The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications
Abstract
:1. Introduction
2. Protein Kinase C-Mitogen Activated Protein Kinase (PKC-MAPK) Signalling Pathways in Diabetes
2.1. PKC Subfamilies
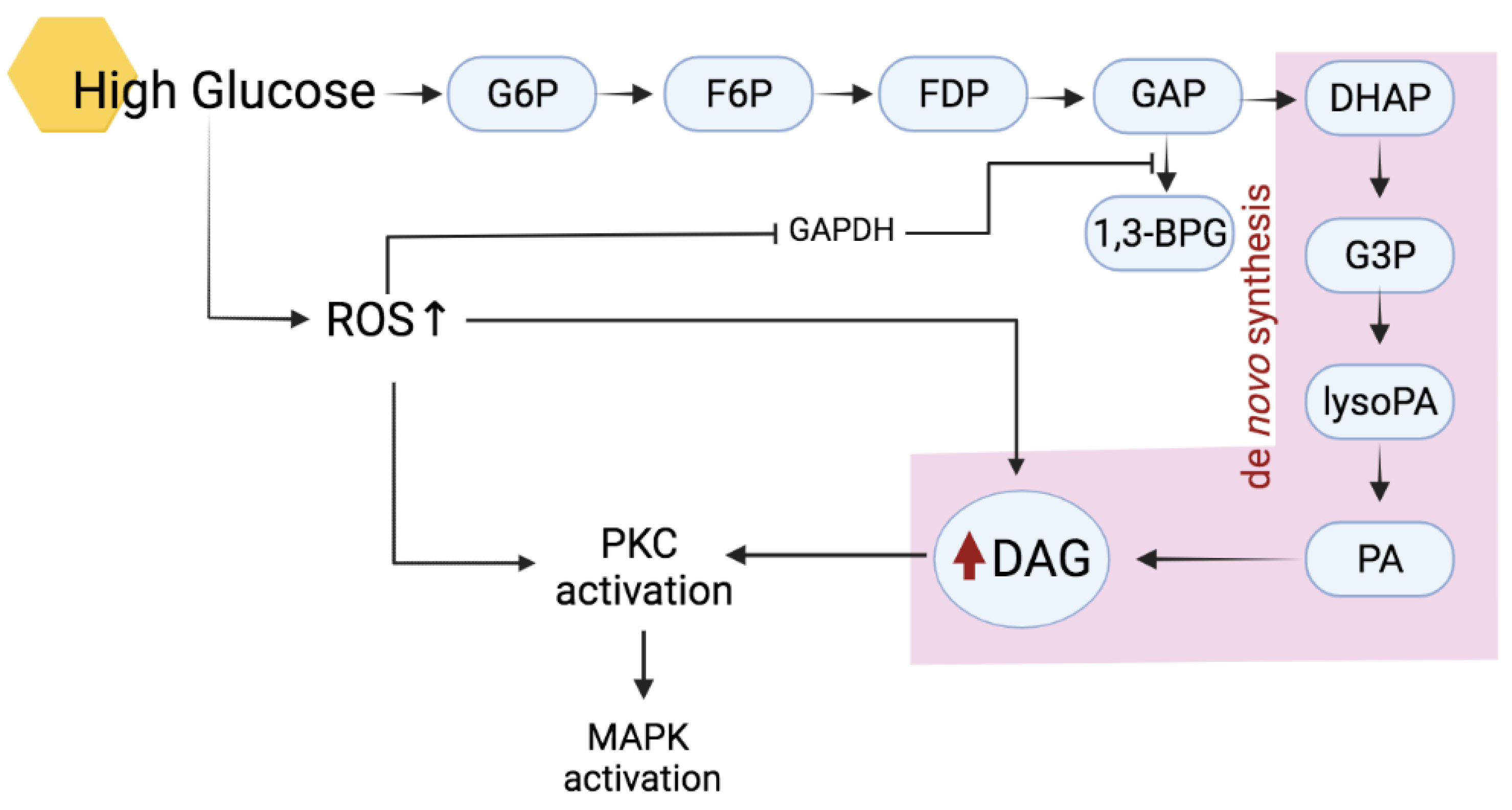
2.2. Activation of PKC in Hyperglycemic Condition
2.3. Roles of PKC in Diabetic Cardiovascular Pathology
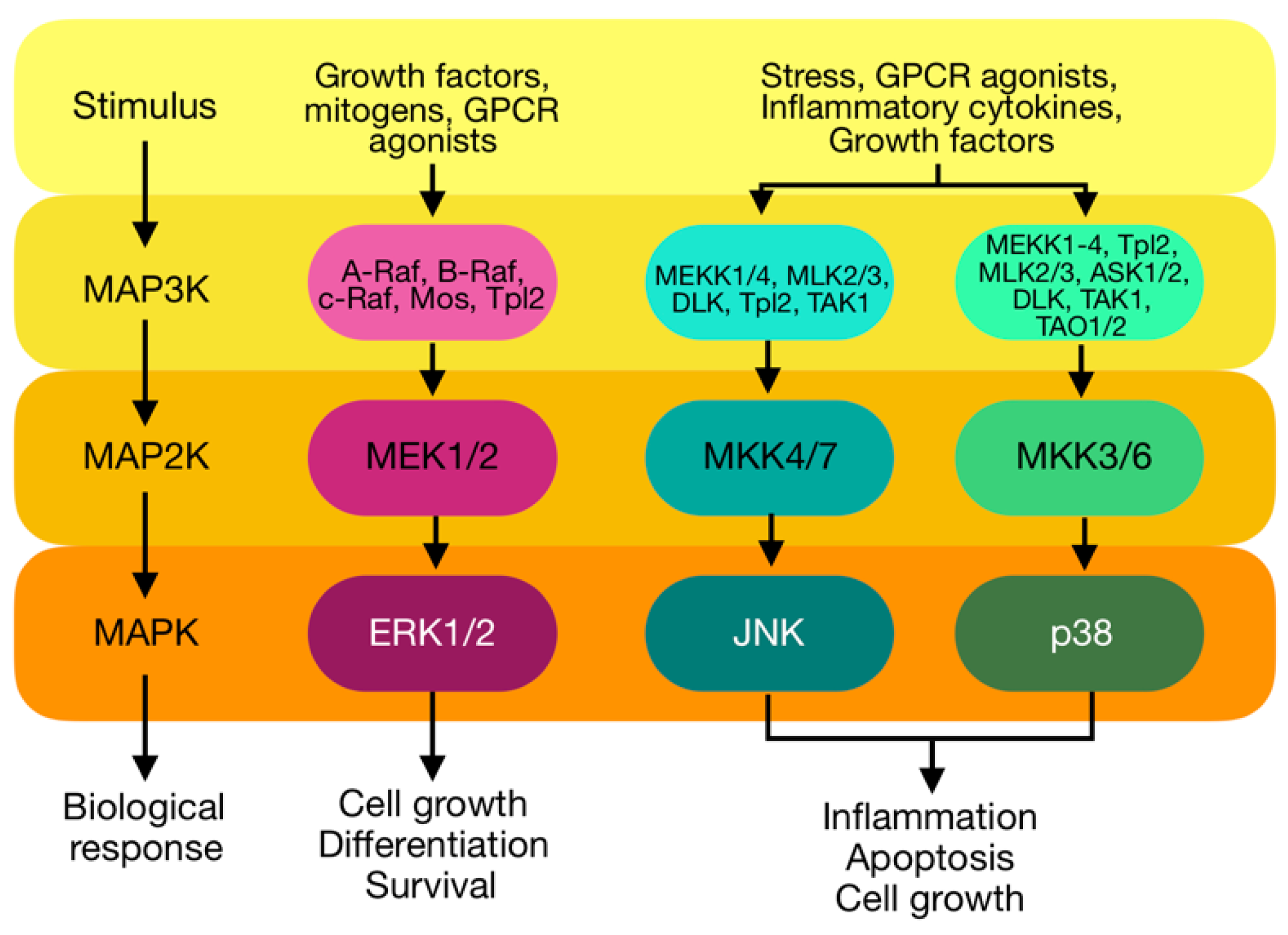
3. Mitogen-Activated Protein Kinases (MAPKs)
3.1. Extracellular-Regulated Kinase (ERK) 1/2
3.2. c-Jun N-Terminal Kinase (JNK)
3.3. p38
3.4. Activation of MAPK by PKC
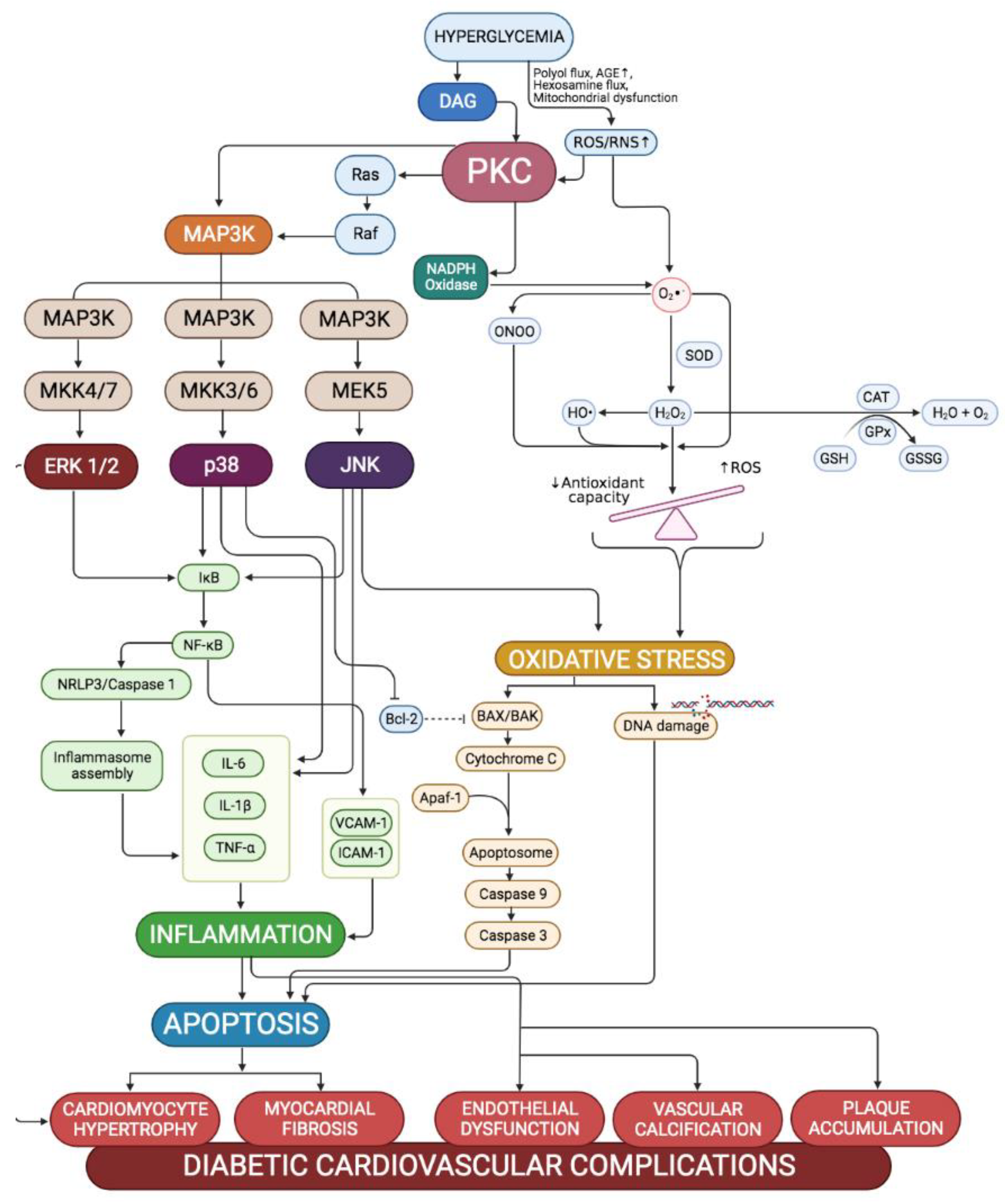
4. Effects of Activation of PKC-MAPK Pathways to Cardiovascular in Hyperglycaemic Conditions
4.1. Cardiovascular Oxidative Stress
4.2. Cardiovascular Inflammation
4.3. Cardiovascular Apoptosis
5. Involvement of PKC-MAPK Pathway in Diabetic Heart Complications
5.1. PKC-MAPK Pathway in Cardiac Hypertrophy
5.2. PKC-MAPK Pathway in Cardiac Fibrosis
5.3. PKC-MAPK Pathway in Endothelial Dysfunction
5.4. PKC-MAPK Pathway in Atherosclerosis
6. PKC-MAPK as Therapeutic Target for Cardiovascular Management in Diabetic Patients
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Federation, I.D. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussel, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- Ambady, R.; Chamukuttan, S.; Nanditha, A. Classification and Diagnosis of Diabetes. In Classification and Diagnosis of Diabetes; Holt, R.I.G., Cockram, C.S., Flyvbjerg, A., Goldstein, B.J., Eds.; Wiley-Blackwell Publishing Ltd: Chichester, UK, 2017; pp. 23–28. ISBN 9781118924860. [Google Scholar]
- Thorn, L.M.; Forsblom, C.; Wadén, J.; Saraheimo, M.; Tolonen, N.; Hietala, K.; Groop, P.H. Metabolic Syndrome as a Risk Factor for Cardiovascular Disease, Mortality, and Progression of Diabetic Nephropathy in Type 1 Diabetes. Diabetes Care 2009, 32, 950–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.-X.; Ma, X.-N.; Guan, C.-H.; Li, Y.-D.; Mauricio, D.; Fu, S.-B. Cardiovascular Disease in Type 2 Diabetes Mellitus: Progress toward Personalized Management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The Potential Role of Flavonoids in Ameliorating Diabetic Cardiomyopathy via Alleviation of Cardiac Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 5094. [Google Scholar] [CrossRef]
- Soetikno, V.; Sari, F.R.; Sukumaran, V.; Lakshmanan, A.P.; Mito, S.; Harima, M.; Thandavarayan, R.A.; Suzuki, K.; Nagata, M.; Takagi, R.; et al. Curcumin Prevents Diabetic Cardiomyopathy in Streptozotocin-Induced Diabetic Rats: Possible Involvement of PKC-MAPK Signaling Pathway. Eur. J. Pharm. Sci. 2012, 47, 604–614. [Google Scholar] [CrossRef]
- Griner, E.M.; Kazanietz, M.G. Protein Kinase C and Other Diacylglycerol Effectors in Cancer. Nat. Rev. Cancer 2007, 7, 281–294. [Google Scholar] [CrossRef]
- Trappanese, D.M.; Sivilich, S.; Ets, H.K.; Kako, F.; Autieri, M.V.; Moreland, R.S. Regulation of Mitogen-Activated Protein Kinase by Protein Kinase C and Mitogen-Activated Protein Kinase Phosphatase-1 in Vascular Smooth Muscle. Am. J. Physiol.-Cell Physiol. 2016, 310, C921–C930. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, M.; Suzuki, D.; Honma, M.; Uehara, G.; Sakai, T.; Umezono, T.; Sakai, H. High Expression of PKC-MAPK Pathway MRNAs Correlates with Glomerular Lesions in Human Diabetic Nephropathy. Kidney Int. 2004, 66, 1107–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.; Ali, A.; Katare, R. Molecular Complexities Underlying the Vascular Complications of Diabetes Mellitus–A Comprehensive Review. J. Diabetes Complicat. 2020, 34, 107613. [Google Scholar] [CrossRef] [PubMed]
- Rask-Madsen, C.; Li, Q.; Freund, B.; Feather, D.; Abramov, R.; Wu, I.H.; Chen, K.; Yamamoto-Hiraoka, J.; Goldenbogen, J.; Sotiropoulos, K.B.; et al. Loss of Insulin Signaling in Vascular Endothelial Cells Accelerates Atherosclerosis in Apolipoprotein e Null Mice. Cell Metab. 2010, 11, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New Insights into Oxidative Stress and Inflammation during Diabetes Mellitus-Accelerated Atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Palaniyandi, S.S.; Sun, L.; Ferreira, J.C.B.; Mochly-Rosen, D. Protein Kinase C in Heart Failure: A Therapeutic Target? Cardiovasc. Res. 2009, 82, 229–239. [Google Scholar] [CrossRef] [Green Version]
- De Geest, B.; Mishra, M. Role of Oxidative Stress in Diabetic Cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein Kinase C: Poised to Signal. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E395–E402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, A.M.; Faenza, I.; Billi, A.M.; Falà, F.; Cocco, L.; Manzoli, L. Nuclear Protein Kinase C Isoforms: Key Players in Multiple Cell Functions? Histol. Histopathol. 2003, 18, 1301–1312. [Google Scholar] [CrossRef]
- Newton, A.C. Protein Kinase C: Structure, Function, and Regulation. J. Biol. Chem. 1995, 270, 28495–28498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, E.; Hatanaka, M. PKC Pathway and ERK / MAPK Pathway Are Required for Induction of Cyclin D1 and P21 Waf1 during Differentiation of Myeloleukemia Cells. Kobe J. Med. Sci. 2006, 52, 181–194. [Google Scholar]
- Rosse, C.; Linch, M.; Kermorgant, S.; Cameron, A.J.M.; Boeckeler, K.; Parker, P.J. PKC and the Control of Localized Signal Dynamics. Nat. Rev. Mol. Cell Biol. 2010, 11, 103–112. [Google Scholar] [CrossRef]
- Noh, H.; King, G.L. The Role of Protein Kinase C Activation in Diabetic Nephropathy. Kidney Int. 2007, 72, S49–S53. [Google Scholar] [CrossRef] [Green Version]
- Gould, C.M.; Newton, A.C. The Life and Death of Protein Kinase C. Curr. Drug Targets 2008, 9, 614–625. [Google Scholar] [CrossRef]
- Liu, M.; Clarke, C.J.; Salama, M.F.; Choi, Y.J.; Obeid, L.M.; Hannun, Y.A. Co-Ordinated Activation of Classical and Novel PKC Isoforms Is Required for PMA-Induced MTORC1 Activation. PLoS ONE 2017, 12, e0184818. [Google Scholar] [CrossRef] [Green Version]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-Evoked Activation of PKC and PKD Isoforms in Regulation of Glucose and Lipid Metabolism: A Review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichmann, T.O.; Lass, A. DAG Tales: The Multiple Faces of Diacylglycerol-Stereochemistry, Metabolism, and Signaling. Cell. Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Anderberg, R.J.; Cooney, S.K.; Meek, R.L. Oxidative Stress Mediates Protein Kinase C Activation and Advanced Glycation End Product Formation in a Mesangial Cell Model of Diabetes and High Protein Diet. Am. J. Nephrol. 2009, 29, 171–180. [Google Scholar] [CrossRef]
- Konishi, H.; Tanaka, M.; Takemura, Y.; Matsuzaki, H.; Ono, Y.; Kikkawa, U.; Nishizuka, Y. Activation of Protein Kinase C by Tyrosine Phosphorylation in Response to H2O2. Proc. Natl. Acad. Sci. USA 1997, 94, 11233–11237. [Google Scholar] [CrossRef] [Green Version]
- Braz, J.C.; Gregory, K.; Pathak, A.; Zhao, W.; Sahin, B.; Klevitsky, R.; Kimball, T.F.; Lorenz, J.N.; Nairn, A.C.; Liggett, S.B.; et al. PKC-α Regulates Cardiac Contractility and Propensity toward Heart Failure. Nat. Med. 2004, 10, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.L.; Heidkamp, M.C.; Patel, N.; Porter, M.; Engman, S.; Samarel, A.M. Alterations in Protein Kinase C Isoenzyme Expression and Autophosphorylation during the Progression of Pressure Overload-Induced Left Ventricular Hypertrophy. Mol. Cell. Biochem. 2003, 242, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Li, H.; Xu, J.; Liu, Y.; Gao, X.; Wang, J.; Ng, K.F.J.; Lau, W.B.; Ma, X.L.; Rodrigues, B.; et al. Hyperglycemia-Induced Protein Kinase C Β2 Activation Induces Diastolic Cardiac Dysfunction in Diabetic Rats by Impairing Caveolin-3 Expression and Akt/ENOS Signaling. Diabetes 2013, 62, 2318–2328. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Fang, Y.; Song, T.; Lv, D.; Wang, Z.; Zhu, L.; Zhao, Z.; Yin, X. FBXL10 Regulates Cardiac Dysfunction in Diabetic Cardiomyopathy via the PKC Β2 Pathway. J. Cell. Mol. Med. 2019, 23, 2558–2567. [Google Scholar] [CrossRef]
- Durpès, M.C.; Morin, C.; Paquin-Veillet, J.; Beland, R.; Paré, M.; Guimond, M.O.; Rekhter, M.; King, G.L.; Geraldes, P. PKC-β Activation Inhibits IL-18-Binding Protein Causing Endothelial Dysfunction and Diabetic Atherosclerosis. Cardiovasc. Res. 2015, 106, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.F.; Chen, S.J.; Tsai, M.C.; Lin, C.S. Potential Role of Protein Kinase C in the Pathophysiology of Diabetes-Associated Atherosclerosis. Front. Pharmacol. 2021, 12, 716332. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qian, X.; Shen, M.; Jiang, R.; Wagner, M.B.; Ding, G.; Chen, G.; Shen, B. Protein Kinase C Promotes Cardiac Fibrosis and Heart Failure by Modulating Galectin-3 Expression. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoletti, R.; Maffei, A.; Madaro, L.; Notte, A.; Stanganello, E.; Cifelli, G.; Carullo, P.; Molinaro, M.; Lembo, G.; Bouché, M. Protein Kinase Cθ Is Required for Cardiomyocyte Survival and Cardiac Remodeling. Cell Death Dis. 2010, 1, e45. [Google Scholar] [CrossRef] [PubMed]
- Takeishi, Y.; Ping, P.; Bolli, R.; Kirkpatrick, D.L.; Hoit, B.D.; Walsh, R.A. Transgenic Overexpression of Constitutively Active Protein Kinase C ε Causes Concentric Cardiac Hypertrophy. Circ. Res. 2000, 86, 1218–1223. [Google Scholar] [CrossRef]
- Churchill, E.N.; Mochly-Rosen, D. The Roles of PKCδ and ε Isoenzymes in the Regulation of Myocardial Ischaemia/Reperfusion Injury. Biochem. Soc. Trans. 2007, 35, 1040–1042. [Google Scholar] [CrossRef]
- Yang, Y.C.; Tsai, C.Y.; Chen, C.L.; Kuo, C.H.; Hou, C.W.; Cheng, S.Y.; Aneja, R.; Huang, C.Y.; Kuo, W.W. Pkcδ Activation Is Involved in ROS-Mediated Mitochondrial Dysfunction and Apoptosis in Cardiomyocytes Exposed to Advanced Glycation End Products (AGEs). Aging Dis. 2018, 9, 647–663. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [Green Version]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK Cascades: Signaling Components, Nuclear Roles and Mechanisms of Nuclear Translocation. Biochim. Biophys. Acta 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.-H.; Wang, X.; Shang, M.-S.; Chen, Z.; Guo, Q.; Li, L.; Wang, H.-Y.; Yu, R.-H.; Ma, C.-S. Crosstalk between PKC and MAPK Pathway Activation in Cardiac Fibroblasts in a Rat Model of Atrial Fibrillation. Biotechnol. Lett. 2020, 42, 1219–1227. [Google Scholar] [CrossRef]
- Rose, B.A.; Force, T.; Wang, Y. Mitogen-Activated Protein Kinase Signaling in the Heart: Angels versus Demons in a Heart-Breaking Tale. Physiol. Rev. 2010, 90, 1507–1546. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed]
- Binion, D.G.; Heidemann, J.; Li, M.S.; Nelson, V.M.; Otterson, M.F.; Rafiee, P. Vascular Cell Adhesion Molecule-1 Expression in Human Intestinal Microvascular Endothelial Cells Is Regulated by PI 3-Kinase/Akt/MAPK/NF-KappaB: Inhibitory Role of Curcumin. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G259–G268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Liu, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Zhang, W.; Elimban, V.; Nijjar, M.S.; Gupta, S.K.; Dhalla, N.S. Role of Mitogen-Activated Protein Kinase in Cardiac Hypertrophy and Heart Failure. Exp. Clin. Cardiol. 2003, 8, 173–183. [Google Scholar]
- Wang, S.; Ding, L.; Ji, H.; Xu, Z.; Liu, Q.; Zheng, Y. The Role of P38 MAPK in the Development of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2016, 17, 1037. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.; Zhong, B.; Luo, Q.; Liu, Q.; Chen, X.; Cao, D.; Li, X.; Yang, S. Phillyrin Attenuates Norepinephrine-Induced Cardiac Hypertrophy and Inflammatory Response by Suppressing P38/ERK1/2 MAPK and AKT/NF-KappaB Pathways. Eur. J. Pharmacol. 2022, 927, 175022. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Deng, Y.; Zhang, S.; Wei, Q.; Yang, Q.; Piao, S.; Bei, W.; Rong, X.; Guo, J. FTZ Ameliorates Diabetic Cardiomyopathy by Inhibiting Inflammation and Cardiac Fibrosis in the Streptozotocin-Induced Model. Evid. Based. Complement. Alternat. Med. 2021, 2021, 5582567. [Google Scholar] [CrossRef]
- El-Sayed, N.; Mostafa, Y.M.; AboGresha, N.M.; Ahmed, A.A.M.; Mahmoud, I.Z.; El-Sayed, N.M. Dapagliflozin Attenuates Diabetic Cardiomyopathy through Erythropoietin Up-Regulation of AKT/JAK/MAPK Pathways in Streptozotocin-Induced Diabetic Rats. Chem. Biol. Interact. 2021, 347, 109617. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Fulton, D.J.R.; Caldwell, R.B.; Caldwell, R.W.; Toque, H.A. Hyperglycemia-Impaired Aortic Vasorelaxation Mediated through Arginase Elevation: Role of Stress Kinase Pathways. Eur. J. Pharmacol. 2019, 844, 26–37. [Google Scholar] [CrossRef]
- Zhang, B.-F.; Jiang, H.; Chen, J.; Guo, X.; Hu, Q.; Yang, S. KDM3A Inhibition Attenuates High Concentration Insulin-induced Vascular Smooth Muscle Cell Injury by Suppressing MAPK/NF-κB Pathways. Int. J. Mol. Sci. 2018, 41, 1265–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montiel, M.; de la Blanca, E.P.; Jiménez, E. P2Y Receptors Activate MAPK/ERK through a Pathway Involving PI3K/PDK1/PKC-Zeta in Human Vein Endothelial Cells. Cell. Physiol. Biochem. 2006, 18, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Puente, L.G.; He, J.-S.; Ostergaard, H.L. A Novel PKC Regulates ERK Activation and Degranulation of Cytotoxic T Lymphocytes: Plasticity in PKC Regulation of ERK. Eur. J. Immunol. 2006, 36, 1009–1018. [Google Scholar] [CrossRef]
- Song, Y.; Xu, J.; Li, Y.; Jia, C.; Ma, X.; Zhang, L.; Xie, X.; Zhang, Y.; Gao, X.; Zhang, Y.; et al. Cardiac Ankyrin Repeat Protein Attenuates Cardiac Hypertrophy by Inhibition of ERK1/2 and TGF-β Signaling Pathways. PLoS ONE 2012, 7, e50436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Song, J.-T.; Ji, X.-F.; Liu, Z.-Q.; Cong, M.-L.; Liu, D.-X. Sodium Ferulate Protects against Angiotensin II-Induced Cardiac Hypertrophy in Mice by Regulating the MAPK/ERK and JNK Pathways. BioMed Res. Int. 2017, 2017, 3754942. [Google Scholar] [CrossRef] [Green Version]
- Roskoski, R. ERK1/2 MAP Kinases: Structure, Function, and Regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef]
- Marampon, F.; Ciccarelli, C.; Zani, B.M. Biological Rationale for Targeting MEK/ERK Pathways in Anti-Cancer Therapy and to Potentiate Tumour Responses to Radiation. Int. J. Mol. Sci. 2019, 20, 2530. [Google Scholar] [CrossRef] [Green Version]
- Chambard, J.-C.; Lefloch, R.; Pouysségur, J.; Lenormand, P. ERK Implication in Cell Cycle Regulation. Biochim. Biophys. Acta 2007, 1773, 1299–1310. [Google Scholar] [CrossRef]
- Sidarala, V.; Kowluru, A. The Regulatory Roles of Mitogen-Activated Protein Kinase (MAPK) Pathways in Health and Diabetes: Lessons Learned from the Pancreatic β-Cell. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 10, 76–84. [Google Scholar] [CrossRef]
- Palomer, X.; Salvadó, L.; Barroso, E.; Vázquez-Carrera, M. An Overview of the Crosstalk between Inflammatory Processes and Metabolic Dysregulation during Diabetic Cardiomyopathy. Int. J. Cardiol. 2013, 168, 3160–3172. [Google Scholar] [CrossRef]
- Miguez, J.S.G.; Dela Justina, V.; Bressan, A.F.M.; Marchi, P.G.F.; Honorio-França, A.C.; Carneiro, F.S.; Clinton Webb, R.; Tostes, R.C.; Giachini, F.R.; Lima, V. V O-Glycosylation with O-Linked β-N-Acetylglucosamine Increases Vascular Contraction: Possible Modulatory Role on Interleukin-10 Signaling Pathway. Life Sci. 2018, 209, 78–84. [Google Scholar] [CrossRef]
- Eishingdrelo, H.; Kongsamut, S. Minireview: Targeting GPCR Activated ERK Pathways for Drug Discovery. Curr. Chem. Genom. Transl. Med. 2013, 7, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saud, K.; Herrera-Molina, R.; Von Bernhardi, R. Pro- and Anti-Inflammatory Cytokines Regulate the ERK Pathway: Implication of the Timing for the Activation of Microglial Cells. Neurotox. Res. 2005, 8, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Song, K. Actin Dysfunction Activates ERK1/2 and Delays Entry into Mitosis in Mammalian Cells. Cell Cycle 2007, 6, 1487–1495. [Google Scholar] [CrossRef]
- Black, A.R.; Black, J.D. Protein Kinase C Signaling and Cell Cycle Regulation. Front. Immunol. 2012, 3, 423. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.J.; Wung, B.S.; Chao, Y.J.; Wang, D.L. Sequential Activation of Protein Kinase C (PKC)-Alpha and PKC-Epsilon Contributes to Sustained Raf/ERK1/2 Activation in Endothelial Cells under Mechanical Strain. J. Biol. Chem. 2001, 276, 31368–31375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinal, N.; Calleja, M.; Morata, G. Pro-Apoptotic and pro-Proliferation Functions of the JNK Pathway of Drosophila: Roles in Cell Competition, Tumorigenesis and Regeneration. Open Biol. 2019, 9, 180256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, B.; Baillet, A.; Poüs, C. Cytoskeleton and Associated Proteins: Pleiotropic JNK Substrates and Regulators. Int. J. Mol. Sci. 2021, 22, 8375. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, D.; Guo, X.; Li, W.; Li, C.; Luo, J.; Zhou, M.; Xue, L. MKK3 Modulates JNK-Dependent Cell Migration and Invasion. Cell Death Dis. 2019, 10, 149. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK Pathways in the Regulation of Metabolic Reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef] [Green Version]
- Zuo, G.; Ren, X.; Qian, X.; Ye, P.; Luo, J.; Gao, X.; Zhang, J.; Chen, S. Inhibition of JNK and P38 MAPK-Mediated Inflammation and Apoptosis by Ivabradine Improves Cardiac Function in Streptozotocin-Induced Diabetic Cardiomyopathy. J. Cell. Physiol. 2019, 234, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Lin, A. Activation of the JNK Signaling Pathway: Breaking the Brake on Apoptosis. Bioessays 2003, 25, 17–24. [Google Scholar] [CrossRef] [PubMed]
- López-Bergami, P.; Habelhah, H.; Bhoumik, A.; Zhang, W.; Wang, L.H.; Ronai, Z. RACK1 Mediates Activation of JNK by Protein Kinase C [Corrected]. Mol. Cell 2005, 19, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.-S.; Rhee, M.H.; Sung, G.-H.; Yoo, B.C.; Cho, J.Y. Functional Roles of P38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses. Mediators Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Shi, L.Z.; Chi, H. Regulation of JNK and P38 MAPK in the Immune System: Signal Integration, Propagation and Termination. Cytokine 2009, 48, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Engel, F.B.; Schebesta, M.; Duong, M.T.; Lu, G.; Ren, S.; Madwed, J.B.; Jiang, H.; Wang, Y.; Keating, M.T. P38 MAP Kinase Inhibition Enables Proliferation of Adult Mammalian Cardiomyocytes. Genes Dev. 2005, 19, 1175–1187. [Google Scholar] [CrossRef] [Green Version]
- Min, D.S.; Shin, E.-Y.; Kim, E.-G. The P38 Mitogen-Activated Protein Kinase Is Involved in Stress-Induced Phospholipase D Activation in Vascular Smooth Muscle Cells. Exp. Mol. Med. 2002, 34, 38–46. [Google Scholar] [CrossRef]
- Nakajima, K.; Tohyama, Y.; Kohsaka, S.; Kurihara, T. Protein Kinase C Alpha Requirement in the Activation of P38 Mitogen-Activated Protein Kinase, Which Is Linked to the Induction of Tumor Necrosis Factor Alpha in Lipopolysaccharide-Stimulated Microglia. Neurochem. Int. 2004, 44, 205–214. [Google Scholar] [CrossRef]
- Yacoub, D.; Théorêt, J.-F.; Villeneuve, L.; Abou-Saleh, H.; Mourad, W.; Allen, B.G.; Merhi, Y. Essential Role of Protein Kinase C Delta in Platelet Signaling, Alpha IIb Beta 3 Activation, and Thromboxane A2 Release. J. Biol. Chem. 2006, 281, 30024–30035. [Google Scholar] [CrossRef] [Green Version]
- Denning, M.F. Protein Kinase C/Mitogen-Activated Protein Kinase Signaling in Keratinocyte Differentiation Control. J. Investig. Dermatol. 2010, 130, 1968–1970. [Google Scholar] [CrossRef] [Green Version]
- Efimova, T.; Deucher, A.; Kuroki, T.; Ohba, M.; Eckert, R.L. Novel Protein Kinase C Isoforms Regulate Human Keratinocyte Differentiation by Activating a P38 Delta Mitogen-Activated Protein Kinase Cascade That Targets CCAAT/Enhancer-Binding Protein Alpha. J. Biol. Chem. 2002, 277, 31753–31760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusanescu, G.; Gotoh, T.; Tian, X.; Feig, L.A. Regulation of Ras Signaling Specificity by Protein Kinase C. Mol. Cell. Biol. 2001, 21, 2650–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, Y.T.; Guo, Y.; Zhu, Y.; Wang, O.L.; Rokosh, G.; Messing, R.O.; Bolli, R. Role of the Protein Kinase C-Epsilon-Raf-1-MEK-1/2-P44/42 MAPK Signaling Cascade in the Activation of Signal Transducers and Activators of Transcription 1 and 3 and Induction of Cyclooxygenase-2 after Ischemic Preconditioning. Circulation 2005, 112, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Chen, W.; Cobb, M.H. Differential Regulation and Properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnoub, A.E.; Weinberg, R.A. Ras Oncogenes: Split Personalities. Nat. Rev. Mol. Cell Biol. 2008, 9, 517–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, M.-J.; Jean, D.; Vézina, A.; Rivard, N. Dual Role of MEK/ERK Signaling in Senescence and Transformation of Intestinal Epithelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G736–G746. [Google Scholar] [CrossRef]
- D’Oria, R.; Laviola, L.; Giorgino, F.; Unfer, V.; Bettocchi, S.; Scioscia, M. PKB/Akt and MAPK/ERK Phosphorylation Is Highly Induced by Inositols: Novel Potential Insights in Endothelial Dysfunction in Preeclampsia. Pregnancy Hypertens. 2017, 10, 107–112. [Google Scholar] [CrossRef]
- Pruitt, K.; Pruitt, W.M.; Bilter, G.K.; Westwick, J.K.; Der, C.J. Raf-Independent Deregulation of P38 and JNK Mitogen-Activated Protein Kinases Are Critical for Ras Transformation. J. Biol. Chem. 2002, 277, 31808–31817. [Google Scholar] [CrossRef] [Green Version]
- Khor, B.-H.; Tiong, H.-C.; Tan, S.C.; Wong, S.K.; Chin, K.-Y.; Karupaiah, T.; Ima-Nirwana, S.; Abdul Gafor, A.H. Effects of Tocotrienols Supplementation on Markers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2021, 16, e0255205. [Google Scholar] [CrossRef]
- Sugimoto, R.; Enjoji, M.; Kohjima, M.; Tsuruta, S.; Fukushima, M.; Iwao, M.; Sonta, T.; Kotoh, K.; Inoguchi, T.; Nakamuta, M. High Glucose Stimulates Hepatic Stellate Cells to Proliferate and to Produce Collagen through Free Radical Production and Activation of Mitogen-Activated Protein Kinase. Liver Int. 2005, 25, 1018–1026. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications Review-Article. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, J.Z.; AlFaris, N.A.; Al-Farga, A.M.; Alshammari, G.M.; BinMowyna, M.N.; Yahya, M.A. Curcumin Reverses Diabetic Nephropathy in Streptozotocin-Induced Diabetes in Rats by Inhibition of PKCβ/P66Shc Axis and Activation of FOXO-3a. J. Nutr. Biochem. 2021, 87, 108515. [Google Scholar] [CrossRef] [PubMed]
- Kadir, R.R.A.; Alwjwaj, M.; McCarthy, Z.; Bayraktutan, U. Therapeutic Hypothermia Augments the Restorative Effects of PKC-β and Nox2 Inhibition on an in Vitro Model of Human Blood-Brain Barrier. Metab. Brain Dis. 2021, 36, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; West, N.E.J.; Pillai, R.; Taggart, D.P.; Channon, K.M. Nitric Oxide Modulates Superoxide Release and Peroxynitrite Formation in Human Blood Vessels. Hypertension 2002, 39, 1088–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rababa’h, A.M.; Guillory, A.N.; Mustafa, R.; Hijjawi, T. Oxidative Stress and Cardiac Remodeling: An Updated Edge. Curr. Cardiol. Rev. 2018, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sapian, S.; Taib, I.S.; Latip, J.; Katas, H.; Chin, K.-Y.; Mohd Nor, N.A.; Jubaidi, F.F.; Budin, S.B. Therapeutic Approach of Flavonoid in Ameliorating Diabetic Cardiomyopathy by Targeting Mitochondrial-Induced Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 11616. [Google Scholar] [CrossRef]
- Zainalabidin, S.; Ramalingam, A.; Mohamed, S.F.A.; Ali, S.S.; Latip, J.; Yap, W.B. S-Allylcysteine Therapy Reduces Adverse Cardiac Remodelling after Myocardial Infarction in a Rat Model. J. Funct. Foods 2020, 66, 103750. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef]
- Maruhashi, T.; Higashi, Y. Pathophysiological Association between Diabetes Mellitus and Endothelial Dysfunction. Antioxidants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Zakaria, E.M.; El-Maraghy, N.N.; Ahmed, A.F.; Ali, A.A.; El-Bassossy, H.M. PARP Inhibition Ameliorates Nephropathy in an Animal Model of Type 2 Diabetes: Focus on Oxidative Stress, Inflammation, and Fibrosis. Naunyn. Schmiedebergs. Arch. Pharmacol. 2017, 390, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, F.; Al-Ozairi, E. Inflammatory Cytokines and the Risk of Cardiovascular Complications in Type 2 Diabetes. Dis. Markers 2013, 35, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Guan, Y.; Raja, R.; Ruiz-Velasco, A.; Liu, W. Mechanisms and Therapeutic Prospects of Diabetic Cardiomyopathy Through the Inflammatory Response. Front. Physiol. 2021, 12, 694864. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Y.; Zhao, Y.; Peng, K.; Li, W.; Wang, Y.; Zhang, J.; Zhou, S.; Liu, Q.; Li, X.; et al. Inhibition of JNK Phosphorylation by a Novel Curcumin Analog Prevents High Glucose-Induced Inflammation and Apoptosis in Cardiomyocytes and the Development of Diabetic Cardiomyopathy. Diabetes 2014, 63, 3497–3511. [Google Scholar] [CrossRef] [Green Version]
- Westermann, D.; Rutschow, S.; Van Linthout, S.; Linderer, A.; Bücker-Gärtner, C.; Sobirey, M.; Riad, A.; Pauschinger, M.; Schultheiss, H.-P.; Tschöpe, C. Inhibition of P38 Mitogen-Activated Protein Kinase Attenuates Left Ventricular Dysfunction by Mediating pro-Inflammatory Cardiac Cytokine Levels in a Mouse Model of Diabetes Mellitus. Diabetologia 2006, 49, 2507–2513. [Google Scholar] [CrossRef]
- Di Filippo, C.; Marfella, R.; Cuzzocrea, S.; Piegari, E.; Petronella, P.; Giugliano, D.; Rossi, F.; D’Amico, M. Hyperglycemia in Streptozotocin-Induced Diabetic Rat Increases Infarct Size Associated with Low Levels of Myocardial HO-1 during Ischemia/Reperfusion. Diabetes 2005, 54, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzik, T.J.; Mussa, S.; Gastaldi, D.; Sadowski, J.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Mechanisms of Increased Vascular Superoxide Production in Human Diabetes Mellitus: Role of NAD(P)H Oxidase and Endothelial Nitric Oxide Synthase. Circulation 2002, 105, 1656–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.-Y.; Chang, S.-S.; Lin, I.-H.; Chen, H.-I. Suppression of Antioxidant Nrf-2 and Downstream Pathway in H9c2 Cells by Advanced Glycation End Products (AGEs) via ERK Phosphorylation. Biochimie 2015, 118, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-G.; Liu, X.-Y.; Ye, J.-M.; Hu, T.-T.; Yang, Y.-Y.; Han, T.; Tan, W. Isosteviol Ameliorates Diabetic Cardiomyopathy in Rats by Inhibiting ERK and NF-ΚB Signaling Pathways. J. Endocrinol. 2018, 238, 47–60. [Google Scholar] [CrossRef]
- Lorenzo, O.; Picatoste, B.; Ares-Carrasco, S.; Ramírez, E.; Egido, J.; Tuñón, J. Potential Role of Nuclear Factor ΚB in Diabetic Cardiomyopathy. Mediators Inflamm. 2011, 2011, 652097. [Google Scholar] [CrossRef] [Green Version]
- Huynh, K.; Kiriazis, H.; Du, X.-J.; Love, J.E.; Gray, S.P.; Jandeleit-Dahm, K.A.; McMullen, J.R.; Ritchie, R.H. Targeting the Upregulation of Reactive Oxygen Species Subsequent to Hyperglycemia Prevents Type 1 Diabetic Cardiomyopathy in Mice. Free Radic. Biol. Med. 2013, 60, 307–317. [Google Scholar] [CrossRef]
- Sari, F.R.; Watanabe, K.; Thandavarayan, R.A.; Harima, M.; Zhang, S.; Muslin, A.J.; Kodama, M.; Aizawa, Y. 14-3-3 Protein Protects against Cardiac Endoplasmic Reticulum Stress (ERS) and ERS-Initiated Apoptosis in Experimental Diabetes. J. Pharmacol. Sci. 2010, 113, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Shiojima, I.; Ikeda, H.; Komuro, I. Chronic Doxorubicin Cardiotoxicity Is Mediated by Oxidative DNA Damage-ATM-P53-Apoptosis Pathway and Attenuated by Pitavastatin through the Inhibition of Rac1 Activity. J. Mol. Cell. Cardiol. 2009, 47, 698–705. [Google Scholar] [CrossRef]
- Narciso, L.; Parlanti, E.; Racaniello, M.; Simonelli, V.; Cardinale, A.; Merlo, D.; Dogliotti, E. The Response to Oxidative DNA Damage in Neurons: Mechanisms and Disease. Neural Plast. 2016, 2016, 3619274. [Google Scholar] [CrossRef] [Green Version]
- Marchenko, N.D.; Moll, U.M. Mitochondrial Death Functions of P53. Mol. Cell. Oncol. 2014, 1, e955995. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Chaabane, W.; User, S.D.; El-Gazzah, M.; Jaksik, R.; Sajjadi, E.; Rzeszowska-Wolny, J.; Los, M.J. Autophagy, Apoptosis, Mitoptosis and Necrosis: Interdependence between Those Pathways and Effects on Cancer. Arch. Immunol. Ther. Exp. 2013, 61, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Conrad, M.; Angeli, J.P.F.; Vandenabeele, P.; Stockwell, B.R. Regulated Necrosis: Disease Relevance and Therapeutic Opportunities. Nat. Rev. Drug Discov. 2016, 15, 348–366. [Google Scholar] [CrossRef]
- Gao, L.; Huang, K.; Jiang, D.-S.; Liu, X.; Huang, D.; Li, H.; Zhang, X.-D.; Huang, K. Novel Role for Caspase-Activated DNase in the Regulation of Pathological Cardiac Hypertrophy. Hypertension 2015, 65, 871–881. [Google Scholar] [CrossRef]
- Kato, K.; Yamanouchi, D.; Esbona, K.; Kamiya, K.; Zhang, F.; Kent, K.C.; Liu, B. Caspase-Mediated Protein Kinase C-Delta Cleavage Is Necessary for Apoptosis of Vascular Smooth Muscle Cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H2253–H2261. [Google Scholar] [CrossRef]
- de Araújo Rocha Reis, J.R.; Cardoso, J.N.; Cardoso, C.M.d.R.; Pereira-Barretto, A.C. Reverse Cardiac Remodeling: A Marker of Better Prognosis in Heart Failure. Arq. Bras. Cardiol. 2015, 104, 502–506. [Google Scholar] [CrossRef]
- Bornstein, A.B.; Rao, S.S.; Marwaha, K. Left Ventricular Hypertrophy; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hunter, J.J.; Tanaka, N.; Rockman, H.A.; Ross, J.; Chien, K.R. Ventricular Expression of a MLC-2v-Ras Fusion Gene Induces Cardiac Hypertrophy and Selective Diastolic Dysfunction in Transgenic Mice. J. Biol. Chem. 1995, 270, 23173–23178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueyama, T.; Kawashima, S.; Sakoda, T.; Rikitake, Y.; Ishida, T.; Kawai, M.; Yamashita, T.; Ishido, S.; Hotta, H.; Yokoyama, M. Requirement of Activation of the Extracellular Signal-Regulated Kinase Cascade in Myocardial Cell Hypertrophy. J. Mol. Cell. Cardiol. 2000, 32, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; Zhang, S.; Treskov, I.; Kovacs, A.; Weinheimer, C.; Muslin, A.J. Raf-1 Kinase Is Required for Cardiac Hypertrophy and Cardiomyocyte Survival in Response to Pressure Overload. Circulation 2004, 110, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Mutlak, M.; Schlesinger-Laufer, M.; Haas, T.; Shofti, R.; Ballan, N.; Lewis, Y.E.; Zuler, M.; Zohar, Y.; Caspi, L.H.; Kehat, I. Extracellular Signal-Regulated Kinase (ERK) Activation Preserves Cardiac Function in Pressure Overload Induced Hypertrophy. Int. J. Cardiol. 2018, 270, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, O.; Watanabe, T.; Nishida, K.; Kashiwase, K.; Higuchi, Y.; Takeda, T.; Hikoso, S.; Hirotani, S.; Asahi, M.; Taniike, M.; et al. Cardiac-Specific Disruption of the c-Raf-1 Gene Induces Cardiac Dysfunction and Apoptosis. J. Clin. Investig. 2004, 114, 937–943. [Google Scholar] [CrossRef] [Green Version]
- Bueno, O.F.; De Windt, L.J.; Tymitz, K.M.; Witt, S.A.; Kimball, T.R.; Klevitsky, R.; Hewett, T.E.; Jones, S.P.; Lefer, D.J.; Peng, C.F.; et al. The MEK1-ERK1/2 Signaling Pathway Promotes Compensated Cardiac Hypertrophy in Transgenic Mice. EMBO J. 2000, 19, 6341–6350. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Kuo, K.-H.; Nagareddy, P.R.; Wang, F.; Guo, Z.; Guo, T.; Jiang, J.; McNeill, J.H. N-Acetylcysteine Attenuates PKCbeta2 Overexpression and Myocardial Hypertrophy in Streptozotocin-Induced Diabetic Rats. Cardiovasc. Res. 2007, 73, 770–782. [Google Scholar] [CrossRef] [Green Version]
- Wakasaki, H.; Koya, D.; Schoen, F.J.; Jirousek, M.R.; Ways, D.K.; Hoit, B.D.; Walsh, R.A.; King, G.L. Targeted Overexpression of Protein Kinase C Beta2 Isoform in Myocardium Causes Cardiomyopathy. Proc. Natl. Acad. Sci. USA 1997, 94, 9320–9325. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Liu, F.; Zhao, Q.; Zhang, J.; Luo, J.; Li, X.; Yang, Y. Constitutive Activation of ERK1/2 Signaling Protects against Myocardial Ischemia via Inhibition of Mitochondrial Fragmentation in the Aging Heart. Ann. Transl. Med. 2021, 9, 479. [Google Scholar] [CrossRef]
- Kehat, I.; Molkentin, J.D. Extracellular Signal-Regulated Kinases 1/2 (ERK1/2) Signaling In Cardiac Hypertrophy. Ann. N. Y. Acad. Sci. 2010, 1188, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, S.; Sah, V.P.; Ross, J.; Brown, J.H.; Han, J.; Chien, K.R. Cardiac Muscle Cell Hypertrophy and Apoptosis Induced by Distinct Members of the P38 Mitogen-Activated Protein Kinase Family. J. Biol. Chem. 1998, 273, 2161–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choukroun, G.; Hajjar, R.; Fry, S.; del Monte, F.; Haq, S.; Guerrero, J.L.; Picard, M.; Rosenzweig, A.; Force, T. Regulation of Cardiac Hypertrophy in Vivo by the Stress-Activated Protein Kinases/c-Jun NH(2)-Terminal Kinases. J. Clin. Investig. 1999, 104, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choukroun, G.; Hajjar, R.; Kyriakis, J.M.; Bonventre, J.V.; Rosenzweig, A.; Force, T. Role of the Stress-Activated Protein Kinases in Endothelin-Induced Cardiomyocyte Hypertrophy. J. Clin. Investig. 1998, 102, 1311–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, G.; Schaefer, A.; Hilfiker-Kleiner, D.; Oppermann, D.; Shukla, P.; Quint, A.; Podewski, E.; Hilfiker, A.; Schröder, F.; Leitges, M.; et al. Increased Collagen Deposition and Diastolic Dysfunction but Preserved Myocardial Hypertrophy after Pressure Overload in Mice Lacking PKCepsilon. Circ. Res. 2005, 96, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Weinheimer, C.; Courtois, M.; Kovacs, A.; Zhang, C.E.; Cheng, A.M.; Wang, Y.; Muslin, A.J. The Role of the Grb2-P38 MAPK Signaling Pathway in Cardiac Hypertrophy and Fibrosis. J. Clin. Investig. 2003, 111, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, K.; Yamaguchi, O.; Hirotani, S.; Hikoso, S.; Higuchi, Y.; Watanabe, T.; Takeda, T.; Osuka, S.; Morita, T.; Kondoh, G.; et al. P38alpha Mitogen-Activated Protein Kinase Plays a Critical Role in Cardiomyocyte Survival but Not in Cardiac Hypertrophic Growth in Response to Pressure Overload. Mol. Cell. Biol. 2004, 24, 10611–10620. [Google Scholar] [CrossRef] [Green Version]
- Petrich, B.G.; Gong, X.; Lerner, D.L.; Wang, X.; Brown, J.H.; Saffitz, J.E.; Wang, Y. C-Jun N-Terminal Kinase Activation Mediates Downregulation of Connexin43 in Cardiomyocytes. Circ. Res. 2002, 91, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Ursitti, J.A.; Petrich, B.G.; Lee, P.C.; Resneck, W.G.; Ye, X.; Yang, J.; Randall, W.R.; Bloch, R.J.; Wang, Y. Role of an Alternatively Spliced Form of AlphaII-Spectrin in Localization of Connexin 43 in Cardiomyocytes and Regulation by Stress-Activated Protein Kinase. J. Mol. Cell. Cardiol. 2007, 42, 572–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachibana, H.; Perrino, C.; Takaoka, H.; Davis, R.J.; Naga Prasad, S.V.; Rockman, H.A. JNK1 Is Required to Preserve Cardiac Function in the Early Response to Pressure Overload. Biochem. Biophys. Res. Commun. 2006, 343, 1060–1066. [Google Scholar] [CrossRef]
- Kyoi, S.; Otani, H.; Matsuhisa, S.; Akita, Y.; Tatsumi, K.; Enoki, C.; Fujiwara, H.; Imamura, H.; Kamihata, H.; Iwasaka, T. Opposing Effect of P38 MAP Kinase and JNK Inhibitors on the Development of Heart Failure in the Cardiomyopathic Hamster. Cardiovasc. Res. 2006, 69, 888–898. [Google Scholar] [CrossRef]
- Fan, G.-C.; Yuan, Q.; Song, G.; Wang, Y.; Chen, G.; Qian, J.; Zhou, X.; Lee, Y.J.; Ashraf, M.; Kranias, E.G. Small Heat-Shock Protein Hsp20 Attenuates Beta-Agonist-Mediated Cardiac Remodeling through Apoptosis Signal-Regulating Kinase 1. Circ. Res. 2006, 99, 1233–1242. [Google Scholar] [CrossRef]
- Fiedler, B.; Feil, R.; Hofmann, F.; Willenbockel, C.; Drexler, H.; Smolenski, A.; Lohmann, S.M.; Wollert, K.C. CGMP-Dependent Protein Kinase Type I Inhibits TAB1-P38 Mitogen-Activated Protein Kinase Apoptosis Signaling in Cardiac Myocytes. J. Biol. Chem. 2006, 281, 32831–32840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, R.; Palm-Leis, A.; Scott, R.C.; Guleria, R.S.; Rachut, E.; Baker, K.M.; Pan, J. All-Trans Retinoic Acid Prevents Development of Cardiac Remodeling in Aortic Banded Rats by Inhibiting the Renin-Angiotensin System. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H633–H644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwik, D.A.; Kuster, G.M.; Brahmbhatt, J.V.; Zaidi, Z.; Malik, J.; Ooi, H.; Ghorayeb, G. EMMPRIN Mediates Beta-Adrenergic Receptor-Stimulated Matrix Metalloproteinase Activity in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2008, 44, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Kuo, W.-W.; Wu, H.-C.; Lai, T.-Y.; Wu, C.-H.; Hwang, J.-M.; Wang, W.-H.; Tsai, F.-J.; Yang, J.-J.; Huang, C.-Y.; et al. ZAK Induces MMP-2 Activity via JNK/P38 Signals and Reduces MMP-9 Activity by Increasing TIMP-1/2 Expression in H9c2 Cardiomyoblast Cells. Mol. Cell. Biochem. 2009, 325, 69–77. [Google Scholar] [CrossRef]
- Liao, P.; Georgakopoulos, D.; Kovacs, A.; Zheng, M.; Lerner, D.; Pu, H.; Saffitz, J.; Chien, K.; Xiao, R.P.; Kass, D.A.; et al. The in Vivo Role of P38 MAP Kinases in Cardiac Remodeling and Restrictive Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2001, 98, 12283–12288. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.D. Cytokine-Induced Modulation of Cardiac Function. Circ. Res. 2004, 95, 1140–1153. [Google Scholar] [CrossRef]
- Birks, E.J.; Burton, P.B.; Owen, V.; Mullen, A.J.; Hunt, D.; Banner, N.R.; Barton, P.J.; Yacoub, M.H. Elevated Tumor Necrosis Factor-Alpha and Interleukin-6 in Myocardium and Serum of Malfunctioning Donor Hearts. Circulation 2000, 102, III352-8. [Google Scholar] [CrossRef] [Green Version]
- Tenhunen, O.; Sármán, B.; Kerkelä, R.; Szokodi, I.; Papp, L.; Tóth, M.; Ruskoaho, H. Mitogen-Activated Protein Kinases P38 and ERK 1/2 Mediate the Wall Stress-Induced Activation of GATA-4 Binding in Adult Heart. J. Biol. Chem. 2004, 279, 24852–24860. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Wang, P.; Zhao, C.X.; Tang, J.; Xiao, X.; Wang, D.W. Decorin Gene Delivery Inhibits Cardiac Fibrosis in Spontaneously Hypertensive Rats by Modulation of Transforming Growth Factor-Beta/Smad and P38 Mitogen-Activated Protein Kinase Signaling Pathways. Hum. Gene Ther. 2009, 20, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sadoshima, J.; Zhai, P.; Hong, C.; Yang, G.; Chen, W.; Yan, L.; Wang, Y.; Vatner, S.F.; Vatner, D.E. Pressure Overload Induces Greater Hypertrophy and Mortality in Female Mice with P38alpha MAPK Inhibition. J. Mol. Cell. Cardiol. 2006, 41, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Vanhoutte, P.M. Macro- and Microvascular Endothelial Dysfunction in Diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef] [Green Version]
- Ugusman, A.; Kumar, J.; Aminuddin, A. Endothelial Function and Dysfunction: Impact of Sodium-Glucose Cotransporter 2 Inhibitors. Pharmacol. Ther. 2021, 224, 107832. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef]
- Huang, A.; Yang, Y.-M.; Yan, C.; Kaley, G.; Hintze, T.H.; Sun, D. Altered MAPK Signaling in Progressive Deterioration of Endothelial Function in Diabetic Mice. Diabetes 2012, 61, 3181–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoguchi, T.; Sonta, T.; Tsubouchi, H.; Etoh, T.; Kakimoto, M.; Sonoda, N.; Sato, N.; Sekiguchi, N.; Kobayashi, K.; Sumimoto, H.; et al. Protein Kinase C-Dependent Increase in Reactive Oxygen Species (ROS) Production in Vascular Tissues of Diabetes: Role of Vascular NAD(P)H Oxidase. J. Am. Soc. Nephrol. 2003, 14, S227–S232. [Google Scholar] [CrossRef] [Green Version]
- Tabit, C.E.; Shenouda, S.M.; Holbrook, M.; Fetterman, J.L.; Kiani, S.; Frame, A.A.; Kluge, M.A.; Held, A.; Dohadwala, M.M.; Gokce, N.; et al. Protein Kinase C-β Contributes to Impaired Endothelial Insulin Signaling in Humans with Diabetes Mellitus. Circulation 2013, 127, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeigler, M.M.; Lam, G.K.; Hunter, M.G.; Eubank, T.D.; Khramtsov, V.V.; Tridandapani, S.; Sen, C.K.; Marsh, C.B. The Role of the NADPH Oxidase Complex, P38 MAPK, and Akt in Regulating Human Monocyte/Macrophage Survival. Am. J. Respir. Cell Mol. Biol. 2007, 36, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-T.; Shih, R.-H.; Lin, C.-C.; Chen, J.-T.; Yang, C.-M. Role of TLR4/NADPH Oxidase/ROS-Activated P38 MAPK in VCAM-1 Expression Induced by Lipopolysaccharide in Human Renal Mesangial Cells. Cell Commun. Signal. 2012, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Xiang, M.; Fan, J.; Fan, J. Association of Toll-like Receptor Signaling and Reactive Oxygen Species: A Potential Therapeutic Target for Posttrauma Acute Lung Injury. Mediators Inflamm. 2010, 2010, 916425. [Google Scholar] [CrossRef]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. NADPH Oxidase Activation in Neutrophils: Role of the Phosphorylation of Its Subunits. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12951. [Google Scholar] [CrossRef] [Green Version]
- Visigalli, R.; Barilli, A.; Parolari, A.; Sala, R.; Rotoli, B.M.; Bussolati, O.; Gazzola, G.C.; Dall’Asta, V. Regulation of Arginine Transport and Metabolism by Protein Kinase Calpha in Endothelial Cells: Stimulation of CAT2 Transporters and Arginase Activity. J. Mol. Cell. Cardiol. 2010, 49, 260–270. [Google Scholar] [CrossRef]
- Wilmes, V.; Scheiper, S.; Roehr, W.; Niess, C.; Kippenberger, S.; Steinhorst, K.; Verhoff, M.A.; Kauferstein, S. Increased Inducible Nitric Oxide Synthase (INOS) Expression in Human Myocardial Infarction. Int. J. Legal Med. 2020, 134, 575–581. [Google Scholar] [CrossRef]
- Zhou, L.; Xue, H.; Wang, Z.; Ni, J.; Yao, T.; Huang, Y.; Yu, C.; Lu, L. Angiotensin-(1-7) Attenuates High Glucose-Induced Proximal Tubular Epithelial-to-Mesenchymal Transition via Inhibiting ERK1/2 and P38 Phosphorylation. Life Sci. 2012, 90, 454–462. [Google Scholar] [CrossRef]
- Kiribayashi, K.; Masaki, T.; Naito, T.; Ogawa, T.; Ito, T.; Yorioka, N.; Kohno, N. Angiotensin II Induces Fibronectin Expression in Human Peritoneal Mesothelial Cells via ERK1/2 and P38 MAPK. Kidney Int. 2005, 67, 1126–1135. [Google Scholar] [CrossRef] [Green Version]
- Perlman, A.; Lawsin, L.M.; Kolachana, P.; Saji, M.; Moore, J.; Ringel, M.D. Angiotensin II Regulation of TGF-Beta in Murine Mesangial Cells Involves Both PI3 Kinase and MAP Kinase. Ann. Clin. Lab. Sci. 2004, 34, 277–286. [Google Scholar]
- Day, R.M.; Yang, Y.; Suzuki, Y.J.; Stevens, J.; Pathi, R.; Perlmutter, A.; Fanburg, B.L.; Lanzillo, J.J. Bleomycin Upregulates Gene Expression of Angiotensin-Converting Enzyme via Mitogen-Activated Protein Kinase and Early Growth Response 1 Transcription Factor. Am. J. Respir. Cell Mol. Biol. 2001, 25, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Kohlstedt, K.; Brandes, R.P.; Müller-Esterl, W.; Busse, R.; Fleming, I. Angiotensin-Converting Enzyme Is Involved in Outside-in Signaling in Endothelial Cells. Circ. Res. 2004, 94, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, K.; Wakino, S.; Tatematsu, S.; Yoshioka, K.; Homma, K.; Sugano, N.; Kimoto, M.; Hayashi, K.; Itoh, H. Role of Asymmetric Dimethylarginine in Vascular Injury in Transgenic Mice Overexpressing Dimethylarginie Dimethylaminohydrolase 2. Circ. Res. 2007, 101, e2–e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Wang, Y.; Cai, L.; Cai, Y.; Hu, J.; Yu, C.; Li, J.; Feng, Z.; Yang, S.; Li, X.; et al. Inhibition of High Glucose-Induced Inflammatory Response and Macrophage Infiltration by a Novel Curcumin Derivative Prevents Renal Injury in Diabetic Rats. Br. J. Pharmacol. 2012, 166, 1169–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Huang, Y.; Wang, Z.; Fang, Q.; Sun, Y.; Tong, C.; Peng, K.; Wang, Y.; Miao, L.; Cai, L.; et al. Inhibition of MAPK-Mediated ACE Expression by Compound C66 Prevents STZ-Induced Diabetic Nephropathy. J. Cell. Mol. Med. 2014, 18, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [Green Version]
- Miname, M.H.; Santos, R.D. Reducing Cardiovascular Risk in Patients with Familial Hypercholesterolemia: Risk Prediction and Lipid Management. Prog. Cardiovasc. Dis. 2019, 62, 414–422. [Google Scholar] [CrossRef]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.-F.; Sobenin, I.A.; Orekhov, A.N. The Atherogenic Role of Circulating Modified Lipids in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular Smooth Muscle Cells in Atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B.; Schurgers, L. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Xie, B.-D.; Sun, L.; Chen, W.; Jiang, S.-L.; Liu, W.; Bian, F.; Tian, H.; Li, R.-K. Phenotypic Switching of Vascular Smooth Muscle Cells in the “normal Region” of Aorta from Atherosclerosis Patients Is Regulated by MiR-145. J. Cell. Mol. Med. 2016, 20, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- Allahverdian, S.; Chaabane, C.; Boukais, K.; Francis, G.A.; Bochaton-Piallat, M.-L. Smooth Muscle Cell Fate and Plasticity in Atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Liu, L.; Chen, H.; Sawamura, T.; Ranganathan, S.; Mehta, J.L. LOX-1 Mediates Oxidized Low-Density Lipoprotein-Induced Expression of Matrix Metalloproteinases in Human Coronary Artery Endothelial Cells. Circulation 2003, 107, 612–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.; Shen, X.; Lin, L.; Leitges, M.; Rosario, R.; Zou, Y.S.; Yan, S.F. PKCβ Promotes Vascular Inflammation and Acceleration of Atherosclerosis in Diabetic ApoE Null Mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1779–1787. [Google Scholar] [CrossRef] [Green Version]
- Schauwienold, D.; Plum, C.; Helbing, T.; Voigt, P.; Bobbert, T.; Hoffmann, D.; Paul, M.; Reusch, H.P. ERK1/2-Dependent Contractile Protein Expression in Vascular Smooth Muscle Cells. Hypertension 2003, 41, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Dietrich, H.; Metzler, B.; Wick, G.; Xu, Q. Hyperexpression and Activation of Extracellular Signal-Regulated Kinases (ERK1/2) in Atherosclerotic Lesions of Cholesterol-Fed Rabbits. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Zuniga, M.C.; Raghuraman, G.; Hitchner, E.; Weyand, C.; Robinson, W.; Zhou, W. PKC-Epsilon and TLR4 Synergistically Regulate Resistin-Mediated Inflammation in Human Macrophages. Atherosclerosis 2017, 259, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Amini, N.; Sarkaki, A.; Dianat, M.; Mard, S.A.; Ahangarpour, A.; Badavi, M. Protective Effects of Naringin and Trimetazidine on Remote Effect of Acute Renal Injury on Oxidative Stress and Myocardial Injury through Nrf-2 Regulation. Pharmacol. Reports 2019, 71, 1059–1066. [Google Scholar] [CrossRef]
- Nishio, H.; Matsui, K.; Tsuji, H.; Tamura, A.; Suzuki, K. Immunohistochemical Study of the Phosphorylated and Activated Form of C-Jun NH2-Terminal Kinase in Human Aorta. Histochem. J. 2001, 33, 167–171. [Google Scholar] [CrossRef]
- Razani, B.; Chakravarthy, M.V.; Semenkovich, C.F. Insulin Resistance and Atherosclerosis. Endocrinol. Metab. Clin. N. Am. 2008, 37, 603–621. [Google Scholar] [CrossRef]
- Lin, C.-S.; Lin, F.-Y.; Ho, L.-J.; Tsai, C.-S.; Cheng, S.-M.; Wu, W.-L.; Huang, C.-Y.; Lian, C.-H.; Yang, S.-P.; Lai, J.-H. PKCδ Signalling Regulates SR-A and CD36 Expression and Foam Cell Formation. Cardiovasc. Res. 2012, 95, 346–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, K.R.; Bakris, G.L.; Toto, R.D.; McGill, J.B.; Hu, K.; Anderson, P.W. The Effect of Ruboxistaurin on Nephropathy in Type 2 Diabetes. Diabetes Care 2005, 28, 2686–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, R.E.; Kim, S.A.; Tuttle, K.R.; Bakris, G.L.; Toto, R.D.; McGill, J.B.; Haney, D.J.; Kelly, D.J.; Anderson, P.W. Effect of Ruboxistaurin on Urinary Transforming Growth Factor-Beta in Patients with Diabetic Nephropathy and Type 2 Diabetes. Diabetes Care 2007, 30, 995–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casellini, C.M.; Barlow, P.M.; Rice, A.L.; Casey, M.; Simmons, K.; Pittenger, G.; Bastyr, E.J.; Wolka, A.M.; Vinik, A.I. A 6-Month, Randomized, Double-Masked, Placebo-Controlled Study Evaluating the Effects of the Protein Kinase C-Beta Inhibitor Ruboxistaurin on Skin Microvascular Blood Flow and Other Measures of Diabetic Peripheral Neuropathy. Diabetes Care 2007, 30, 896–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladage, D.; Tilemann, L.; Ishikawa, K.; Correll, R.N.; Kawase, Y.; Houser, S.R.; Molkentin, J.D.; Hajjar, R.J. Inhibition of PKCα/β with Ruboxistaurin Antagonizes Heart Failure in Pigs after Myocardial Infarction Injury. Circ. Res. 2011, 109, 1396–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in Patients with BRAF-Mutant Melanoma (COLUMBUS): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet. Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib Combined with Vemurafenib in Advanced BRAF(V600)-Mutant Melanoma (CoBRIM): Updated Efficacy Results from a Randomised, Double-Blind, Phase 3 Trial. Lancet. Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Xu, Z.; Tong, Q.; Zhang, Z.; Wang, S.; Zheng, Y.; Liu, Q.; Qian, L.-B.; Chen, S.-Y.; Sun, J.; Cai, L. Inhibition of HDAC3 Prevents Diabetic Cardiomyopathy in OVE26 Mice via Epigenetic Regulation of DUSP5-ERK1/2 Pathway. Clin. Sci. 2017, 131, 1841–1857. [Google Scholar] [CrossRef] [Green Version]
- Emami, H.; Vucic, E.; Subramanian, S.; Abdelbaky, A.; Fayad, Z.A.; Du, S.; Roth, E.; Ballantyne, C.M.; Mohler, E.R.; Farkouh, M.E.; et al. The Effect of BMS-582949, a P38 Mitogen-Activated Protein Kinase (P38 MAPK) Inhibitor on Arterial Inflammation: A Multicenter FDG-PET Trial. Atherosclerosis 2015, 240, 490–496. [Google Scholar] [CrossRef]
- Newby, L.K.; Marber, M.S.; Melloni, C.; Sarov-Blat, L.; Aberle, L.H.; Aylward, P.E.; Cai, G.; de Winter, R.J.; Hamm, C.W.; Heitner, J.F.; et al. Losmapimod, a Novel P38 Mitogen-Activated Protein Kinase Inhibitor, in Non-ST-Segment Elevation Myocardial Infarction: A Randomised Phase 2 Trial. Lancet 2014, 384, 1187–1195. [Google Scholar] [CrossRef]
- Cheriyan, J.; Webb, A.J.; Sarov-Blat, L.; Elkhawad, M.; Wallace, S.M.L.; Mäki-Petäjä, K.M.; Collier, D.J.; Morgan, J.; Fang, Z.; Willette, R.N.; et al. Inhibition of P38 Mitogen-Activated Protein Kinase Improves Nitric Oxide-Mediated Vasodilatation and Reduces Inflammation in Hypercholesterolemia. Circulation 2011, 123, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkhawad, M.; Rudd, J.H.F.; Sarov-Blat, L.; Cai, G.; Wells, R.; Davies, L.C.; Collier, D.J.; Marber, M.S.; Choudhury, R.P.; Fayad, Z.A.; et al. Effects of P38 Mitogen-Activated Protein Kinase Inhibition on Vascular and Systemic Inflammation in Patients with Atherosclerosis. JACC. Cardiovasc. Imaging 2012, 5, 911–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, X.; Zhou, S.; Jia, Y.; Li, Y.; Song, Y.; Wang, J.; Wu, H. SP600125 Suppresses Keap1 Expression and Results in NRF2-Mediated Prevention of Diabetic Nephropathy. J. Mol. Endocrinol. 2018, 60, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Hein, T.W.; Xu, X.; Ren, Y.; Xu, W.; Tsai, S.-H.; Thengchaisri, N.; Kuo, L. Requisite Roles of LOX-1, JNK, and Arginase in Diabetes-Induced Endothelial Vasodilator Dysfunction of Porcine Coronary Arterioles. J. Mol. Cell. Cardiol. 2019, 131, 82–90. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Miao, X.; Zhou, S.; Tan, Y.; Liang, G.; Zheng, Y.; Liu, Q.; Sun, J.; Cai, L. Inhibition of JNK by Compound C66 Prevents Pathological Changes of the Aorta in STZ-Induced Diabetes. J. Cell. Mol. Med. 2014, 18, 1203–1212. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Abdul Hamid, Z.; Mohamad Anuar, N.N.; Jalil, J.; Mohd Nor, N.A.; Budin, S.B. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. Int. J. Mol. Sci. 2022, 23, 8582. https://doi.org/10.3390/ijms23158582
Jubaidi FF, Zainalabidin S, Taib IS, Abdul Hamid Z, Mohamad Anuar NN, Jalil J, Mohd Nor NA, Budin SB. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. International Journal of Molecular Sciences. 2022; 23(15):8582. https://doi.org/10.3390/ijms23158582
Chicago/Turabian StyleJubaidi, Fatin Farhana, Satirah Zainalabidin, Izatus Shima Taib, Zariyantey Abdul Hamid, Nur Najmi Mohamad Anuar, Juriyati Jalil, Nor Anizah Mohd Nor, and Siti Balkis Budin. 2022. "The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications" International Journal of Molecular Sciences 23, no. 15: 8582. https://doi.org/10.3390/ijms23158582







