla, L.; Fernández-Celis, A.; Arrieta, V.; García-Peña, A.; Gainza, A.; Álvarez, V.; Sádaba, R.; et al. Targeting Fatty Acid-Binding Protein 4 Improves Pathologic Features of Aortic Stenosis. Int. J. Mol. Sci. 2022, 23, 8439. https://doi.org/10.3390/ijms23158439
Garaikoetxea M, Martín-Núñez E, Navarro A, Matilla L, Fernández-Celis A, Arrieta V, García-Peña A, Gainza A, Álvarez V, Sádaba R, et al. Targeting Fatty Acid-Binding Protein 4 Improves Pathologic Features of Aortic Stenosis. International Journal of Molecular Sciences. 2022; 23(15):8439. https://doi.org/10.3390/ijms23158439
Chicago/Turabian StyleGaraikoetxea, Mattie, Ernesto Martín-Núñez, Adela Navarro, Lara Matilla, Amaya Fernández-Celis, Vanessa Arrieta, Amaia García-Peña, Alicia Gainza, Virginia Álvarez, Rafael Sádaba, and et al. 2022. "Targeting Fatty Acid-Binding Protein 4 Improves Pathologic Features of Aortic Stenosis" International Journal of Molecular Sciences 23, no. 15: 8439. https://doi.org/10.3390/ijms23158439
APA StyleGaraikoetxea, M., Martín-Núñez, E., Navarro, A., Matilla, L., Fernández-Celis, A., Arrieta, V., García-Peña, A., Gainza, A., Álvarez, V., Sádaba, R., Jover, E., & López-Andrés, N. (2022). Targeting Fatty Acid-Binding Protein 4 Improves Pathologic Features of Aortic Stenosis. International Journal of Molecular Sciences, 23(15), 8439. https://doi.org/10.3390/ijms23158439






