Utilization of Aminoguanidine Prevents Cytotoxic Effects of Semen
Abstract
1. Introduction
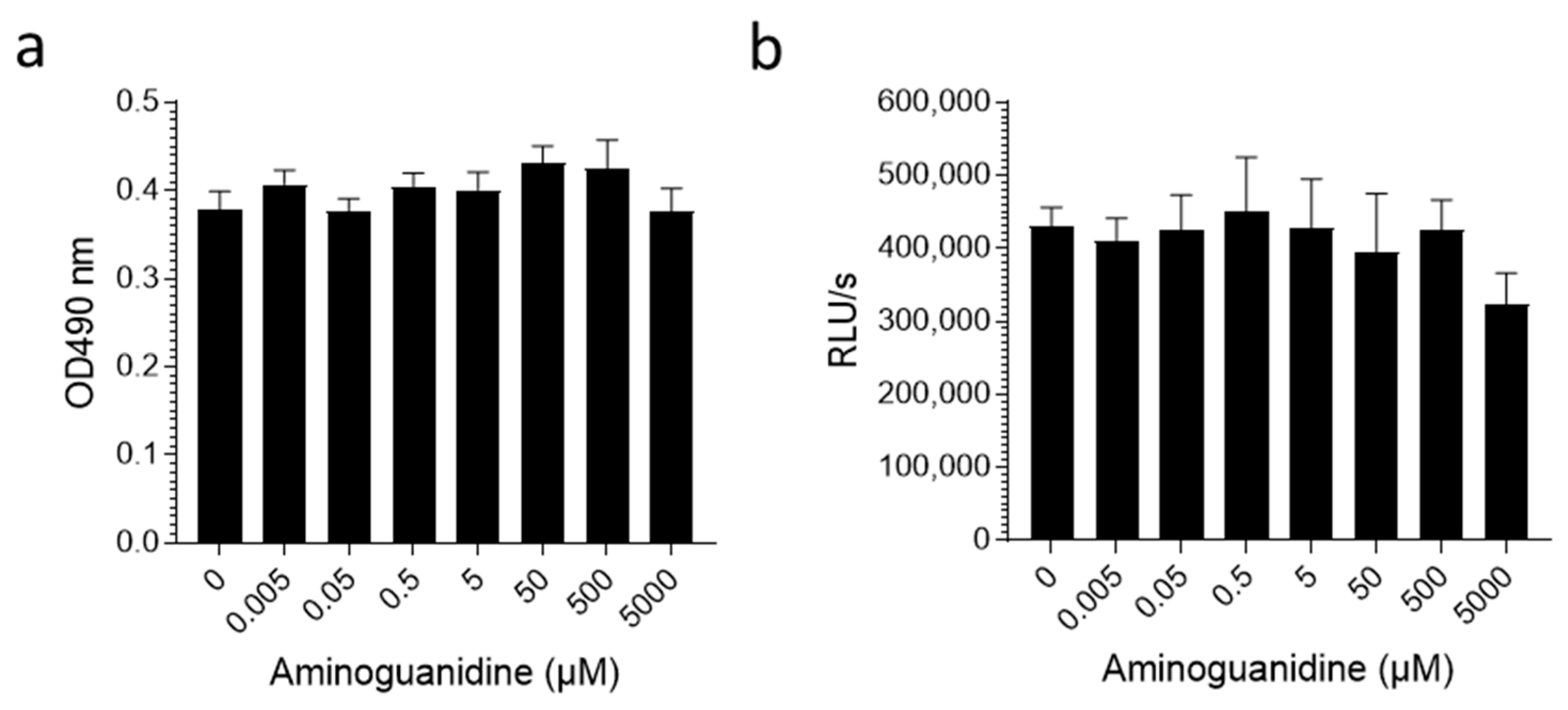
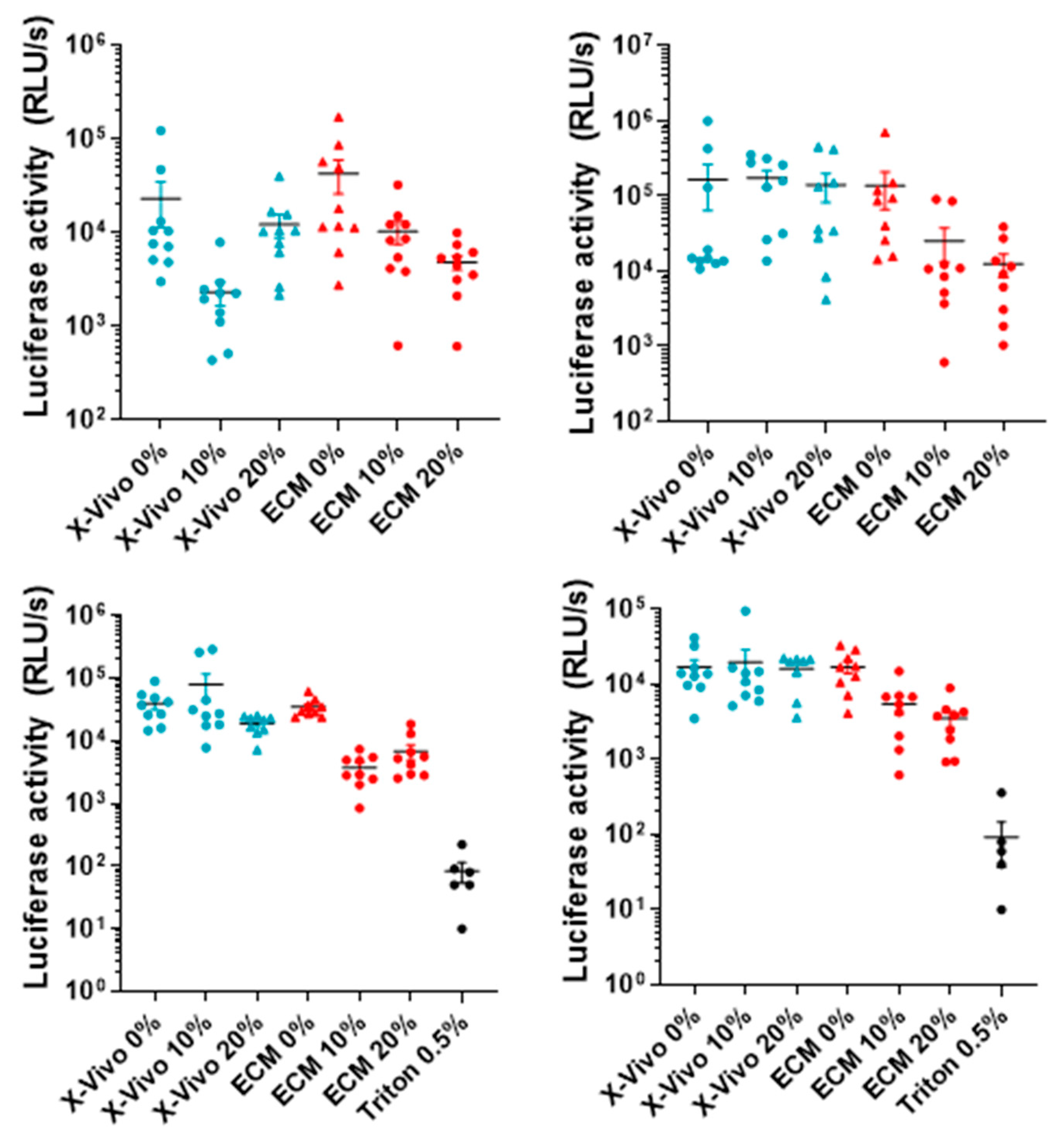
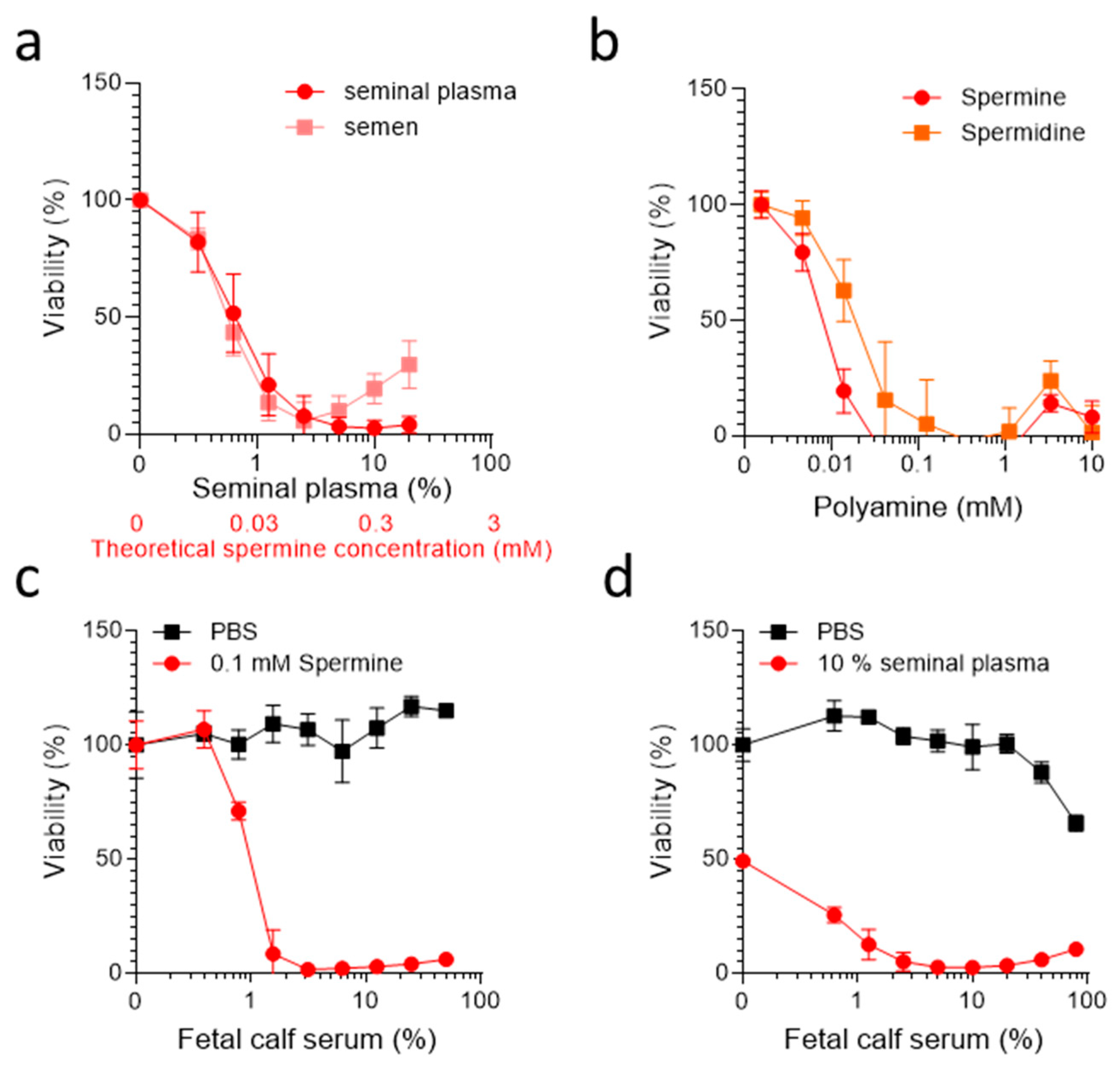
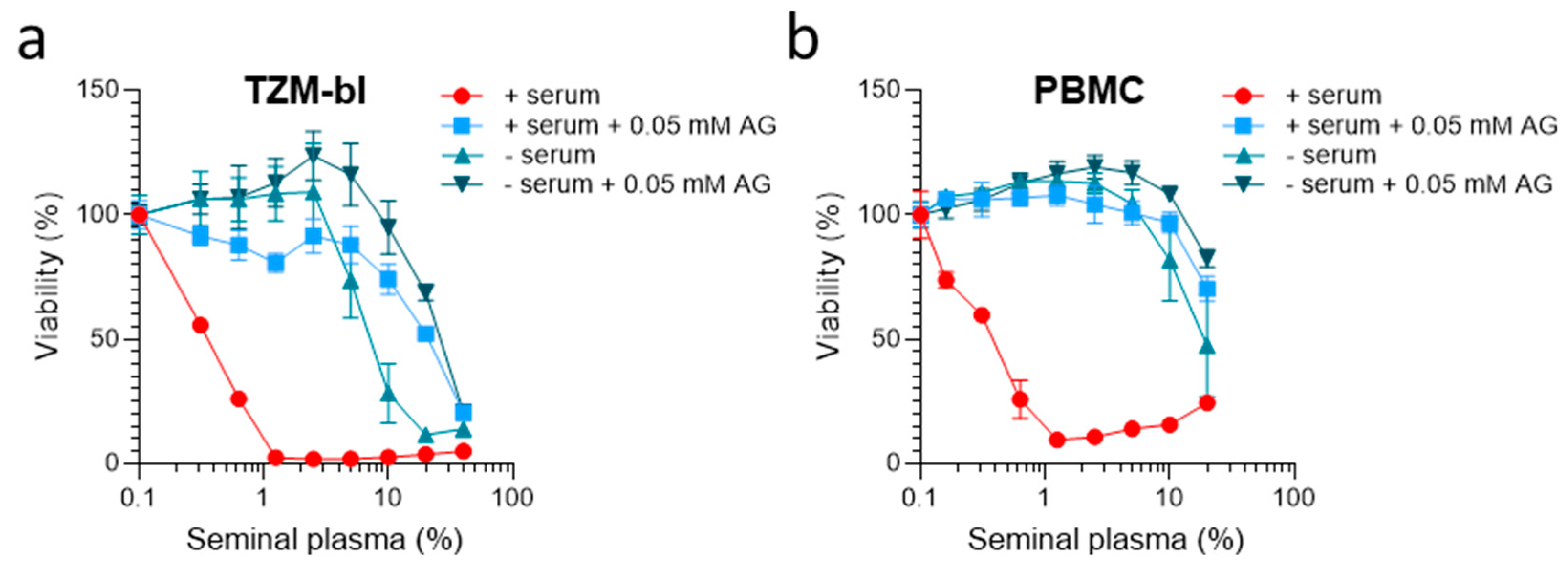
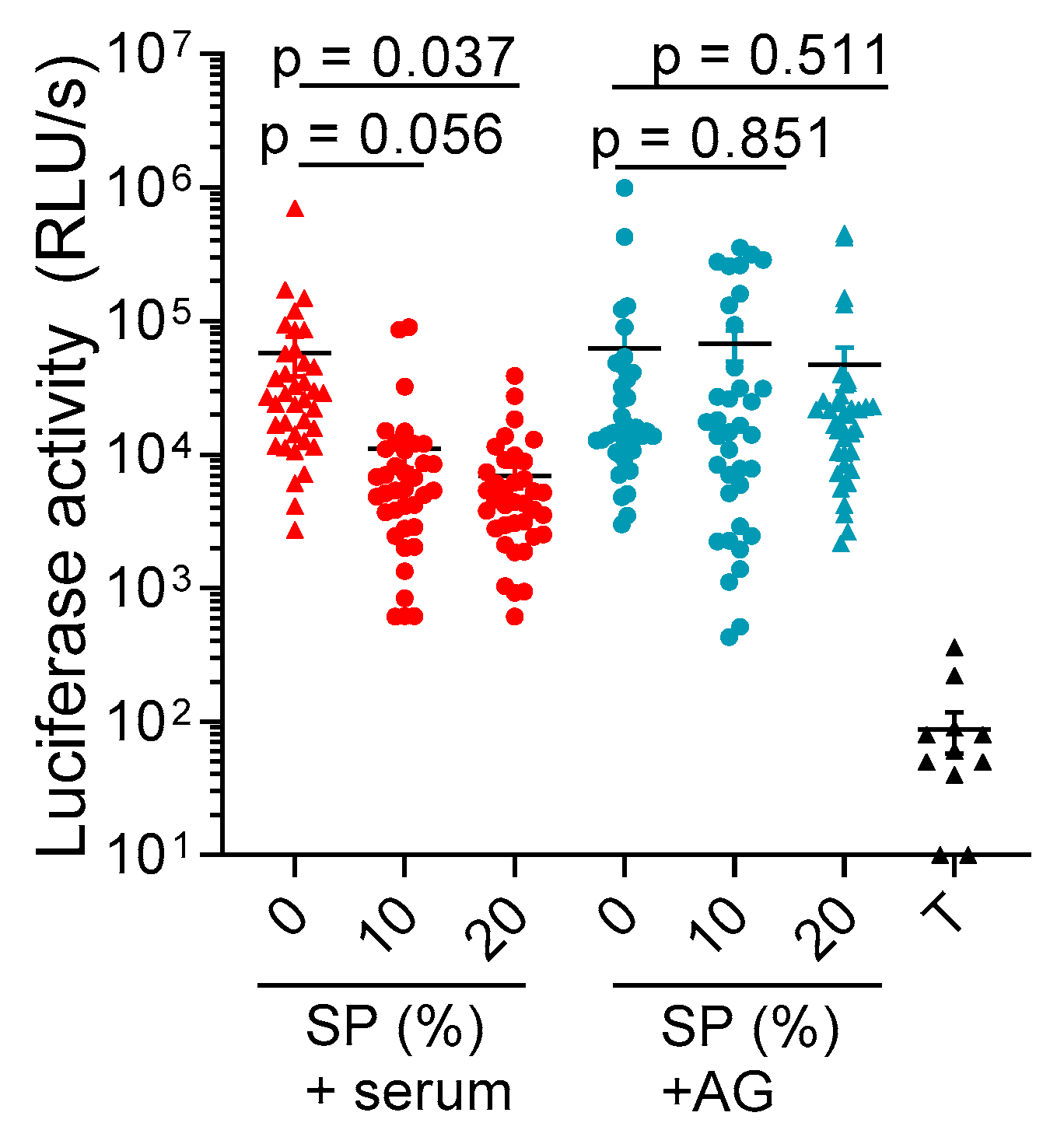
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Semen and Seminal Plasma
4.3. Cell Culture and Primary Tissue
4.4. Statement
4.5. Aminoguanidine Treatment of Serum and Medium
4.6. Toxicity Assays for Cells
4.7. Toxicity Assay for Primary Tissues
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Haugen, T.B.; Grotmol, T. PH of Human Semen. Int. J. Androl. 1998, 21, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Szczykutowicz, J.; Kałuza, A.; Kaźmierowska-Niemczuk, M.; Ferens-Sieczkowska, M. The Potential Role of Seminal Plasma in the Fertilization Outcomes. BioMed Res. Int. 2019, 2019, 5397804. [Google Scholar] [CrossRef] [PubMed]
- Valsa, J.; Skandhan, K.P.; Sumangala, B.; Jaya, V. Time Bound Changes (in 24 h) in Human Sperm Motility and Level of Calcium and Magnesium in Seminal Plasma. Alex. J. Med. 2016, 52, 235–241. [Google Scholar] [CrossRef][Green Version]
- Gao, J.; Yuan, R.; Yang, S.; Wang, Y.; Huang, Y.; Yan, L.; Jiang, H.; Qiao, J. Age-Related Changes in Human Conventional Semen Parameters and Sperm Chromatin Structure Assay-Defined Sperm DNA/Chromatin Integrity. Reprod. Biomed. Online 2021, 42, 973–982. [Google Scholar] [CrossRef]
- Ricci, E.; Al-Beitawi, S.; Cipriani, S.; Alteri, A.; Chiaffarino, F.; Candiani, M.; Gerli, S.; Viganó, P.; Parazzini, F. Dietary Habits and Semen Parameters: A Systematic Narrative Review. Andrology 2018, 6, 104–116. [Google Scholar] [CrossRef]
- Zirafi, O.; Kim, K.A.; Roan, N.R.; Kluge, S.F.; Müller, J.A.; Jiang, S.; Mayer, B.; Greene, W.C.; Kirchhoff, F.; Münch, J. Semen Enhances HIV Infectivity and Impairs the Antiviral Efficacy of Microbicides. Sci. Transl. Med. 2014, 6, 262ra157. [Google Scholar] [CrossRef]
- Vallely, P.J.; Rees, R.C. Seminal Plasma Suppression of Human Lymphocyte Responses in Vitro Requires the Presence of Bovine Serum Factors. Clin. Exp. Immunol. 1986, 66, 181–187. [Google Scholar] [PubMed]
- Vallely, P.J.; Sharrard, R.M.; Rees, R.C. The Identification of Factors in Seminal Plasma Responsible for Suppression of Natural Killer Cell Activity. Immunology 1988, 63, 451–456. [Google Scholar] [PubMed]
- Szymaniec, S.; Quayle, A.J.; Hargreave, T.B.; James, K. Human Seminal Plasma Suppresses Lymphocyte Responses in Vitro in Serum-Free Medium. J. Reprod. Immunol. 1987, 12, 191–200. [Google Scholar] [CrossRef]
- Kiessling, A.A. Isolation of Human Immunodeficiency Virus Type 1 from Semen and Vaginal Fluids. Methods Mol. Biol. 2005, 304, 71–86. [Google Scholar] [CrossRef]
- Allen, R.D.; Roberts, T.K. Role of Spermine in the Cytotoxic Effects of Seminal Plasma. Am. J. Reprod. Immunol. Microbiol. 1987, 13, 4–8. [Google Scholar] [CrossRef]
- Roan, N.R.; Müller, J.A.; Liu, H.; Chu, S.; Arnold, F.; Stürzel, C.M.; Walther, P.; Dong, M.; Witkowska, H.E.; Kirchhoff, F.; et al. Peptides Released by Physiological Cleavage of Semen Coagulum Proteins Form Amyloids That Enhance HIV Infection. Cell Host Microbe 2011, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Yolamanova, M.; Zirafi, O.; Roan, N.R.; Staendker, L.; Forssmann, W.G.; Burgener, A.; Dejucq-Rainsford, N.; Hahn, B.H.; Shaw, G.M.; et al. Semen-Mediated Enhancement of HIV Infection Is Donor-Dependent and Correlates with the Levels of SEVI. Retrovirology 2010, 7, 55. [Google Scholar] [CrossRef]
- Münch, J.; Rücker, E.; Ständker, L.; Adermann, K.; Goffinet, C.; Schindler, M.; Wildum, S.; Chinnadurai, R.; Rajan, D.; Specht, A.; et al. Semen-Derived Amyloid Fibrils Drastically Enhance HIV Infection. Cell 2007, 131, 1059–1071. [Google Scholar] [CrossRef]
- Rametse, C.L.; Adefuye, A.O.; Olivier, A.J.; Curry, L.; Gamieldien, H.; Burgers, W.A.; Lewis, D.A.; Williamson, A.L.; Katz, A.A.; Passmore, J.A.S. Inflammatory Cytokine Profiles of Semen Influence Cytokine Responses of Cervicovaginal Epithelial Cells. Front. Immunol. 2018, 9, 2721. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.D. Semen Polyamines in AIDS Pathogenesis. Nature 1984, 310, 103. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Páez, L.; Aguirre-Alvarado, C.; Oviedo, N.; Alcántara-Farfán, V.; Lara-Ramírez, E.E.; Jimenez-Gutierrez, G.E.; Cordero-Martínez, J. Polyamines Influence Mouse Sperm Channels Activity. Int. J. Mol. Sci. 2021, 22, 441. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Toxicity of Polyamines and Their Metabolic Products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, H.; Rui, H.; Thomassen, Y.; Hald, T.; Purvis, K. Polyamines and Other Accessory Sex Gland Secretions in Human Seminal Plasma 8 Years after Vasectomy. J. Reprod. Fertil. 1989, 87, 39–45. [Google Scholar] [CrossRef][Green Version]
- Shohat, B.; Maayan, R.; Singer, R.; Sagiv, M.; Kaufman, H.; Zukerman, Z. Immunosuppressive Activity and Polyamine Levels of Seminal Plasma in Azo-Ospermic, Oligospermic, and Normospermic Men. Arch. Androl. 1990, 24, 41–50. [Google Scholar] [CrossRef]
- Sharmin, S.; Sakata, K.; Kashiwagi, K.; Ueda, S.; Iwasaki, S.; Shirahata, A.; Igarashi, K. Polyamine Cytotoxicity in the Presence of Bovine Serum Amine Oxidase. Biochem. Biophys. Res. Commun. 2001, 282, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A.; Agostinelli, E.; Przybytkowski, E.; Mondovi, B. Aldehyde Dehydrogenase and Cytotoxicity of Purified Bovine Serum Amine Oxidase and Spermine in Chinese Hamster Ovary Cells. Biochem. Cell Biol. 1994, 72, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gahl, W.A.; Pitot, H.C. Reversal by Aminoguanidine of the Inhibition of Proliferation of Human Fibroblasts by Spermidine and Spermine. Chem. Biol. Interact. 1978, 22, 91–98. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, Metabolism, and Biomolecular Interactions Relevant to Human Health and Disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Parchment, R.E.; Lewellyn, A.; Swartzendruber, D.; Pierce, G.B. Serum Amine Oxidase Activity Contributes to Crisis in Mouse Embryo Cell Lines. Proc. Natl. Acad. Sci. USA 1990, 87, 4340–4344. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.O. Biological Effects of Aminoguanidine: An Update. Inflamm. Res. 1999, 48, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Shore, P.A.; Cohn, V.H. Comparative Effects of Monoamine Oxidase Inhibitors on Monoamine Oxidase and Diamine Oxidase. Biochem. Pharmacol. 1960, 5, 91–95. [Google Scholar] [CrossRef]
- Tamura, H.; Horiike, K.; Fukuda, H.; Watanabe, T. Kinetic Studies on the Inhibition Mechanism of Diamine Oxidase from Porcine Kidney by Aminoguanidine. J. Biochem. 1989, 105, 299–306. [Google Scholar] [CrossRef]
- Holbert, C.E.; Dunworth, M.; Foley, J.R.; Dunston, T.T.; Stewart, T.M.; Casero, R.A. Autophagy Induction by Exogenous Polyamines Is an Artifact of Bovine Serum Amine Oxidase Activity in Culture Serum. J. Biol. Chem. 2020, 295, 9061–9068. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Qi, C.; Shen, L.; Wang, J.; Liu, X.; Zhang, N.; Bing, T.; Shangguan, D. Oxidative Degradation of Polyamines by Serum Supplement Causes Cytotoxicity on Cultured Cells. Sci. Rep. 2018, 8, 10384. [Google Scholar] [CrossRef]
- Wei, X.; Decker, J.M.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilby, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of Resistant Human Immunodeficiency Virus Type 1 in Patients Receiving Fusion Inhibitor (T-20) Monotherapy. Antimicrob. Agents Chemother. 2002, 46, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.A.; Harms, M.; Schubert, A.; Mayer, B.; Jansen, S.; Herbeuval, J.P.; Michel, D.; Mertens, T.; Vapalahti, O.; Schmidt-Chanasit, J.; et al. Development of a High-Throughput Colorimetric Zika Virus Infection Assay. Med. Microbiol. Immunol. 2017, 206, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gilad, G.M.; Gilad, V.H. Polyamines Affect Growth of Cultured Rat Cerebellar Neurons in Different Sera. Int. J. Dev. Neurosci. 1986, 4, 195–208. [Google Scholar] [CrossRef]
- Magnes, C.; Fauland, A.; Gander, E.; Narath, S.; Ratzer, M.; Eisenberg, T.; Madeo, F.; Pieber, T.; Sinner, F. Polyamines in Biological Samples: Rapid and Robust Quantification by Solid-Phase Extraction Online-Coupled to Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1331, 44–51. [Google Scholar] [CrossRef]
- Okamoto, M.; Byrn, R.; Eyre, R.C.; Mullen, T.; Church, P.; Kiessling, A. a Seminal Plasma Induces Programmed Cell Death in Cultured Peripheral Blood Mononuclear Cells. AIDS Res. Hum. Retrovir. 2002, 18, 797–803. [Google Scholar] [CrossRef]
- Selva, K.J.; Kent, S.J.; Parsons, M.S. Modulation of Innate and Adaptive Cellular Immunity Relevant to HIV-1 Vaccine Design by Seminal Plasma. AIDS 2017, 31, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and Organization of the Human Antimicrobial Peptide LL-37 in Phospholipid Membranes: Relevance to the Molecular Basis for Its Non-Cell-Selective Activity. Biochem. J. 1999, 341, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Martellini, J.A.; Cole, A.L.; Venkataraman, N.; Quinn, G.A.; Svoboda, P.; Gangrade, B.K.; Pohl, J.; Sørensen, O.E.; Cole, A.M. Cationic Polypeptides Contribute to the Anti-HIV-1 Activity of Human Seminal Plasma. FASEB J. 2009, 23, 3609–3618. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, U.; Rosenkovitch, E. Effect of Oxidized Spermine and Other Aldehydes on the Infectivity of Vaccinia Virus. Appl. Microbiol. 1972, 23, 232–235. [Google Scholar] [CrossRef]
- Balandya, E.; Sheth, S.; Sanders, K.; Wieland-Alter, W.; Lahey, T. Semen Protects CD4+ Target Cells from HIV Infection but Promotes the Preferential Transmission of R5 Tropic HIV. J. Immunol. 2010, 185, 7596–7604. [Google Scholar] [CrossRef] [PubMed]
- Grivel, J.C.; Margolis, L. Use of Human Tissue Explants to Study Human Infectious Agents. Nat. Protoc. 2009, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.A.; Harms, M.; Krüger, F.; Groß, R.; Joas, S.; Hayn, M.; Dietz, A.N.; Lippold, S.; Von Einem, J.; Schubert, A.; et al. Semen Inhibits Zika Virus Infection of Cells and Tissues from the Anogenital Region. Nat. Commun. 2018, 9, 2207. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harms, M.; von Maltitz, P.; Groß, R.; Mayer, B.; Deniz, M.; Müller, J.; Münch, J. Utilization of Aminoguanidine Prevents Cytotoxic Effects of Semen. Int. J. Mol. Sci. 2022, 23, 8563. https://doi.org/10.3390/ijms23158563
Harms M, von Maltitz P, Groß R, Mayer B, Deniz M, Müller J, Münch J. Utilization of Aminoguanidine Prevents Cytotoxic Effects of Semen. International Journal of Molecular Sciences. 2022; 23(15):8563. https://doi.org/10.3390/ijms23158563
Chicago/Turabian StyleHarms, Mirja, Pascal von Maltitz, Rüdiger Groß, Benjamin Mayer, Miriam Deniz, Janis Müller, and Jan Münch. 2022. "Utilization of Aminoguanidine Prevents Cytotoxic Effects of Semen" International Journal of Molecular Sciences 23, no. 15: 8563. https://doi.org/10.3390/ijms23158563
APA StyleHarms, M., von Maltitz, P., Groß, R., Mayer, B., Deniz, M., Müller, J., & Münch, J. (2022). Utilization of Aminoguanidine Prevents Cytotoxic Effects of Semen. International Journal of Molecular Sciences, 23(15), 8563. https://doi.org/10.3390/ijms23158563





