Human Multipotent Mesenchymal Stromal Cell–Derived Extracellular Vesicles Enhance Neuroregeneration in a Rat Model of Sciatic Nerve Crush Injury
Abstract
1. Introduction
2. Results
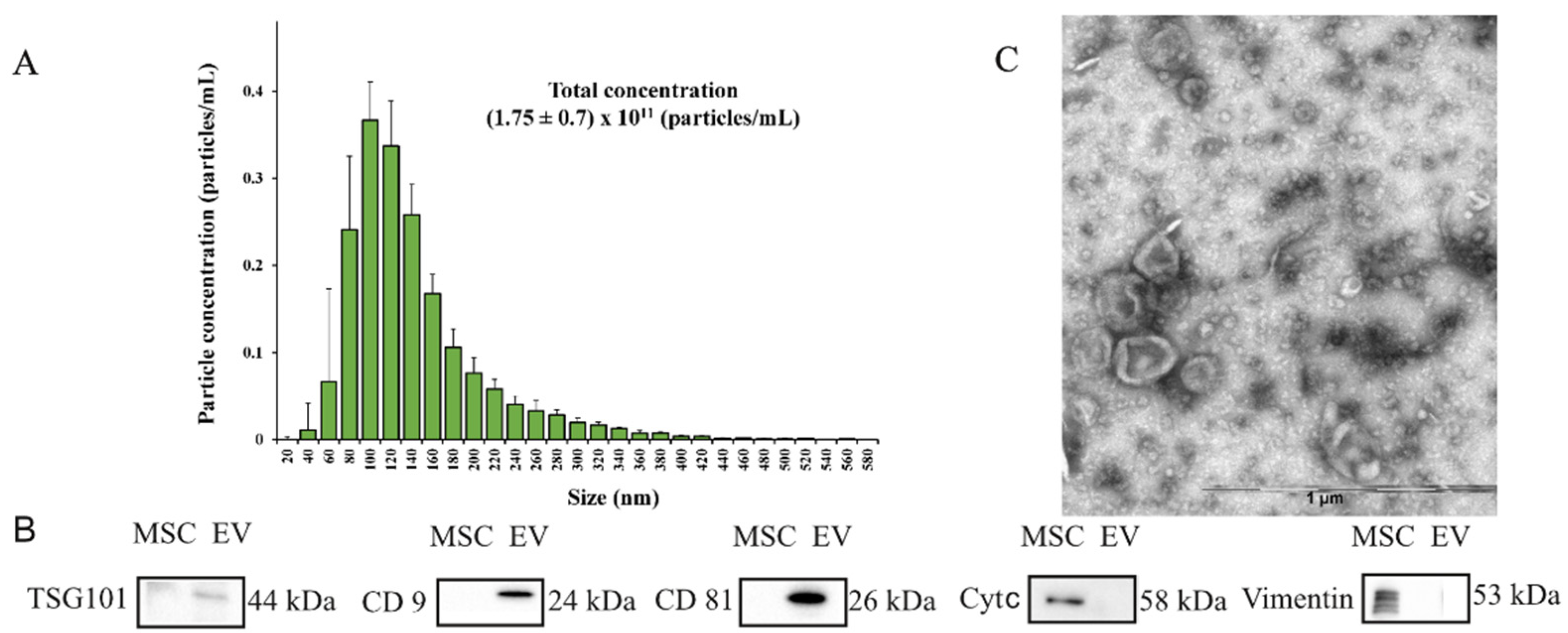
2.1. Characterization of Extracellular Vesicles
2.2. Effect of Extracellular Vesicles on the Atrophy of the Gastrocnemius Muscle in the Injured Limb
2.3. Functional Deficit of the Injured Limb
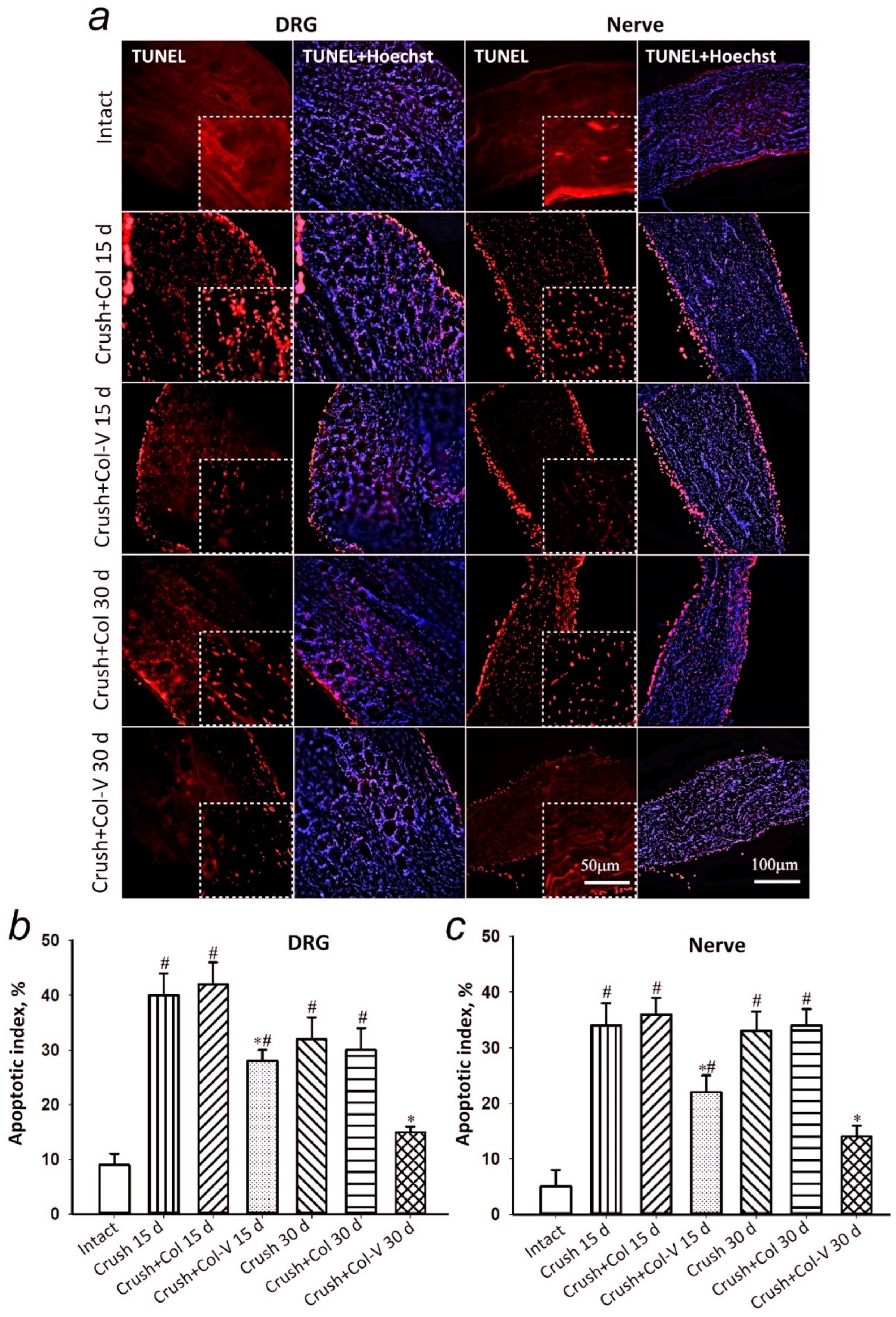
2.4. Assessment of the Number of Apoptotic Cells in Dorsal Root Ganglia and Sciatic Nerve Segment in Rats
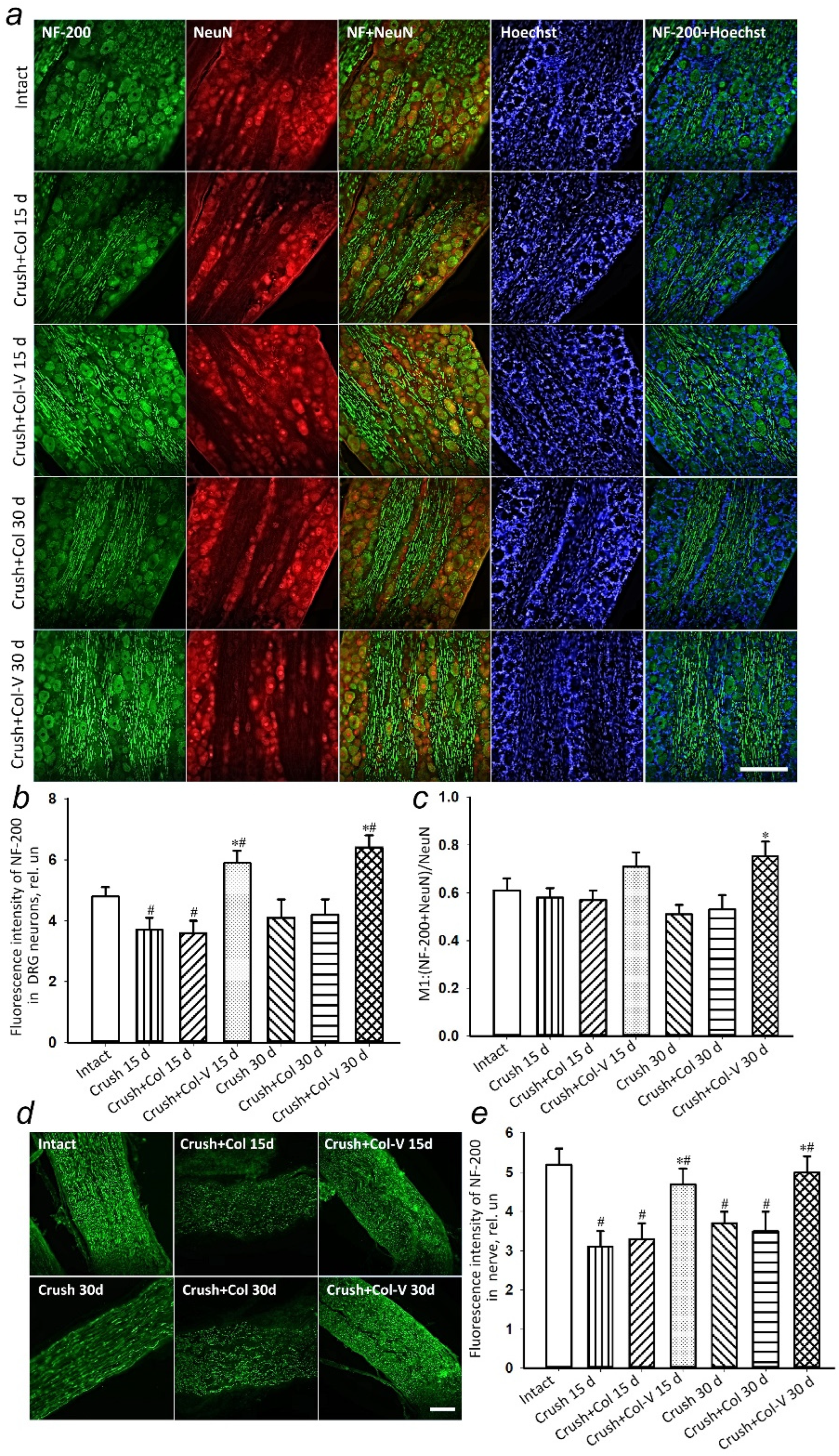
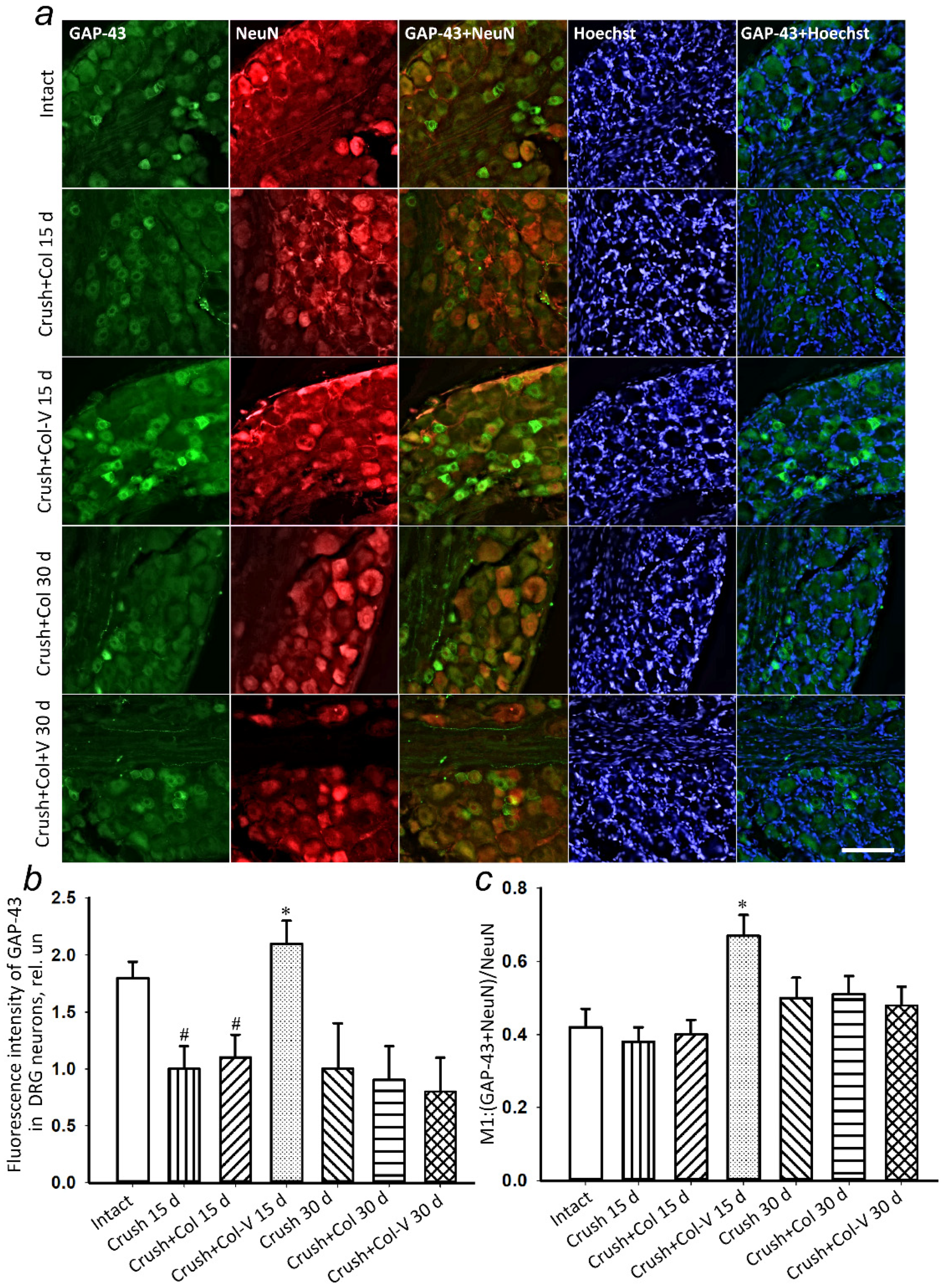
2.5. Changes in the Levels of Regeneration-Associated Proteins
3. Discussion
4. Materials and Methods
4.1. Primary Culture of MSCs
4.2. Isolation of Extracellular Vesicles by Differential Centrifugation
4.3. Analysis of the Size of Extracellular Vesicles
4.4. Animals
4.5. Rat Sciatic Nerve Crushing Model
4.6. Preparing Collagen-Gel-Based Vesicle Substance
4.7. Estimation of Gastrocnemius Muscle Mass Change in Damaged Limb in 30 Days after Sciatic Nerve Crush
4.8. Extensor Postural Thrust Test
4.9. Preparing Muscle Tissues for Histological Study
4.10. Estimation of Apoptotic Cell Number
4.11. Immunofluorescence Microscopy of Rat Spinal Ganglia and Damaged Nerve Fragments
4.12. Data Processing and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sayad Fathi, S.; Zaminy, A. Stem cell therapy for nerve injury. World J. Stem Cells 2017, 9, 144–151. [Google Scholar] [CrossRef]
- Palomo, R.; Sánchez, R. Physiotherapy applied to the upper extremity in 0 to 10-year-old children with obstetric brachial palsy: A systematic review. Rev. Neurol. 2020, 71, 1–10. [Google Scholar] [CrossRef]
- Van der Looven, R.; Le Roy, L.; Tanghe, E.; Samijn, B.; Roets, E.; Pauwels, N.; Deschepper, E.; De Muynck, M.; Vingerhoets, G.; Van den Broeck, C. Risk factors for neonatal brachial plexus palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2020, 62, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Barsaoui, M.; Safi, H.; Said, W.; Nessib, M.N. Nerve surgery in obstetric brachial plexus palsy, report of 68 cases. Tunis. Med. 2017, 95, 196–200. [Google Scholar] [PubMed]
- Moucharafieh, R.C.; Badra, M.I.; Boulos, K.A.; Mansour, J.I.; Daher, J.C.; Wardani, H.M.; Nour, H.G.A.; El Sayde, E.G.; Nehme, A.H. Nerve transfers in the upper extremity: A review. Injury 2020, 51, 2804–2810. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Tu, Y.-K. Surgical reconstructions for adult brachial plexus injuries. Part I: Treatments for combined C5 and C6 injuries, with or without C7 injuries. Injury 2020, 51, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Guo, Q.; Guo, J.; Xu, X.; Xu, K.; Li, Y.; Yang, T.; Gu, X.; Cao, R.; Cui, S. Brachial plexus bridging with specific extracellular matrix-modified chitosan/silk scaffold: A new expand of tissue engineered nerve graft. J. Neural Eng. 2022, 19, 026010. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Williams, D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6456. [Google Scholar] [CrossRef] [PubMed]
- Schlosshauer, B.; Dreesmann, L.; Schaller, H.-E.; Sinis, N. Synthetic nerve guide implants in humans: A comprehensive survey. Neurosurgery 2006, 59, 740–747; discussion 747–748. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Joosten, E.A.J.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Mauck, R.L.; Gorman, J.H.; Gorman, R.C. Acellular biomaterials: An evolving alternative to cell-based therapies. Sci. Transl. Med. 2013, 5, 176ps4. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C. V Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int. J. Mol. Sci. 2016, 17, 2101. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Sánchez, D.N.; de Lima Resende, L.A.; Boff Araujo Pinto, G.; de Carvalho Bovolato, A.L.; Possebon, F.S.; Deffune, E.; Amorim, R.M. Canine Adipose-Derived Mesenchymal Stromal Cells Enhance Neuroregeneration in a Rat Model of Sciatic Nerve Crush Injury. Cell Transplant. 2019, 28, 47–54. [Google Scholar] [CrossRef]
- Lopatina, T.; Bruno, S.; Tetta, C.; Kalinina, N.; Porta, M.; Camussi, G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Chopp, M. Exosomes in stroke pathogenesis and therapy. J. Clin. Investig. 2016, 126, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Blanc, L.; Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Boltze, J.; Arnold, A.; Walczak, P.; Jolkkonen, J.; Cui, L.; Wagner, D.-C. The Dark Side of the Force—Constraints and Complications of Cell Therapies for Stroke. Front. Neurol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Yu, T.; Xu, Y.; Ahmad, M.A.; Javed, R.; Hagiwara, H.; Tian, X. Exosomes as a Promising Therapeutic Strategy for Peripheral Nerve Injury. Curr. Neuropharmacol. 2021, 19, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, T.; Sakai, K.; Watanabe, J.; Katagiri, W.; Hibi, H. Dental pulp-derived stem cell conditioned medium to regenerate peripheral nerves in a novel animal model of dysphagia. PLoS ONE 2018, 13, e0208938. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. Biomed Res. Int. 2019, 2019, 6458237. [Google Scholar] [CrossRef] [PubMed]
- Guseva, D.; Chelyshev, Y. The Plasticity of the DRG Neurons Belonging to Different Subpopulations After Dorsal Rhizotomy. Cell. Mol. Neurobiol. 2006, 26, 1223–1232. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z. The Effects of Target Skeletal Muscle Cells on Dorsal Root Ganglion Neuronal Outgrowth and Migration In Vitro. PLoS ONE 2013, 8, e52849. [Google Scholar] [CrossRef] [PubMed]
- Lekan, H.A.; Chung, K.; Yoon, Y.W.; Chung, J.M.; Coggeshall, R.E. Loss of dorsal root ganglion cells concomitant with dorsal root axon sprouting following segmental nerve lesions. Neuroscience 1997, 81, 527–534. [Google Scholar] [CrossRef]
- Murata, R.; Ohtori, S.; Ochiai, N.; Takahashi, N.; Saisu, T.; Moriya, H.; Takahashi, K.; Wada, Y. Extracorporeal shockwaves induce the expression of ATF3 and GAP-43 in rat dorsal root ganglion neurons. Auton. Neurosci. 2006, 128, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, P.; Oblinger, M. Sensory neurons selectively upregulate synthesis and transport of the beta III-tubulin protein during axonal regeneration. J. Neurosci. 1995, 15, 1545–1555. [Google Scholar] [CrossRef]
- Fernandes, K.J.; Fan, D.P.; Tsui, B.J.; Cassar, S.L.; Tetzlaff, W. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: Differential regulation of GAP-43, tubulins, and neurofilament-M. J. Comp. Neurol. 1999, 414, 495–510. [Google Scholar] [CrossRef]
- Maday, S.; Twelvetrees, A.E.; Moughamian, A.J.; Holzbaur, E.L.F. Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron 2014, 84, 292–309. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, C.; Huang, L.; Chen, J.; Xu, N. Protective effects of intravitreal administration of mesenchymal stem cell-derived exosomes in an experimental model of optic nerve injury. Exp. Cell Res. 2021, 407, 112792. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- McKay Hart, A.; Brannstrom, T.; Wiberg, M.; Terenghi, G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat. Exp. Brain Res. 2002, 142, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Vigneswara, V.; Berry, M.; Logan, A.; Ahmed, Z. Caspase-2 Is Upregulated after Sciatic Nerve Transection and Its Inhibition Protects Dorsal Root Ganglion Neurons from Apoptosis after Serum Withdrawal. PLoS ONE 2013, 8, e57861. [Google Scholar] [CrossRef] [PubMed]
- Dzreyan, V.; Rodkin, S.; Nikul, V.; Pitinova, M.; Uzdensky, A. The Expression of E2F1, p53, and Caspase 3 in the Rat Dorsal Root Ganglia After Sciatic Nerve Transection. J. Mol. Neurosci. 2021, 71, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Sommervaille, T.; Reynolds, M.L.; Woolf, C.J. Time-dependent differences in the increase in GAP-43 expression in dorsal root ganglion cells after peripheral axotomy. Neuroscience 1991, 45, 213–220. [Google Scholar] [CrossRef]
- McKerracher, L.; Essagian, C.; Aguayo, A. Marked increase in beta-tubulin mRNA expression during regeneration of axotomized retinal ganglion cells in adult mammals. J. Neurosci. 1993, 13, 5294–5300. [Google Scholar] [CrossRef]
- Braun, H.; Schäfer, K.; Höllt, V. BetaIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J. Neurotrauma 2002, 19, 975–983. [Google Scholar] [CrossRef]
- Chung, T.-W.; Yang, M.-C.; Tseng, C.-C.; Sheu, S.-H.; Wang, S.-S.; Huang, Y.-Y.; Chen, S.-D. Promoting regeneration of peripheral nerves in-vivo using new PCL-NGF/Tirofiban nerve conduits. Biomaterials 2011, 32, 734–743. [Google Scholar] [CrossRef]
- Burton, V.J.; Butler, L.M.; McGettrick, H.M.; Stone, P.C.; Jeffery, H.C.; Savage, C.O.; Rainger, G.E.; Nash, G.B. Delay of migrating leukocytes by the basement membrane deposited by endothelial cells in long-term culture. Exp. Cell Res. 2011, 317, 276–292. [Google Scholar] [CrossRef] [PubMed]
- McGrady, N.R.; Pasini, S.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Restoring the Extracellular Matrix: A Neuroprotective Role for Collagen Mimetic Peptides in Experimental Glaucoma. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Nogimura, D.; Mizushige, T.; Taga, Y.; Nagai, A.; Shoji, S.; Azuma, N.; Kusubata, M.; Adachi, S.; Yoshizawa, F.; Kabuyama, Y. Prolyl-hydroxyproline, a collagen-derived dipeptide, enhances hippocampal cell proliferation, which leads to antidepressant-like effects in mice. FASEB J. 2020, 34, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Silachev, D.; Goryunov, K.; Shpilyuk, M.; Beznoschenko, O.; Morozova, N.; Kraevaya, E.; Popkov, V.; Pevzner, I.; Zorova, L.; Evtushenko, E.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef]
- Varejão, A.S.P.; Melo-Pinto, P.; Meek, M.F.; Filipe, V.M.; Bulas-Cruz, J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol. Res. 2004, 26, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]



| Groups | 3 d | 7 d | 15 d | 30 d |
|---|---|---|---|---|
| Crush | −100 ± 2 | −100 ± 2 | −87 ± 8 | −66 ± 12 |
| Crush+Col | −100 ± 3 | −100 ± 2 | −100 ± 3 | −50 ± 13 |
| Crush+Col-V | −100 ± 1 | −100 ± 2 | −81 ± 10 | −40 ± 12 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demyanenko, S.V.; Pitinova, M.A.; Kalyuzhnaya, Y.N.; Khaitin, A.M.; Batalshchikova, S.A.; Dobaeva, N.M.; Shevtsova, Y.A.; Goryunov, K.V.; Plotnikov, E.Y.; Pashkevich, S.G.; et al. Human Multipotent Mesenchymal Stromal Cell–Derived Extracellular Vesicles Enhance Neuroregeneration in a Rat Model of Sciatic Nerve Crush Injury. Int. J. Mol. Sci. 2022, 23, 8583. https://doi.org/10.3390/ijms23158583
Demyanenko SV, Pitinova MA, Kalyuzhnaya YN, Khaitin AM, Batalshchikova SA, Dobaeva NM, Shevtsova YA, Goryunov KV, Plotnikov EY, Pashkevich SG, et al. Human Multipotent Mesenchymal Stromal Cell–Derived Extracellular Vesicles Enhance Neuroregeneration in a Rat Model of Sciatic Nerve Crush Injury. International Journal of Molecular Sciences. 2022; 23(15):8583. https://doi.org/10.3390/ijms23158583
Chicago/Turabian StyleDemyanenko, Svetlana V., Maria A. Pitinova, Yulia N. Kalyuzhnaya, Andrey M. Khaitin, Svetlana A. Batalshchikova, Natalya M. Dobaeva, Yulia A. Shevtsova, Kirill V. Goryunov, Egor Y. Plotnikov, Svetlana G. Pashkevich, and et al. 2022. "Human Multipotent Mesenchymal Stromal Cell–Derived Extracellular Vesicles Enhance Neuroregeneration in a Rat Model of Sciatic Nerve Crush Injury" International Journal of Molecular Sciences 23, no. 15: 8583. https://doi.org/10.3390/ijms23158583
APA StyleDemyanenko, S. V., Pitinova, M. A., Kalyuzhnaya, Y. N., Khaitin, A. M., Batalshchikova, S. A., Dobaeva, N. M., Shevtsova, Y. A., Goryunov, K. V., Plotnikov, E. Y., Pashkevich, S. G., Sukhikh, G. T., & Silachev, D. N. (2022). Human Multipotent Mesenchymal Stromal Cell–Derived Extracellular Vesicles Enhance Neuroregeneration in a Rat Model of Sciatic Nerve Crush Injury. International Journal of Molecular Sciences, 23(15), 8583. https://doi.org/10.3390/ijms23158583








