Thiosulfate-Cyanide Sulfurtransferase a Mitochondrial Essential Enzyme: From Cell Metabolism to the Biotechnological Applications
Abstract
:1. Introduction
2. Structure and Function of TST
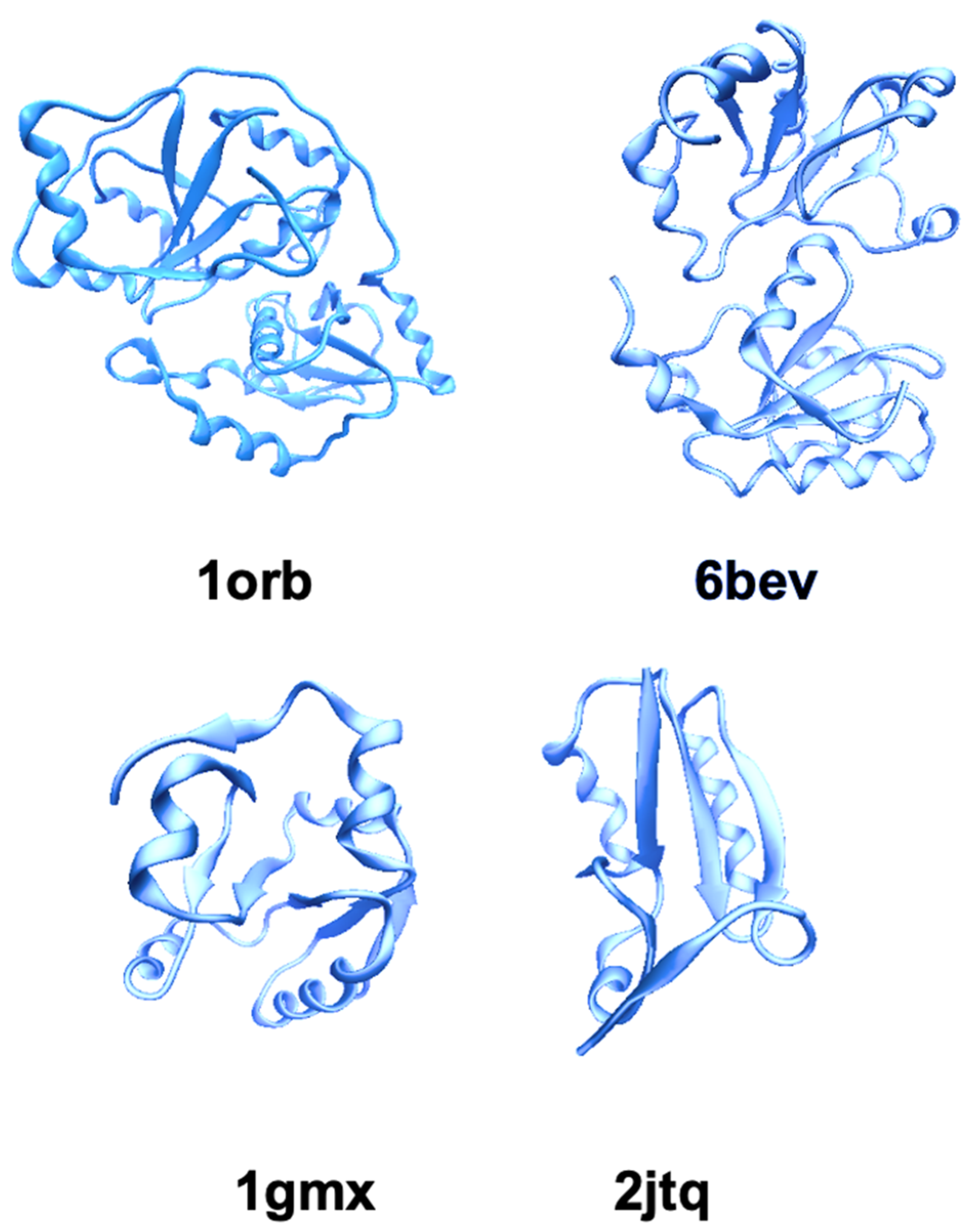
2.1. TST/Rhodanese Structure: A Model for Studying the Protein Folding
2.2. Functional Role of TST in Cell Metabolism
3. TST in Diseases
3.1. Lebers Hereditary Optic Neuropathy
3.2. Colonic Diseases and Cancer
3.3. Diabetes Mellitus Type 2 and Friedreich’s Ataxia
4. Biotechnological Application of TST
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, K. Die Rhodanbildung Im Tierkorper [Thiocyanogen in the Bodies of Animals]. FEBS J. 1933, 259, 243–256. [Google Scholar]
- Tabita, R.; Silver, M.; Lundgren, D. The Rhodanese Enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can. J. Biochem. 1969, 47, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Westley, J. Rhodanese. Adv. Enzymol. Relat. Areas Mol. Biol. 1973, 39, 736–737. [Google Scholar]
- Aita, N.; Ishii, K.; Akamatsu, Y.; Ogasawara, Y.; Tanabe, S. Cloning and Expression of Human Liver Rhodanese CDNA. Biochem. Biophys. Res. Commun. 1997, 231, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Sorbo, B. On the Properties of Rhodanese. Acta Chem. Scand. 1951, 5, 724–726. [Google Scholar]
- Himwich, W.A.; Saunders, J.P. Enzymatic Conversion of Cyanide to Thiocyanate. Am. J. Physiol. Leg. Content 1948, 153, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Hatzfeld, Y.; Saito, K. Evidence for the Existence of Rhodanese (Thiosulfate: Cyanide Sulfurtransferase) in Plants: Preliminary Characterization of Two Rhodanese CDNAs from Arabidopsis Thaliana. FEBS Lett. 2000, 470, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Tomati, U.; Giovannozzi-Sermanni, G.; Duprè, S.; Cannella, C. NADH: Nitrate Reductase Activity Restoration by Rhodanese. Phytochemistry 1976, 15, 597–598. [Google Scholar] [CrossRef]
- Green, J.R.; Westley, J. Mechanism of Rhodanese Action: Polarographic Studies. J. Biol. Chem. 1961, 236, 3047–3050. [Google Scholar] [CrossRef]
- Hall, A.H.; Saiers, J.; Baud, F. Which Cyanide Antidote? Crit. Rev. Toxicol. 2009, 39, 541–552. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Xia, Y.; Xun, L.; Liu, H. Saccharomyces Cerevisiae Rhodanese RDL2 Uses the Arg Residue of the Active-Site Loop for Thiosulfate Decomposition. Antioxidants 2021, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Sriraman, A.; Banerjee, R. Polymorphic Variants of Human Rhodanese Exhibit Differences in Thermal Stability and Sulfur Transfer Kinetics. J. Biol. Chem. 2015, 290, 23579–23588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahara, N.; Okazaki, T.; Nishino, T. Cytosolic Mercaptopyruvate Sulfurtransferase Is Evolutionarily Related to Mitochondrial Rhodanese: Striking similarity in active site amino acid sequence and the increase in the mercaptopyruvate sulfurtransferase activity of rhodanese by site-directed mutagenesis. J. Biol. Chem. 1995, 270, 16230–16235. [Google Scholar] [PubMed] [Green Version]
- Hendry-Hofer, T.B.; Ng, P.C.; Witeof, A.E.; Mahon, S.B.; Brenner, M.; Boss, G.R.; Bebarta, V.S. A Review on Ingested Cyanide: Risks, Clinical Presentation, Diagnostics, and Treatment Challenges. J. Med. Toxicol. 2019, 15, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Cressey, P.; Saunders, D.A.; Goodman, J.M. Cyanogenic Glycosides in Plant-Based Foods Available in New Zealand. Food Addit. Contam. Part A 2013, 30, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Shahbazkia, H.R.; Aminlari, M.; Tavana, M. Distribution of the Enzyme Rhodanese in Tissues of the Cat (Felis catus). J. Feline Med. Surg. 2009, 11, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Aminlari, M.; Li, A.; Kunanithy, V.; Scaman, C.H. Rhodanese Distribution in Porcine (Sus scrofa) Tissues. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 132, 309–313. [Google Scholar] [CrossRef]
- Yip, W.-K.; Yang, S.F. Cyanide Metabolism in Relation to Ethylene Production in Plant Tissues. Plant Physiol. 1988, 88, 473–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploegman, J.H.; Drent, G.; Kalk, K.H.; Hol, W.G.; Heinrikson, R.L.; Keim, P.; Weng, L.; Russell, J. The Covalent and Tertiary Structure of Bovine Liver Rhodanese. Nature 1978, 273, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Bordo, D.; Bork, P. The Rhodanese/Cdc25 Phosphatase Superfamily. EMBO Rep. 2002, 3, 741–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spallarossa, A.; Donahue, J.L.; Larson, T.J.; Bolognesi, M.; Bordo, D. Escherichia Coli GlpE Is a Prototype Sulfurtransferase for the Single-Domain Rhodanese Homology Superfamily. Structure 2001, 9, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.; Teertstra, W.; Koster, M.; Tommassen, J. PspE (Phage-Shock Protein E) of Escherichia Coli Is a Rhodanese. FEBS Lett. 2002, 518, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Cipollone, R.; Ascenzi, P.; Visca, P. Common Themes and Variations in the Rhodanese Superfamily. IUBMB Life 2007, 59, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Melideo, S.L.; Jackson, M.R.; Jorns, M.S. Biosynthesis of a Central Intermediate in Hydrogen Sulfide Metabolism by a Novel Human Sulfurtransferase and Its Yeast Ortholog. Biochemistry 2014, 53, 4739–4753. [Google Scholar] [CrossRef]
- Pagani, S.; Sessa, G.; Sessa, F.; Colnaghi, R. Properties of Azotobacter Vinelandii Rhodanese. Biochem. Mol. Biol. Int. 1993, 29, 595–604. [Google Scholar] [PubMed]
- Melino, S.; Cicero, D.O.; Forlani, F.; Pagani, S.; Paci, M. The N-Terminal Rhodanese Domain from Azotobacter Vinelandii Has a Stable and Folded Structure Independently of the C-Terminal Domain. FEBS Lett. 2004, 577, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the Human Mitochondrial Hydrogen Sulfide Oxidation Pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [Green Version]
- Ray, W.K.; Zeng, G.; Potters, M.B.; Mansuri, A.M.; Larson, T.J. Characterization of a 12-Kilodalton Rhodanese Encoded by GlpE of Escherichia Coli and Its Interaction with Thioredoxin. J. Bacteriol. 2000, 182, 2277–2284. [Google Scholar] [CrossRef] [Green Version]
- Palenchar, P.M.; Buck, C.J.; Cheng, H.; Larson, T.J.; Mueller, E.G. Evidence That ThiI, an Enzyme Shared between Thiamin and 4-Thiouridine Biosynthesis, May Be a Sulfurtransferase That Proceeds through a Persulfide Intermediate. J. Biol. Chem. 2000, 275, 8283–8286. [Google Scholar] [CrossRef] [Green Version]
- Colnaghi, R.; Pagani, S.; Kennedy, C.; Drummond, M. Cloning, Sequence Analysis and Overexpression of the Rhodanese Gene of Azotobacter Vinelandii. Eur. J. Biochem. 1996, 236, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Forlani, F.; Carpen, A.; Bordo, D.; Colnaghi, R. Mutagenic Analysis of Thr-232 in Rhodanese from Azotobacter Vinelandii Highlighted the Differences of This Prokaryotic Enzyme from the Known Sulfurtransferases. FEBS Lett. 2000, 472, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Melino, S.; Cicero, D.O.; Orsale, M.; Forlani, F.; Pagani, S.; Paci, M. Azotobacter Vinelandii Rhodanese: Selenium Loading and Ion Interaction Studies. Eur. J. Biochem. 2003, 270, 4208–4215. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Motl, N.; Akey, D.L.; Sakamoto, N.; Fearon, E.R.; Smith, J.L.; Banerjee, R. Thiosulfate Sulfurtransferase-like Domain-Containing 1 Protein Interacts with Thioredoxin. J. Biol. Chem. 2018, 293, 2675–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Keithly, M.E.; Armstrong, R.N.; Higgins, K.A.; Edmonds, K.A.; Giedroc, D.P. Staphylococcus Aureus CstB Is a Novel Multidomain Persulfide Dioxygenase-Sulfurtransferase Involved in Hydrogen Sulfide Detoxification. Biochemistry 2015, 54, 4542–4554. [Google Scholar] [CrossRef] [Green Version]
- Motl, N.; Skiba, M.A.; Kabil, O.; Smith, J.L.; Banerjee, R. Structural and Biochemical Analyses Indicate That a Bacterial Persulfide Dioxygenase–Rhodanese Fusion Protein Functions in Sulfur Assimilation. J. Biol. Chem. 2017, 292, 14026–14038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza, A.N.; Cambui, C.C.N.; Moreno, A.C.R.; Fessel, M.R.; Balan, A. Mycobacterium Tuberculosis CysA2 Is a Dual Sulfurtransferase with Activity against Thiosulfate and 3-Mercaptopyruvate and Interacts with Mammalian Cells. Sci. Rep. 2019, 9, 16791. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.A.; Rogers, E.; Lorimer, G.H.; Horowitz, P.M. Chaperonins Facilitate the in Vitro Folding of Monomeric Mitochondrial Rhodanese. J. Biol. Chem. 1991, 266, 13044–13049. [Google Scholar] [CrossRef]
- Hofmann, H.; Hillger, F.; Pfeil, S.H.; Hoffmann, A.; Streich, D.; Haenni, D.; Nettels, D.; Lipman, E.A.; Schuler, B. Single-Molecule Spectroscopy of Protein Folding in a Chaperonin Cage. Proc. Natl. Acad. Sci. USA 2010, 107, 11793–11798. [Google Scholar] [CrossRef] [Green Version]
- Koculi, E.; Thirumalai, D. Retardation of Folding Rates of Substrate Proteins in the Nanocage of GroEL. Biochemistry 2021, 60, 460–464. [Google Scholar] [CrossRef]
- Bhattacharyya, A.M.; Horowitz, P.M. The Aggregation State of Rhodanese during Folding Influences the Ability of GroEL to Assist Reactivation. J. Biol. Chem. 2001, 276, 28739–28743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinker, A.; Pfeifer, G.; Kerner, M.J.; Naylor, D.J.; Hartl, F.U.; Hayer-Hartl, M. Dual Function of Protein Confinement in Chaperonin-Assisted Protein Folding. Cell 2001, 107, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, A.M.; Horowitz, P.M. Rhodanese Can Partially Refold in Its GroEL−GroES−ADP Complex and Can Be Released to Give a Homogeneous Product. Biochemistry 2002, 41, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Trevino, R.; Gliubich, F.; Berni, R.; Cianci, M.; Chirgwin, J.; Zanotti, G.; Horowitz, P. NH2-Terminal Sequence Truncation Decreases the Stability of Bovine Rhodanese, Minimally Perturbs Its Crystal Structure, and Enhances Interaction with GroEL under Native Conditions. J. Biol. Chem. 1999, 274, 13938–13947. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, J.; Butler, M.; Horowitz, P. Characterization of a Stable, Reactivatable Complex between Chaperonin 60 and Mitochondrial Rhodanese. J. Biol. Chem. 1992, 267, 24648–24654. [Google Scholar] [CrossRef]
- Gliubich, F.; Berni, R.; Colapietro, M.; Barba, L.; Zanotti, G. Structure of Sulfur-Substituted Rhodanese at 1.36 Å Resolution. Acta Crystallogr. Sect. D 1998, 54, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianci, M.; Gliubich, F.; Zanotti, G.; Berni, R. Specific Interaction of Lipoate at the Active Site of Rhodanese11Atomic Model Coordinates Have Been Deposited at the Protein Data Bank with the Accession Code 1DP2. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 2000, 1481, 103–108. [Google Scholar] [CrossRef]
- Horowitz, P.M.; Patel, K. Some Comparisons between Solution and Crystal Properties of Thiosulfate Sulfurtransferase. Biochem. Biophys. Res. Commun. 1980, 94, 419–423. [Google Scholar] [CrossRef]
- Westley, J. [37] Thiosulfate: Cyanide Sulfurtransferase (Rhodanese). In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981; Volume 77, pp. 285–291. ISBN 0076-6879. [Google Scholar]
- Davidson, B.; Westley, J. Tryptophan in the Active Site of Rhodanese. J. Biol. Chem. 1965, 240, 4463–4469. [Google Scholar] [CrossRef]
- Finazzi Agrò, A.; Federici, G.; Giovagnoli, C.; Cannella, C.; Cavallini, D. Effect of Sulfur Binding on Rhodanese Fluorescence. Eur. J. Biochem. 1972, 28, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Cannella, C.; Berni, R.; Rosato, N.; Finazzi-Agro, A. Active Site Modifications Quench Intrinsic Fluorescence of Rhodanese by Different Mechanisms. Biochemistry 1986, 25, 7319–7323. [Google Scholar] [CrossRef] [PubMed]
- Guido, K.; Horowitz, P.M. Contact versus Energy Transfer Fluorescence Quenching in the Sulfur Substituted Form of the Enzyme Rhodanese: A Study Using Cesium Ion Resolved Emission Spectra. Biochem. Biophys. Res. Commun. 1975, 67, 670–676. [Google Scholar] [CrossRef]
- Blicharska, B.; Koloczek, H.; Wasylewski, Z. A Nuclear Magnetic Relaxation Study of Conformational Changes Induced by Substrate and Temperature in Bovine Liver Thiosulfate Sulfurtransferase and Yeast Hexokinase. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1982, 708, 326–329. [Google Scholar] [CrossRef]
- Man, M.; Bryant, R.G. Nuclear Magnetic Resonance Investigation of Rhodanese Sulfhydryl Groups: A CHLORIDE ION PROBE STUDY. J. Biol. Chem. 1974, 249, 1109–1112. [Google Scholar] [CrossRef]
- Cicero, D.O.; Melino, S.; Orsale, M.; Brancato, G.; Amadei, A.; Forlani, F.; Pagani, S.; Paci, M. Structural Rearrangements of the Two Domains of Azotobacter Vinelandii Rhodanese upon Sulfane Sulfur Release: Essential Molecular Dynamics, 15N NMR Relaxation and Deuterium Exchange on the Uniformly Labeled Protein. Int. J. Biol. Macromol. 2003, 33, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Melino, S.; Melis, R.; Paci, M.; Cicero, D.O. Backbone NMR Assignment of the 29.6 KDa Rhodanese Protein from Azotobacter Vinelandii. J. Biomol. NMR 2006, 36, 73. [Google Scholar] [CrossRef] [PubMed]
- Volini, M.; Wang, S.-F. The Interdependence of Substrate and Protein Transformations in Rhodanese Catalysis: II. Enzyme conformational changes significant for catalysis. J. Biol. Chem. 1973, 248, 7386–7391. [Google Scholar] [CrossRef]
- Li, H.; Yang, F.; Kang, X.; Xia, B.; Jin, C. Solution Structures and Backbone Dynamics of Escherichia Coli Rhodanese PspE in Its Sulfur-Free and Persulfide-Intermediate Forms: Implications for the Catalytic Mechanism of Rhodanese. Biochemistry 2008, 47, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Bucher, P.; Kajava, A.V. A Model of Cdc25 Phosphatase Catalytic Domain and Cdk-Interaction Surface Based on the Presence of a Rhodanese Homology Domain. J. Mol. Biol. 1998, 282, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, Y.; Lacourciere, G.; Stadtman, T.C. Formation of a Selenium-Substituted Rhodanese by Reaction with Selenite and Glutathione: Possible Role of a Protein Perselenide in a Selenium Delivery System. Proc. Natl. Acad. Sci. USA 2001, 98, 9494–9498. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, M.D.; Ahmed, F.; Lacourciere, G.M.; Lauhon, C.T.; Stadtman, T.C.; Larson, T.J. Functional Diversity of the Rhodanese Homology Domain: The Escherichia Coli YbbB Gene Encodes a Selenophosphate-Dependent TRNA 2-Selenouridine Synthase. J. Biol. Chem. 2004, 279, 1801–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnov, A.; Comte, C.; Mager-Heckel, A.-M.; Addis, V.; Krasheninnikov, I.A.; Martin, R.P.; Entelis, N.; Tarassov, I. Mitochondrial Enzyme Rhodanese Is Essential for 5 S Ribosomal RNA Import into Human Mitochondria. J. Biol. Chem. 2010, 285, 30792–30803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cereda, A.; Carpen, A.; Picariello, G.; Iriti, M.; Faoro, F.; Ferranti, P.; Pagani, S. Effects of the Deficiency of the Rhodanese-like Protein RhdA in Azotobacter Vinelandii. FEBS Lett. 2007, 581, 1625–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonomi, F.; Pagani, S.; Cerletti, P.; Cannella, C. Rhodanese-mediated Sulfur Transfer to Succinate Dehydrogenase. Eur. J. Biochem. 1977, 72, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Wróbel, M. H2S, Polysulfides, and Enzymes: Physiological and Pathological Aspects. Biomolecules 2020, 10, 640. [Google Scholar] [CrossRef] [Green Version]
- Kruithof, P.D.; Lunev, S.; Lozano, S.P.A.; de Assis Batista, F.; Al-Dahmani, Z.M.; Joles, J.A.; Dolga, A.M.; Groves, M.R.; van Goor, H. Unraveling the Role of Thiosulfate Sulfurtransferase in Metabolic Diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165716. [Google Scholar] [CrossRef] [PubMed]
- Rydz, L.; Wróbel, M.; Jurkowska, H. Sulfur Administration in Fe–S Cluster Homeostasis. Antioxidants 2021, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Galante, Y.M. Interaction of Rhodanese with Mitochondrial NADH Dehydrogenase. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1983, 742, 278–284. [Google Scholar] [CrossRef]
- Nishino, T.; Usami, C.; Tsushima, K. Reversible Interconversion between Sulfo and Desulfo Xanthine Oxidase in a System Containing Rhodanese, Thiosulfate, and Sulfhydryl Reagent. Proc. Natl. Acad. Sci. USA 1983, 80, 1826–1829. [Google Scholar] [CrossRef] [Green Version]
- Pagani, S.; Bonomi, F.; Cerletti, P. Sulfide Insertion into Spinach Ferredoxin by Rhodanese. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1982, 700, 154–164. [Google Scholar] [CrossRef]
- Krueger, K.; Koch, K.; Jühling, A.; Tepel, M.; Scholze, A. Low Expression of Thiosulfate Sulfurtransferase (Rhodanese) Predicts Mortality in Hemodialysis Patients. Clin. Biochem. 2010, 43, 95–101. [Google Scholar] [CrossRef]
- Venkatraman, A.; Landar, A.; Davis, A.J.; Ulasova, E.; Page, G.; Murphy, M.P.; Darley-Usmar, V.; Bailey, S.M. Oxidative Modification of Hepatic Mitochondria Protein Thiols: Effect of Chronic Alcohol Consumption. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G521–G527. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N.; Katayama, A. Post-Translational Regulation of Mercaptopyruvate Sulfurtransferase via a Low Redox Potential Cysteine-Sulfenate in the Maintenance of Redox Homeostasis. J. Biol. Chem. 2005, 280, 34569–34576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, K.A.; Peng, H.; Luebke, J.L.; Chang, F.-M.J.; Giedroc, D.P. Conformational Analysis and Chemical Reactivity of the Multidomain Sulfurtransferase, Staphylococcus Aureus CstA. Biochemistry 2015, 54, 2385–2398. [Google Scholar] [CrossRef] [PubMed]
- de Paula, C.P.; Dos Santos, M.C.; Tairum, C.A.; Breyer, C.A.; Toledo-Silva, G.; Toyama, M.H.; Mori, G.M.; de Oliveira, M.A. Glutaredoxin-like Protein (GLP)—A Novel Bacteria Sulfurtransferase That Protects Cells against Cyanide and Oxidative Stresses. Appl. Microbiol. Biotechnol. 2020, 104, 5477–5492. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Tanaka, M.; Tanaka, Y.; Ito, T. Novel Characterization of Antioxidant Enzyme, 3-Mercaptopyruvate Sulfurtransferase-Knockout Mice: Overexpression of the Evolutionarily-Related Enzyme Rhodanese. Antioxidants 2019, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Ogata, K.; Dai, X.; Volini, M. Bovine Mitochondrial Rhodanese Is a Phosphoprotein. J. Biol. Chem. 1989, 264, 2718–2725. [Google Scholar] [CrossRef]
- Weng, L.; Heinrikson, R.L.; Westley, J. Active Site Cysteinyl and Arginyl Residues of Rhodanese. A Novel Formation of Disulfide Bonds in the Active Site Promoted by Phenylglyoxal. J. Biol. Chem. 1978, 253, 8109–8119. [Google Scholar] [CrossRef]
- Cerletti, P. Seeking a Better Job for an Under-Employed Enzyme: Rhodanese. Trends Biochem. Sci. 1986, 11, 369–372. [Google Scholar] [CrossRef]
- Kaczor, M.; Sura, P.; Bronowicka-Adamska, P.; Wróbel, M. Exposure to Lead in Water and Cysteine Non-Oxidative Metabolism in Pelophylax Ridibundus Tissues. Aquat. Toxicol. 2013, 127, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Hwang, J.H.; Lee, Y.S.; Cho, K.S. Purification and Characterization of Mouse Liver Rhodanese. BMB Rep. 1995, 28, 170–176. [Google Scholar]
- Oi, S. Inhibition of Rat Liver Rhodanese by Di-, Tricarboxylic, and α-Keto Acids1. J. Biochem. 1975, 78, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.L.; Horowitz, P.M.; Westley, J. Rhodanese as a Thioredoxin Oxidase. Int. J. Biochem. Cell Biol. 2000, 32, 465–473. [Google Scholar] [CrossRef]
- Turkowsky, A.; Blotevogel, K.-H.; Fischer, U. Properties of a Soluble Thiosulfate Sulfur Transferase (Rhodanese) of the Marine Methanogen Methanosarcina Frisia. FEMS Microbiol. Lett. 1991, 81, 251–255. [Google Scholar] [CrossRef]
- Anosike, E.O.; Jack, A.S. A Comparison of Some Biochemical Properties of Liver Thiosulphate Sulphurtransferase from Guinea Pig (Lepus caniculus) & Albino Rat. Indian J. Biochem. Biophys. 1982, 19, 13–16. [Google Scholar]
- Ramasamy, S.; Singh, S.; Taniere, P.; Langman, M.; Eggo, M. Sulfide-Detoxifying Enzymes in the Human Colon Are Decreased in Cancer and Upregulated in Differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G288–G296. [Google Scholar] [CrossRef] [Green Version]
- Birkenkamp-Demtroder, K.; Christensen, L.L.; Olesen, S.H.; Frederiksen, C.M.; Laiho, P.; Aaltonen, L.A.; Laurberg, S.; Sørensen, F.B.; Hagemann, R.; Ørntoft, T.F. Gene Expression in Colorectal Cancer. Cancer Res. 2002, 62, 4352–4363. [Google Scholar]
- Birkenkamp-Demtroder, K.; Olesen, S.H.; Sørensen, F.B.; Laurberg, S.; Laiho, P.; Aaltonen, L.A.; Ørntoft, T.F. Differential Gene Expression in Colon Cancer of the Caecum versus the Sigmoid and Rectosigmoid. Gut 2005, 54, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Sabelli, R.; Iorio, E.; De Martino, A.; Podo, F.; Ricci, A.; Viticchiè, G.; Rotilio, G.; Paci, M.; Melino, S. Rhodanese–Thioredoxin System and Allyl Sulfur Compounds. FEBS J. 2008, 275, 3884–3899. [Google Scholar] [CrossRef]
- Nakajima, T.; Taki, K.; Wang, B.; Ono, T.; Matsumoto, T.; Oghiso, Y.; Tanaka, K.; Ichinohe, K.; Nakamura, S.; Tanaka, S. Induction of Rhodanese, a Detoxification Enzyme, in Livers from Mice after Long-Term Irradiation with Low-Dose-Rate Gamma-Rays. J. Radiat. Res. 2008, 49, 661–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Chen, Z.; Zhang, X.; Xin, Y.; Xia, Y.; Xun, L.; Liu, H. Rhodanese Rdl2 Produces Reactive Sulfur Species to Protect Mitochondria from Reactive Oxygen Species. Free Radic. Biol. Med. 2021, 177, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Kaya, V.; Yazkan, R.; Yıldırım, M.; Doğuç, D.K.; Süren, D.; Bozkurt, K.K.; Yüksel, Ö.; Demirpence, Ö.; Şen, C.A.; Yalçın, A.Y. The Relation of Radiation-Induced Pulmonary Fibrosis with Stress and the Efficiency of Antioxidant Treatment: An Experimental Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 290. [Google Scholar]
- Demiryilmaz, I.; Sener, E.; Cetin, N.; Altuner, D.; Suleyman, B.; Albayrak, F.; Akcay, F.; Suleyman, H. Biochemically and Histopathologically Comparative Review of Thiamine’s and Thiamine Pyrophosphate’s Oxidative Stress Effects Generated with Methotrexate in Rat Liver. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, BR475. [Google Scholar] [CrossRef]
- Nakajima, T. Roles of Sulfur Metabolism and Rhodanese in Detoxification and Anti-Oxidative Stress Functions in the Liver: Responses to Radiation Exposure. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1721. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, T.M.; Grieshaber, M.K. Three Enzymatic Activities Catalyze the Oxidation of Sulfide to Thiosulfate in Mammalian and Invertebrate Mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Characterization of Patient Mutations in Human Persulfide Dioxygenase (ETHE1) Involved in H2S Catabolism. J. Biol. Chem. 2012, 287, 44561–44567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarejo, M.J.; Westley, J.W. Rhodanese-Catalyzed Reduction of Thiosulfate by Reduced Lipoic Acid. J. Biol. Chem. 1963, 238, 1185–1186. [Google Scholar] [CrossRef]
- Iciek, M.; Kowalczyk-Pachel, D.; Bilska-Wilkosz, A.; Kwiecień, I.; Górny, M.; Włodek, L. S-Sulfhydration as a Cellular Redox Regulation. Biosci. Rep. 2016, 36, e00304. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The Quantitative Significance of the Transsulfuration Enzymes for H2S Production in Murine Tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S Biogenesis by Human Cystathionine γ-Lyase Leads to the Novel Sulfur Metabolites Lanthionine and Homolanthionine and Is Responsive to the Grade of Hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative Contributions of Cystathionine β-Synthase and γ-Cystathionase to H2S Biogenesis via Alternative Trans-Sulfuration Reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.L.; Ferraz, J.G.; Muscara, M.N. Hydrogen Sulfide: An Endogenous Mediator of Resolution of Inflammation and Injury. Antioxid. Redox Signal. 2012, 17, 58–67. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen Sulfide (H2S) Metabolism in Mitochondria and Its Regulatory Role in Energy Production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of Mitochondrial Bioenergetic Function by Hydrogen Sulfide. Part I. Biochemical and Physiological Mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular Biology of Hydrogen Sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.M.; Elsas, L.J.; Nikoskelainen, E.K. Mitochondrial DNA Mutation Associated with Leber’s Hereditary Optic Neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [CrossRef]
- Lin, C.S.; Sharpley, M.S.; Fan, W.; Waymire, K.G.; Sadun, A.A.; Carelli, V.; Ross-Cisneros, F.N.; Baciu, P.; Sung, E.; McManus, M.J. Mouse MtDNA Mutant Model of Leber Hereditary Optic Neuropathy. Proc. Natl. Acad. Sci. USA 2012, 109, 20065–20070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielson, S.R.; Wong, A.; Carelli, V.; Martinuzzi, A.; Schapira, A.H.; Cortopassi, G.A. Cells Bearing Mutations Causing Leber’s Hereditary Optic Neuropathy Are Sensitized to Fas-Induced Apoptosis. J. Biol. Chem. 2002, 277, 5810–5815. [Google Scholar] [CrossRef] [Green Version]
- Cagianut, B.; Rhyner, K.; Furrer, W.; Schnebli, H. Thiosulphate-Sulphur Transferase (Rhodanese) Deficiency in Leber’s Hereditary Optic Atrophy. Lancet 1981, 318, 981–982. [Google Scholar] [CrossRef]
- Poole, C.; Kind, P. Deficiency of Thiosulphate Sulphurtransferase (Rhodanese) in Leber’s Hereditary Optic Neuropathy. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 1229–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoskelainen, E.; Hoyt, W.F.; Nummelin, K.; Schatz, H. Fundus Findings in Leber’s Hereditary Optic Neuroretinopathy: III. Fluorescein Angiographic Studies. Arch. Ophthalmol. 1984, 102, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, D.; Poole, C.; Kind, P.; Hopkinson, D. Rhodanese Isozymes in Three Subjects with Leber’s Optic Neuropathy. J. Med. Genet. 1989, 26, 113–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Hwang, J.-M.; Chang, B.-L.; Park, S.S. Spectrum of the Mitochondrial DNA Mutations of Leber’s Hereditary Optic Neuropathy in Koreans. J. Neurol. 2003, 250, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhou, X.; Hu, Y.; Tong, Y.; Li, R.; Lu, F.; Yang, H.; Mo, J.Q.; Qu, J.; Guan, M.-X. Clinical Evaluation and Mitochondrial DNA Sequence Analysis in Three Chinese Families with Leber’s Hereditary Optic Neuropathy. Biochem. Biophys. Res. Commun. 2005, 332, 614–621. [Google Scholar] [CrossRef]
- Torroni, A.; Petrozzi, M.; D’Urbano, L.; Sellitto, D.; Zeviani, M.; Carrara, F.; Carducci, C.; Leuzzi, V.; Carelli, V.; Barboni, P. Haplotype and Phylogenetic Analyses Suggest That One European-Specific MtDNA Background Plays a Role in the Expression of Leber Hereditary Optic Neuropathy by Increasing the Penetrance of the Primary Mutations 11778 and 14484. Am. J. Hum. Genet. 1997, 60, 1107. [Google Scholar]
- Levine, J.; Ellis, C.J.; Furne, J.K.; Springfield, J.; Levitt, M.D. Fecal Hydrogen Sulfide Production in Ulcerative Colitis. Am. J. Gastroenterol. 1998, 93, 83–87. [Google Scholar] [CrossRef]
- Picton, R.; Eggo, M.; Merrill, G.; Langman, M.; Singh, S. Mucosal Protection against Sulphide: Importance of the Enzyme Rhodanese. Gut 2002, 50, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, E.; Matsunami, M.; Kimura, T.; Yonezawa, D.; Ishiki, T.; Sekiguchi, F.; Nishikawa, H.; Maeda, Y.; Ishikura, H.; Kawabata, A. Rhodanese, but Not Cystathionine-γ-Lyase, Is Associated with Dextran Sulfate Sodium-Evoked Colitis in Mice: A Sign of Impaired Colonic Sulfide Detoxification? Toxicology 2009, 264, 96–103. [Google Scholar] [CrossRef]
- Yi, H.; Li, X.-H.; Yi, B.; Zheng, J.; Zhu, G.; Li, C.; Li, M.-Y.; Zhang, P.-F.; Li, J.-L.; Chen, Z.-C.; et al. Identification of Rack1, EF-Tu and Rhodanese as Aging-Related Proteins in Human Colonic Epithelium by Proteomic Analysis. J. Proteome Res. 2010, 9, 1416–1423. [Google Scholar] [CrossRef]
- Wróbel, M.; Włodek, L. Effects of Thiazolidine-4 (R)-Carboxylates and Other Low-Molecular-Weight Sulfur Compounds on the Activity of Mercaptopyruvate Sulfurtransferase, Rhodanese and Cystathionase in Ehrlich Ascites Tumor Cells and Tumor-Bearing Mouse Liver. Amino Acids 1997, 12, 309–314. [Google Scholar] [CrossRef]
- Jurkowska, H.; Wróbel, M. N-Acetyl-L-Cysteine as a Source of Sulfane Sulfur in Astrocytoma and Astrocyte Cultures: Correlations with Cell Proliferation. Amino Acids 2008, 34, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Jurkowska, H.; Placha, W.; Nagahara, N.; Wróbel, M. The Expression and Activity of Cystathionine-γ-Lyase and 3-Mercaptopyruvate Sulfurtransferase in Human Neoplastic Cell Lines. Amino Acids 2011, 41, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Kamińska, M.; Kaminski, K.; Wróbel, M. The Expression and Activity of Rhodanese, 3-Mercaptopyruvate Sulfurtransferase, Cystathionine γ-Lyase in the Most Frequently Chosen Cellular Research Models. Biomolecules 2021, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S.; Creemers, J.W.M.; Ohagi, S.; Raffin-Sanson, M.-L.; Sanders, L.; Montague, C.T.; Hutton, J.C.; O’Rahilly, S. Obesity and Impaired Prohormone Processing Associated with Mutations in the Human Prohormone Convertase 1 Gene. Nat. Genet. 1997, 16, 303–306. [Google Scholar] [CrossRef]
- Clément, K.; Vaisse, C.; Lahlou, N.; Cabrol, S.; Pelloux, V.; Cassuto, D.; Gourmelen, M.; Dina, C.; Chambaz, J.; Lacorte, J.-M.; et al. A Mutation in the Human Leptin Receptor Gene Causes Obesity and Pituitary Dysfunction. Nature 1998, 392, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.F.A.; Thorleifsson, G.; Reynisdottir, I.; Benediktsson, R.; Manolescu, A.; Sainz, J.; Helgason, A.; Stefansson, H.; Emilsson, V.; Helgadottir, A.; et al. Variant of Transcription Factor 7-like 2 (TCF7L2) Gene Confers Risk of Type 2 Diabetes. Nat. Genet. 2006, 38, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, Y.; Oda, N.; Cox, N.J.; Li, X.; Orho-Melander, M.; Hara, M.; Hinokio, Y.; Lindner, T.H.; Mashima, H.; Schwarz, P.E.H.; et al. Genetic Variation in the Gene Encoding Calpain-10 Is Associated with Type 2 Diabetes Mellitus. Nat. Genet. 2000, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Morton, N.M.; Beltram, J.; Carter, R.N.; Michailidou, Z.; Gorjanc, G.; McFadden, C.; Barrios-Llerena, M.E.; Rodriguez-Cuenca, S.; Gibbins, M.T.G.; Aird, R.E.; et al. Genetic Identification of Thiosulfate Sulfurtransferase as an Adipocyte-Expressed Antidiabetic Target in Mice Selected for Leanness. Nat. Med. 2016, 22, 771–779, Corrigendum in: Nat. Med. 2018, 24, 525. [Google Scholar] [CrossRef] [PubMed]
- Lainšček, D.; Šuštar, U.; Carter, R.N.; Morton, N.M.; Horvat, S. Tst Gene Mediates Protection against Palmitate-Induced Inflammation in 3T3-L1 Adipocytes. Biochem. Biophys. Res. Commun. 2020, 527, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, B.; Cai, B.; Liao, F.; Zheng, Y.; Zeng, Q.; Fan, X.; Gong, Y.; Yang, J.; Cui, Q.H.; Tang, C.; et al. Increase or Decrease Hydrogen Sulfide Exert Opposite Lipolysis, but Reduce Global Insulin Resistance in High Fatty Diet Induced Obese Mice. PLoS ONE 2013, 8, e73892. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.-T.; Price, J.W., III; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 Emission and Cellular Redox State Link Excess Fat Intake to Insulin Resistance in Both Rodents and Humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, J.; Jiang, P.; Li, M.; Liu, Q.; Cao, Y.; Wang, S. Serum Fatty Acid Binding Protein 4 Is Positively Associated with Early Stroke Recurrence in Nondiabetic Ischemic Stroke. Aging 2019, 11, 1977. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hertzel, A.V.; Steen, K.A.; Wang, Q.; Suttles, J.; Bernlohr, D.A. Uncoupling Lipid Metabolism from Inflammation through Fatty Acid Binding Protein-Dependent Expression of UCP2. Mol. Cell. Biol. 2015, 35, 1055–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritov, V.B.; Menshikova, E.V.; Azuma, K.; Wood, R.; Toledo, F.G.S.; Goodpaster, B.H.; Ruderman, N.B.; Kelley, D.E. Deficiency of Electron Transport Chain in Human Skeletal Muscle Mitochondria in Type 2 Diabetes Mellitus and Obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E49–E58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phielix, E.; Schrauwen-Hinderling, V.B.; Mensink, M.; Lenaers, E.; Meex, R.; Hoeks, J.; Kooi, M.E.; Moonen-Kornips, E.; Sels, J.-P.; Hesselink, M.K.C.; et al. Lower Intrinsic ADP-Stimulated Mitochondrial Respiration Underlies In Vivo Mitochondrial Dysfunction in Muscle of Male Type 2 Diabetic Patients. Diabetes 2008, 57, 2943–2949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Dahmani, Z.M.; Li, X.; Wiggenhauser, L.M.; Ott, H.; Kruithof, P.D.; Lunev, S.; Batista, F.A.; Luo, Y.; Dolga, A.M.; Morton, N.M.; et al. Thiosulfate Sulfurtransferase Prevents Hyperglycemic Damage to the Zebrafish Pronephros in an Experimental Model for Diabetes. Sci. Rep. 2022, 12, 12077. [Google Scholar] [CrossRef]
- Campuzano, V.; Montermini, L.; Molto, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s Ataxia: Autosomal Recessive Disease Caused by an Intronic GAA Triplet Repeat Expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef]
- Tan, G.; Napoli, E.; Taroni, F.; Cortopassi, G. Decreased Expression of Genes Involved in Sulfur Amino Acid Metabolism in Frataxin-Deficient Cells. Hum. Mol. Genet. 2003, 12, 1699–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Akhter, Y.; Chatterjee, S. A Review on Remediation of Cyanide Containing Industrial Wastes Using Biological Systems with Special Reference to Enzymatic Degradation. World J. Microbiol. Biotechnol. 2019, 35, 70. [Google Scholar] [CrossRef] [PubMed]
- Ademakinwa, A.N.; Agunbiade, M.O.; Fagbohun, O. Biodegradation of Cyanide in Cassava Wastewater Using a Novel Thermodynamically-Stable Immobilized Rhodanese. Prep. Biochem. Biotechnol. 2021, 51, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Szilasi, M.; Budai, M.; Budai, L.; Petrikovics, I. Nanoencapsulated and Microencapsulated Enzymes in Drug Antidotal Therapy. Toxicol. Ind. Health 2012, 28, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Ciocci, M.; Savio, S.; Antonaroli, S.; Seliktar, D.; Melino, S.; Congestri, R. Trichormus Variabilis (Cyanobacteria) Biomass: From the Nutraceutical Products to Novel EPS-Cell/Protein Carrier Systems. Mar. Drugs 2018, 16, 298. [Google Scholar] [CrossRef] [Green Version]
- Supapong, C.; Cherdthong, A. Rhodaneses Enzyme Addition Could Reduce Cyanide Concentration and Enhance Fiber Digestibility via In Vitro Fermentation Study. Fermentation 2021, 7, 207. [Google Scholar] [CrossRef]
- de Araújo, F.T.; Caseli, L. Rhodanese Incorporated in Langmuir and Langmuir–Blodgett Films of Dimyristoylphosphatidic Acid: Physical Chemical Properties and Improvement of the Enzyme Activity. Colloids Surf. B Biointerfaces 2016, 141, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Luque-Almagro, V.M.; Cabello, P.; Sáez, L.P.; Olaya-Abril, A.; Moreno-Vivián, C.; Roldán, M.D. Exploring Anaerobic Environments for Cyanide and Cyano-Derivatives Microbial Degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1067–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovecka, P.; Thimova, M.; Grznarova, P.; Lipov, J.; Knejzlik, Z.; Stiborova, H.; Nindhia, T.G.T.; Demnerova, K.; Ruml, T. Study of Cytotoxic Effects of Benzonitrile Pesticides. BioMed Res. Int. 2015, 2015, 381264. [Google Scholar] [CrossRef] [Green Version]
- Alvillo-Rivera, A.; Garrido-Hoyos, S.; Buitrón, G.; Thangarasu-Sarasvathi, P.; Rosano-Ortega, G. Biological Treatment for the Degradation of Cyanide: A Review. J. Mater. Res. Technol. 2021, 12, 1418–1433. [Google Scholar] [CrossRef]
- Akcil, A.; Mudder, T. Microbial Destruction of Cyanide Wastes in Gold Mining: Process Review. Biotechnol. Lett. 2003, 25, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Botz, M.; Mudder, T.; Akcil, A. Cyanide Treatment: Physical, Chemical, and Biological Processes. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 619–645. [Google Scholar]
- White, D.M.; Schnabel, W. Treatment of cyanide waste in a sequencing batch biofilm reactor. Water Res. 1998, 32, 254–257. [Google Scholar] [CrossRef]
- McCutcheon, S.; Schnoor, J. Overview of Phytotransformation and Control of Wastes. Phytoremediation Transform. Control. Contam. 2003, 3–58. [Google Scholar]
- Dash, R.R.; Gaur, A.; Balomajumder, C. Cyanide in Industrial Wastewaters and Its Removal: A Review on Biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef]
- Patil, Y.; Paknikar, K. Biodetoxification of Silver–Cyanide from Electroplating Industry Wastewater. Lett. Appl. Microbiol. 2000, 30, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, R.; Ascenzi, P.; Frangipani, E.; Visca, P. Cyanide Detoxification by Recombinant Bacterial Rhodanese. Chemosphere 2006, 63, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Kaewkannetra, P.; Imai, T.; Garcia-Garcia, F.J.; Chiu, T.Y. Cyanide Removal from Cassava Mill Wastewater Using Azotobactor Vinelandii TISTR 1094 with Mixed Microorganisms in Activated Sludge Treatment System. J. Hazard. Mater. 2009, 172, 224–228. [Google Scholar] [CrossRef]
- Kandasamy, S.; Dananjeyan, B.; Krishnamurthy, K.; Benckiser, G. Aerobic Cyanide Degradation by Bacterial Isolates from Cassava Factory Wastewater. Braz. J. Microbiol. 2015, 46, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, R.C.; Padmaja, G.; Balagopalan, C. Extracellular Rhodanese Production by Rhizopus Oryzae. Zent. Mikrobiol. 1990, 145, 259–268. [Google Scholar] [CrossRef]
- Oi, S. Purification and Some Properties of Trametes Sanguinea Rhodanese. Agric. Biol. Chem. 1973, 37, 629–635. [Google Scholar] [CrossRef]
- Nok, A.J.; Nasir, S.M.; Sa’Adatu, Y. Immobilized Rhodanese: Some Aspects of Anion Inhibition Kinetics and Modulation by Cations. J. Biochem. Toxicol. 1993, 8, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, P.; Bowman, S. Reversible Thermal Denaturation of Immobilized Rhodanese. J. Biol. Chem. 1987, 262, 5587–5591. [Google Scholar] [CrossRef]
- McGuinn, W.; Baxter, L.; Pei, L.; Petrikovics, I.; Cannon, E.; Way, J. Antagonism of the Lethal Effects of Cyanide by a Synthetic Water-Soluble Cobalt (III) Porphyrin Compound. Fundam. Appl. Toxicol. 1994, 23, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Way, J.L. Cyanide Antagonism. Fundam. Appl. Toxicol. 1983, 3, 383–386. [Google Scholar] [CrossRef]
- Way, J.L. Cyanide Intoxication and Its Mechanism of Antagonism. Annu. Rev. Pharmacol. Toxicol. 1984, 24, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, L. Enzyme Therapy in Cyanide Poisoning: Effect of Rhodanese and Sulfur Compounds. Arch. Toxicol. 1980, 45, 315–323. [Google Scholar] [CrossRef]
- Graham, L.M.; Nguyen, T.M.; Lee, S.B. Nanodetoxification: Emerging Role of Nanomaterials in Drug Intoxication Treatment. Nanomedicine 2011, 6, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, J.; Lu, Y. Enzyme Therapeutics for Systemic Detoxification. Adv. Drug Deliv. Rev. 2015, 90, 24–39. [Google Scholar] [CrossRef]
- Jin, Y.; Fan, X.; Li, X.; Zhang, Z.; Sun, L.; Fu, Z.; Lavoie, M.; Pan, X.; Qian, H. Distinct Physiological and Molecular Responses in Arabidopsis Thaliana Exposed to Aluminum Oxide Nanoparticles and Ionic Aluminum. Environ. Pollut. 2017, 228, 517–527. [Google Scholar] [CrossRef]
- Cannon, E.; Leung, P.; Hawkins, A.; Petrikovics, I.; DeLoach, J.; Way, J. Antagonism of Cyanide Intoxication with Murine Carrier Erythrocytes Containing Bovine Rhodanese and Sodium Thiosulfate. J. Toxicol. Environ. Health Part A Curr. Issues 1994, 41, 267–274. [Google Scholar] [CrossRef]
- Leung, P.; Cannon, E.P.; Petrikovics, I.; Hawkins, A.; Way, J.L. In vivo studies on rhodanese encapsulation in mouse carrier erythrocytes. Toxicol. Appl. Pharmacol. 1991, 110, 268–274. [Google Scholar] [CrossRef]
- Leung, P.; Davis, R.W.; Yao, C.C.; Cannon, E.P.; Way, J.L. Rhodanese and sodium thiosulfate encapsulated in mouse carrier erythrocytes. Fundam. Appl. Toxicol. 1991, 16, 559–566. [Google Scholar] [CrossRef]
- Petrikovics, I.; Cannon, E.; McGuinn, W.; Pei, L.; Pu, L.; Lindner, L.; Way, J. Cyanide Antagonism with Carrier Erythrocytes and Organic Thiosulfonates. Fundam. Appl. Toxicol. 1995, 24, 86–93. [Google Scholar] [CrossRef]
- Petrikovics, I.; Budai, M.; Baskin, S.; Rockwood, G.; Childress, J.; Budai, L.; Gróf, P.; Klebovich, I.; Szilasi, M. Characterization of Liposomal Vesicles Encapsulating Rhodanese for Cyanide Antagonism. Drug Deliv. 2009, 16, 312–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrikovics, I.; Jayanna, P.; Childress, J.; Budai, M.; Martin, S.; Kuzmitcheva, G.; Rockwood, G. Optimization of Liposomal Lipid Composition for a New, Reactive Sulfur Donor, and in Vivo Efficacy Studies on Mice to Antagonize Cyanide Intoxication. J. Drug Deliv. 2011, 2011, 928626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrikovics, I.; Baskin, S.I.; Beigel, K.M.; Schapiro, B.J.; Rockwood, G.A.; Manage, A.B.; Budai, M.; Szilasi, M. Nano-Intercalated Rhodanese in Cyanide Antagonism. Nanotoxicology 2010, 4, 247–254. [Google Scholar] [CrossRef]
- Sörbo, B. A Colorimetric Method for the Determination of Thiosulfate. Biochim. Et Biophys. Acta 1957, 23, 412–416. [Google Scholar] [CrossRef]
- Vetter, J. Plant Cyanogenic Glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef]
- Latif, S.; Zimmermann, S.; Barati, Z.; Müller, J. Detoxification of Cassava Leaves by Thermal, Sodium Bicarbonate, Enzymatic, and Ultrasonic Treatments. J. Food Sci. 2019, 84, 1986–1991. [Google Scholar] [CrossRef]
- Groom, C.; Luong, J. A Flow through Analysis Biosensor System for Cyanide. J. Biotechnol. 1991, 21, 161–171. [Google Scholar] [CrossRef]
- Ikebukuro, K.; Miyata, A.; Cho, S.J.; Nomura, Y.; Yamauchi, Y.; Hasebe, Y.; Uchiyama, S.; Karube, I. Microbial Cyanide Sensor for Monitoring River Water. J. Biotechnol. 1996, 48, 73–80. [Google Scholar] [CrossRef]
- Mak, K.K.; Yanase, H.; Renneberg, R. Cyanide Fishing and Cyanide Detection in Coral Reef Fish Using Chemical Tests and Biosensors. Biosens. Bioelectron. 2005, 20, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Filipović-Kovačević, Ž.; Mikšaj, M.; Šalamon, D. Cyanide Determination in Fruit Brandies by an Amperometric Biosensor with Immobilised Saccharomyces Cerevisiae. Eur. Food Res. Technol. 2002, 215, 347–352. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vanajothi, R.; Vishnupriya, S.; Premkumar, K.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Enzyme Immobilization on Nanomaterials for Biosensor and Biocatalyst in Food and Biomedical Industry. Curr. Pharm. Des. 2019, 25, 2661–2676. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauretti, A.; Neri, A.; Kossover, O.; Seliktar, D.; Nardo, P.D.; Melino, S. Design of a Novel Composite H2S-releasing Hydrogel for Cardiac Tissue Repair. Macromol. Biosci. 2016, 16, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Buonvino, S.; Ciocci, M.; Seliktar, D.; Melino, S. Photo-Polymerization Damage Protection by Hydrogen Sulfide Donors for 3d-Cell Culture Systems Optimization. Int. J. Mol. Sci. 2021, 22, 6095. [Google Scholar] [CrossRef]



| TST | Applications | References |
|---|---|---|
| Aureobasidium pullulans |
| [144] |
| Azotobacter vinelandii |
| [146,159,188] |
| Bacillus pumilus and Pseudomonas putida |
| [160] |
| Bos taurus |
| [147,148,164,168,172,173,174,176,177,178] |
| Bos taurus and Saccharomyces cerevisiae |
| [182,183,185] |
| Pseudomonas aeruginosa |
| [23,158] |
| Rattus norvegicus |
| [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonvino, S.; Arciero, I.; Melino, S. Thiosulfate-Cyanide Sulfurtransferase a Mitochondrial Essential Enzyme: From Cell Metabolism to the Biotechnological Applications. Int. J. Mol. Sci. 2022, 23, 8452. https://doi.org/10.3390/ijms23158452
Buonvino S, Arciero I, Melino S. Thiosulfate-Cyanide Sulfurtransferase a Mitochondrial Essential Enzyme: From Cell Metabolism to the Biotechnological Applications. International Journal of Molecular Sciences. 2022; 23(15):8452. https://doi.org/10.3390/ijms23158452
Chicago/Turabian StyleBuonvino, Silvia, Ilaria Arciero, and Sonia Melino. 2022. "Thiosulfate-Cyanide Sulfurtransferase a Mitochondrial Essential Enzyme: From Cell Metabolism to the Biotechnological Applications" International Journal of Molecular Sciences 23, no. 15: 8452. https://doi.org/10.3390/ijms23158452
APA StyleBuonvino, S., Arciero, I., & Melino, S. (2022). Thiosulfate-Cyanide Sulfurtransferase a Mitochondrial Essential Enzyme: From Cell Metabolism to the Biotechnological Applications. International Journal of Molecular Sciences, 23(15), 8452. https://doi.org/10.3390/ijms23158452







