Increased Expression of Alpha-, Beta-, and Gamma-Synucleins in Brainstem Regions of a Non-Human Primate Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
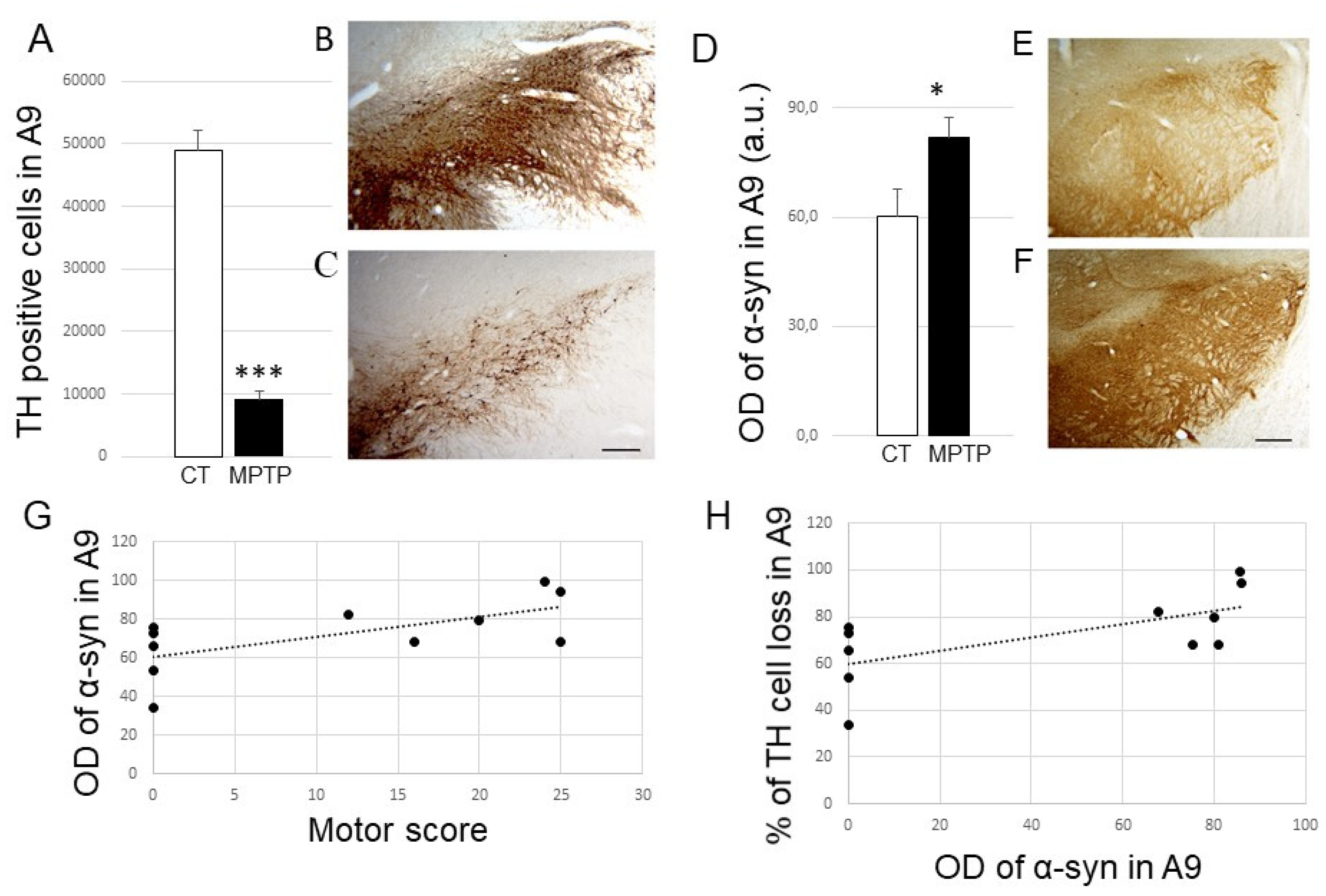
2.1. Impact of MPTP Intoxication on Parkinsonism, DA Cell Loss, and Synuclein Levels in the SN
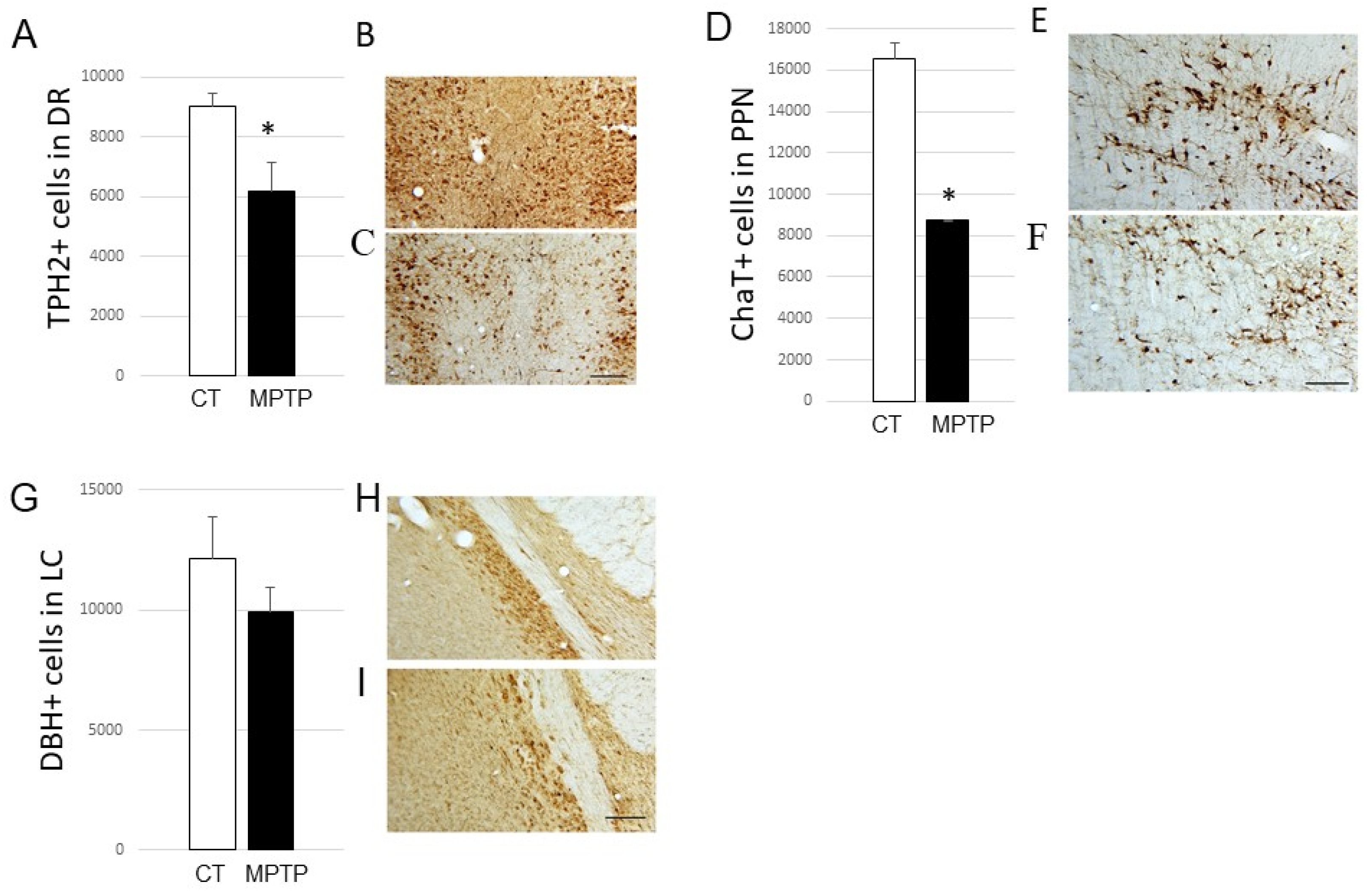
2.2. Impact of MPTP on Cell Loss in the DR, PPN, and LC
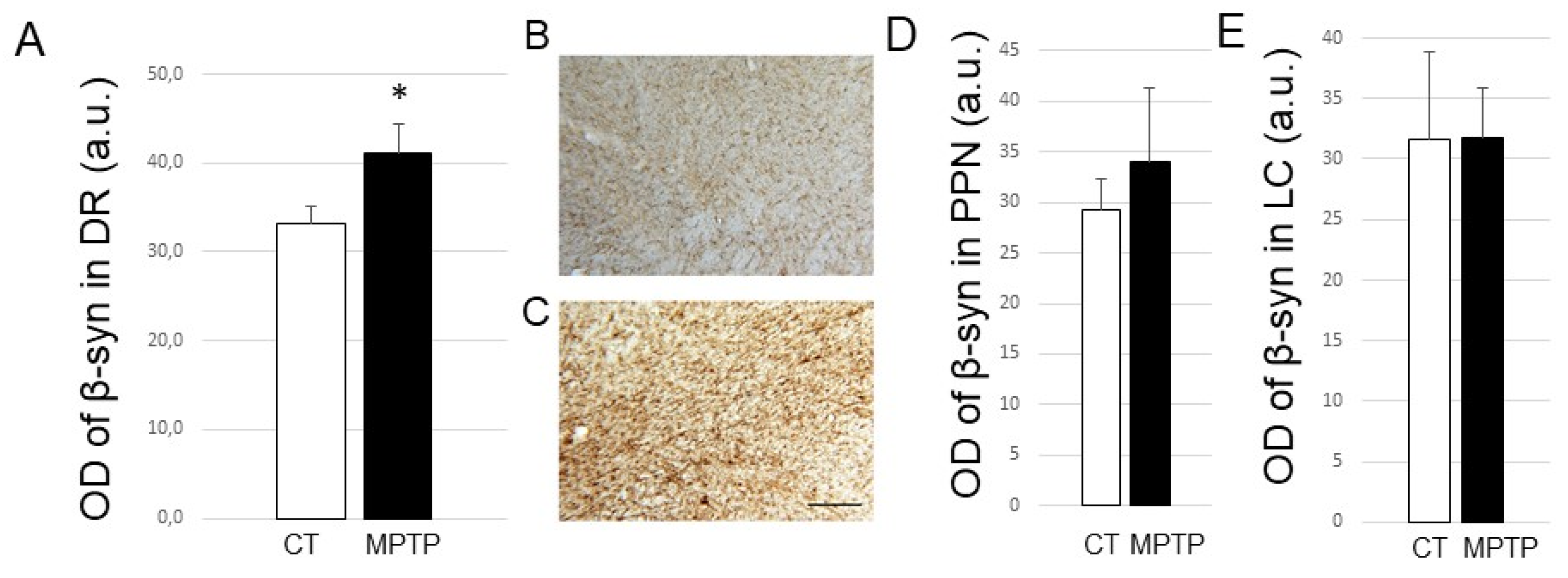
2.3. Impact of MPTP Treatment on α-Synuclein Expression Levels in the DR, PPN, and LC
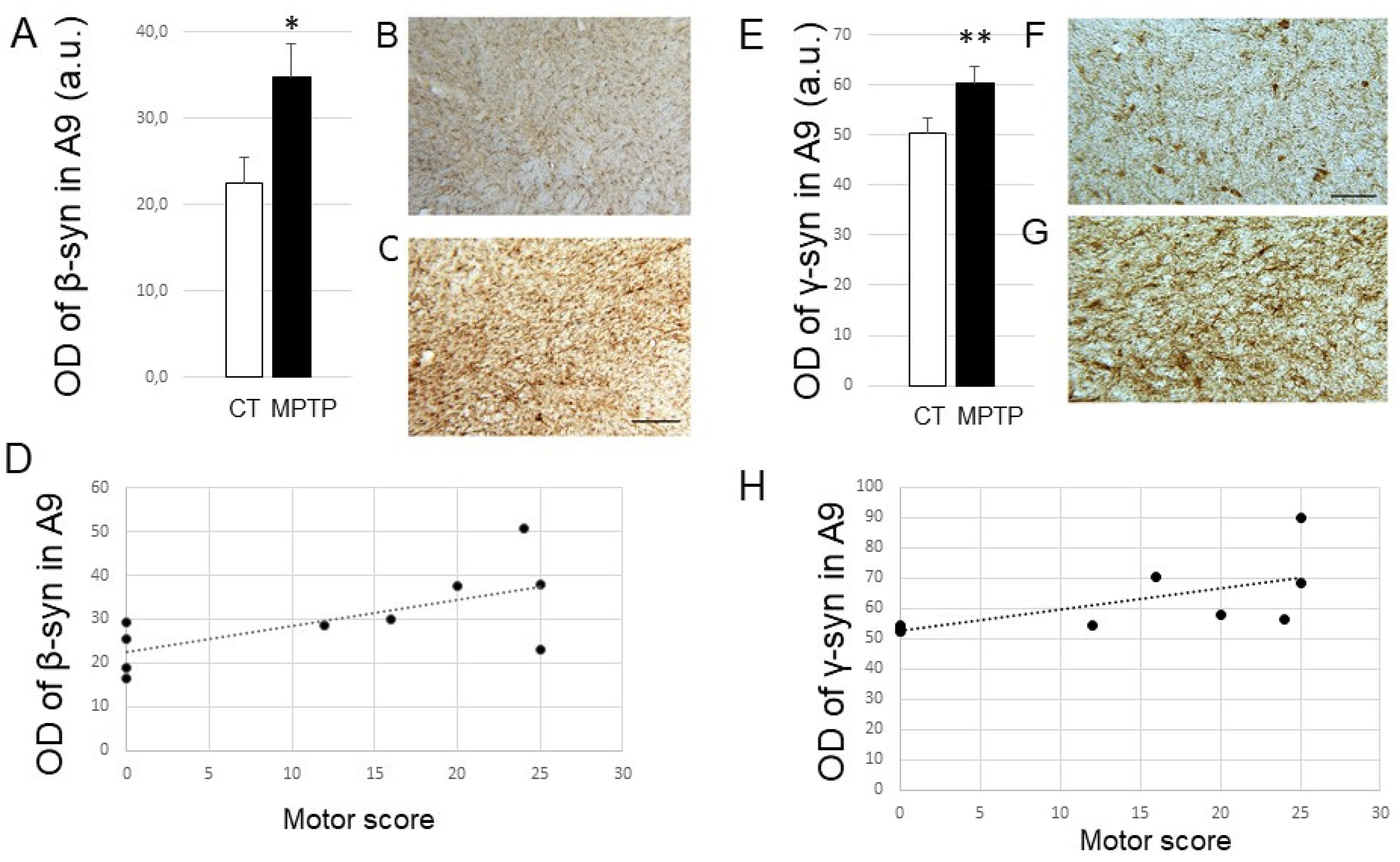
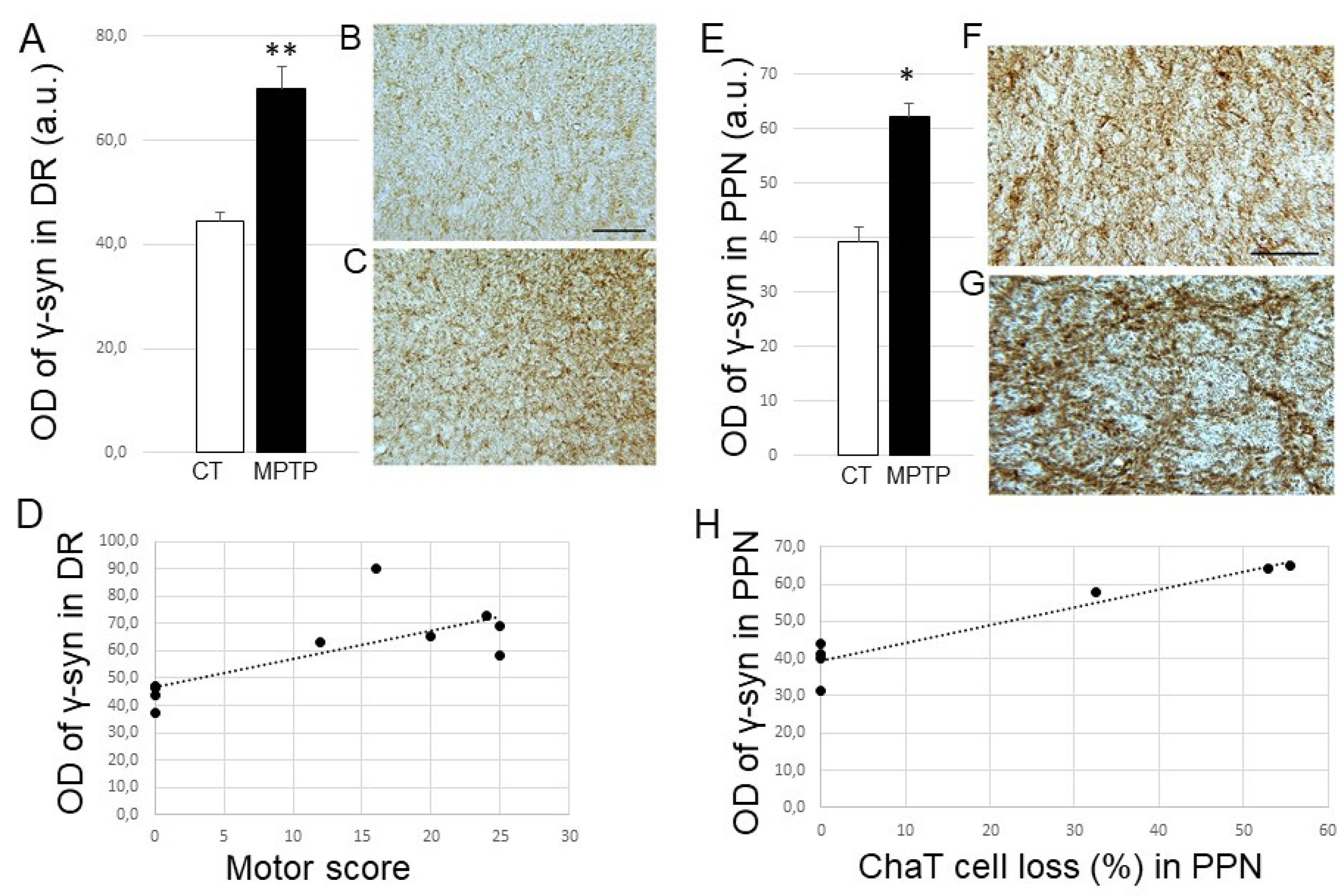
2.4. Impact of MPTP treatment on β- and γ-Synuclein expression levels in the DR, PPN, and LC
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Animals
4.3. MPTP Intoxication
4.4. Parkinsonism Assessment
4.5. Immunohistochemistry
4.5.1. Tissue Preparation
4.5.2. Immunostaining
4.6. Soma Quantification
4.7. Optical Density Measurements
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Przedborski, S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Politis, M. Neuroimaging in Parkinson disease: From research setting to clinical practice. Nat. Rev. Neurol. 2014, 10, 708–722. [Google Scholar] [CrossRef]
- Braak, E.; Sandmann-Keil, D.; Rüb, U.; Gai, W.P.; de Vos, R.A.; Steur, E.N.; Arai, K.; Braak, H. α-synuclein immunopositive Parkinson’s disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol. 2001, 101, 195–201. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Seidel, K.; Mahlke, J.; Siswanto, S.; Krüger, R.; Heinsen, H.; Auburger, G.; Bouzrou, M.; Grinberg, L.T.; Wicht, H.; Korf, H.W.; et al. The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol. 2015, 25, 121–135. [Google Scholar] [CrossRef]
- Huynh, B.; Fu, Y.; Kirik, D.; Shine, J.M.; Halliday, G.M. Comparison of Locus Coeruleus Pathology with Nigral and Forebrain Pathology in Parkinson’s Disease. Mov. Disord. 2021, 36, 2085–2093. [Google Scholar] [CrossRef]
- Jellinger, K.A. Pathology of Parkinson’s disease—changes other than the nigrostriatal pathway. Mol. Chem. Neuropathol. 1991, 14, 153–197. [Google Scholar] [CrossRef] [PubMed]
- Chinaglia, G.; Landwehrmeyer, B.; Probst, A.; Palacios, J.M. Serotoninergic terminal transporters are differentially affected in Parkinson’s disease and progressive Supranuclear palsy: An autoradiographic study with [3H]citalopram. Neuroscience 1993, 54, 691–699. [Google Scholar] [CrossRef]
- Kish, S.J.; Tong, J.; Hornykiewicz, O.; Rajput, A.; Chang, L.J.; Guttman, M.; Furukawa, Y. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain 2008, 131, 120–131. [Google Scholar]
- de Natale, E.R.; Wilson, H.; Politis, M. Serotonergic imaging in Parkinson’s disease. Prog. Brain Res. 2021, 261, 303–338. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Graybiel, A.M.; Duyckaerts, C.; Javoy-Agid, F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc. Natl. Acad. Sci. USA 1987, 84, 5976–5980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sébille, S.B.; Rolland, A.S.; Faillot, M.; Perez-Garcia, F.; Colomb-Clerc, A.; Lau, B.; Dumas, S.; Fernandez Vidal, S.; Welter, M.L.; Francois, C.; et al. Normal and pathological neuronal distribution of the human mesencephalic locomotor region. Mov. Disord. 2019, 34, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Pahapill, P.A.; Lozano, A.M. The pedunculopontine nucleus and Parkinson’s disease. Brain 2000, 123 Pt 9, 1767–1783. [Google Scholar] [CrossRef]
- Rinne, J.O.; Ma, S.Y.; Lee, M.S.; Collan, Y.; Röyttä, M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson’s disease is related to disability of the patients. Parkinsonism Relat. Disord. 2008, 14, 553–557. [Google Scholar] [CrossRef]
- Chan-Palay, V. Alterations in the locus coeruleus in dementias of Alzheimer’s and Parkinson’s disease. Prog. Brain Res. 1991, 88, 625–630. [Google Scholar] [CrossRef]
- Paredes-Rodriguez, E.; Vegas-Suarez, S.; Morera-Herreras, T.; De Deurwaerdere, P.; Miguelez, C. The Noradrenergic System in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 435. [Google Scholar] [CrossRef] [Green Version]
- Doppler, C.E.J.; Kinnerup, M.B.; Brune, C.; Farrher, E.; Betts, M.; Fedorova, T.D.; Schaldemose, J.L.; Knudsen, K.; Ismail, R.; Seger, A.D.; et al. Regional locus coeruleus degeneration is uncoupled from noradrenergic terminal loss in Parkinson’s disease. Brain 2021, 144, 2732–2744. [Google Scholar] [CrossRef]
- Garcia-Lorenzo, D.; Longo-Dos Santos, C.; Ewenczyk, C.; Leu-Semenescu, S.; Gallea, C.; Quattrocchi, G.; Pita Lobo, P.; Poupon, C.; Benali, H.; Arnulf, I.; et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 2013, 136 Pt 7, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, J.; Prasuhn, M.; Fellbrich, A.; Strautz, R.; Lemmer, F.; Dreischmeier, S.; Kasten, M.; Münte, T.F.; Hanssen, H.; Heldmann, M.; et al. Association of locus coeruleus and substantia nigra pathology with cognitive and motor functions in patients with Parkinson disease. Neurology 2021, 97, e1007–e1016. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Tanji, K.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology 2007, 27, 494–506. [Google Scholar] [CrossRef]
- Shahmoradian, S.H.; Lewis, A.J.; Genoud, C.; Hench, J.; Moors, T.E.; Navarro, P.P.; Castaño-Díez, D.; Schweighauser, G.; Graff-Meyer, A.; Goldie, K.N.; et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [Green Version]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. Alpha-synuclein locus triplication causes Parkinson’s disease. Science 2003, 302, 841. [Google Scholar] [CrossRef] [Green Version]
- Chartier-Harlin, M.C.; Kachergus, J.; Roumier, C.; Mouroux, V.; Douay, X.; Lincoln, S.; Levecque, C.; Larvor, L.; Andrieux, J.; Hulihan, M.; et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 2004, 364, 1167–1169. [Google Scholar] [CrossRef]
- Ibáñez, P.; Bonnet, A.M.; Débarges, B.; Lohmann, E.; Tison, F.; Pollak, P.; Agid, Y.; Dürr, A.; Brice, A. Causal relation between α-synuclein gene duplication and familial Parkinson’s disease. Lancet 2004, 364, 1169–1171. [Google Scholar] [CrossRef]
- Wilhelm, B.G.; Mandad, S.; Truckenbrodt, S.; Kröhnert, K.; Schäfer, C.; Rammner, B.; Koo, S.J.; Claßen, G.A.; Krauss, M.; Haucke, V.; et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 2014, 344, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Sudhof, T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oaks, A.W.; Sidhu, A. Synuclein modulation of monoamine transporters. FEBS Lett. 2011, 585, 1001–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.; Wong, Y.C.; Ysselstein, D.; Severino, A.; Krainc, D. Synaptic, mitochondrial, and lysosomal dysfunction in Parkinson’s disease. Trends Neurosci. 2019, 42, 140–149. [Google Scholar] [CrossRef]
- Kontopoulos, E.; Parvin, J.D.; Feany, M.B. α-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 2006, 15, 3012–3023. [Google Scholar] [CrossRef] [Green Version]
- Schaser, A.J.; Osterberg, V.R.; Dent, S.E.; Stackhouse, T.L.; Wakeham, C.M.; Boutros, S.W.; Weston, L.J.; Owen, N.; Weissman, T.A.; Luna, E.; et al. Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders. Sci. Rep. 2019, 9, 10919. [Google Scholar] [CrossRef] [Green Version]
- Barba, L.; Paolini Paoletti, F.; Bellomo, G.; Gaetani, L.; Halbgebauer, S.; Oeckl, P.; Otto, M.; Parnetti, L. Alpha and Beta Synucleins: From Pathophysiology to Clinical Application as Biomarkers. Mov. Disord. 2022, 37, 669–683. [Google Scholar] [CrossRef]
- Lau, A.; So, R.W.L.; Lau, H.H.C.; Sang, J.C.; Ruiz-Riquelme, A.; Fleck, S.C.; Stuart, E.; Menon, S.; Visanji, N.P.; Meisl, G.; et al. α-Synuclein strains target distinct brain regions and cell types. Nat. Neurosci. 2020, 23, 21–31. [Google Scholar] [CrossRef]
- Chu, Y.; Kordower, J.H. Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol. Dis. 2007, 25, 134–149. [Google Scholar] [CrossRef]
- Mori, F.; Nishie, M.; Kakita, A.; Yoshimoto, M.; Takahashi, H.; Wakabayashi, K. Relationship among α-synuclein accumulation, dopamine synthesis, and neurodegeneration in Parkinson disease substantia nigra. J. Neuropathol. Exp. Neurol. 2006, 65, 808–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkstra, A.A.; Voorn, P.; Berendse, H.W.; Groenewegen, H.J.; Netherlands Brain Bank; Rozemuller, A.J.M.; van de Berg, W.D.J. Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov. Disord. 2014, 29, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Valero, A.; Beyer, K. Alternative Splicing of Alpha- and Beta-Synuclein Genes Plays Differential Roles in Synucleinopathies. Genes 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spillantini, M.G.; Goedert, M. Neurodegeneration and the ordered assembly of α-synuclein. Cell Tissue Res. 2018, 373, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Galvin, J.E.; Uryu, K.; Lee, V.M.; Trojanowski, J.Q. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc. Natl. Acad. Sci. USA 1999, 96, 13450–13455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surgucheva, I.; Newell, K.L.; Burns, J.; Surguchov, A. New α- and γ-synuclein immunopathological lesions in human brain. Acta Neuropathol. Commun. 2014, 2, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raina, A.; Leite, K.; Guerin, S.; Mahajani, S.U.; Chakrabarti, K.S.; Voll, D.; Becker, S.; Griesinger, C.; Bähr, M.; Kügler, S. Dopamine promotes the neurodegenerative potential of β-synuclein. J. Neurochem. 2021, 156, 674–691. [Google Scholar] [CrossRef]
- Ninkina, N.; Millership, S.J.; Peters, O.M.; Connor-Robson, N.; Chaprov, K.; Kopylov, A.T.; Montoya, A.; Kramer, H.; Withers, D.J.; Buchman, V.L. β-synuclein potentiates synaptic vesicle dopamine uptake and rescues dopaminergic neurons from MPTP-induced death in the absence of other synucleins. J. Biol. Chem. 2021, 297, 101375. [Google Scholar] [CrossRef]
- Uversky, V.N.; Li, J.; Souillac, P.; Millett, I.S.; Doniach, S.; Jakes, R.; Goedert, M.; Fink, A.L. Biophysical properties of the synucleins and their propensities to fibrillate: Inhibition of α-synuclein assembly by beta- and gamma-synucleins. J. Biol. Chem. 2002, 277, 11970–11978. [Google Scholar] [CrossRef] [Green Version]
- Taschenberger, G.; Toloe, J.; Tereshchenko, J.; Akerboom, J.; Wales, P.; Benz, R.; Becker, S.; Outeiro, T.F.; Looger, L.L.; Bähr, M.; et al. β-synuclein aggregates and induces neurodegeneration in dopaminergic neurons. Ann. Neurol. 2013, 74, 109–118. [Google Scholar] [CrossRef]
- Ninkina, N.; Peters, O.; Millership, S.; Salem, H.; van der Putten, H.; Buchman, V.L. Gamma-synucleinopathy: Neurodegeneration associated with overexpression of the mouse protein. Hum. Mol. Genet. 2009, 18, 1779–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavia-Collado, R.; Rodríguez-Aller, R.; Alarcón-Arís, D.; Miquel-Rio, L.; Ruiz-Bronchal, E.; Paz, V.; Campa, L.; Galofré, M.; Sgambato, V.; Bortolozzi, A. Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice. Int. J. Mol. Sci. 2022, 23, 1807. [Google Scholar] [CrossRef] [PubMed]
- Carnazza, K.E.; Komer, L.E.; Xie, Y.X.; Pineda, A.; Briano, J.A.; Gao, V.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; et al. Synaptic vesicle binding of α-synuclein is modulated by β- and γ-synucleins. Cell Rep. 2022, 39, 110675. [Google Scholar] [CrossRef]
- Beyer, K.; Ispierto, L.; Latorre, P.; Tolosa, E.; Ariza, A. Alpha- and beta-synuclein expression in Parkinson disease with and without dementia. J. Neurol. Sci. 2011, 310, 112–117. [Google Scholar] [CrossRef]
- Oeckl, P.; Metzger, F.; Nagl, M.; von Arnim, C.A.; Halbgebauer, S.; Steinacker, P.; Ludolph, A.C.; Otto, M. Alpha-, beta-, and gamma-synuclein quantification in cerebrospinal fluid by multiple reaction monitoring reveals increased concentrations in Alzheimer’s and Creutzfeldt-Jakob disease, but no alteration in synucleinopathies. Mol. Cell. Proteom. 2016, 15, 3126–3138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudoin-Gobert, M.; Epinat, J.; Météreau, E.; Duperrier, S.; Neumane, S.; Ballanger, B.; Lavenne, F.; Liger, F.; Tourvielle, C.; Bonnefoi, F.; et al. Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain 2015, 138 Pt 9, 2632–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, G.; Herrero, M.T.; Murphy, K.; McCann, H.; Ros-Bernal, F.; Barcia, C.; Mori, H.; Blesa, F.J.; Obeso, J.A. No Lewy pathology in monkeys with over 10 years of severe MPTP Parkinsonism. Mov. Disord. 2009, 24, 1519–1523. [Google Scholar] [CrossRef]
- Kimura, K.; Inoue, K.-I.; Kuroiwa, Y.; Tanaka, F.; Takada, M. Propagated but Topologically Distributed Forebrain Neurons Expressing Alpha-Synuclein in Aged Macaques. PLoS ONE 2016, 11, e0166861. [Google Scholar] [CrossRef]
- Huang, B.; Wu, S.; Wang, Z.; Ge, L.; Rizak, J.D.; Wu, J.; Li, J.; Xu, L.; Lv, L.; Yin, Y.; et al. Phosphorylated α-Synuclein Accumulations and Lewy Body-like Pathology Distributed in Parkinson’s Disease-Related Brain Areas of Aged Rhesus Monkeys Treated with MPTP. Neuroscience 2018, 379, 302–315. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Li, X.; Chen, M.; Liu, C.; Li, J.; Yu, S. Alpha-synuclein oligomerization and dopaminergic degeneration occur synchronously in the brain and colon of MPTP-intoxicated parkinsonian monkeys. Neurosci. Lett. 2020, 716, 134640. [Google Scholar] [CrossRef] [PubMed]
- Kowall, N.W.; Hantraye, P.; Brouillet, E.; Beal, M.F.; McKee, A.C.; Ferrante, R.J. MPTP induces α-synuclein aggregation in the substantia nigra of baboons. NeuroReport 2000, 11, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Purisai, M.G.; McCormack, A.L.; Langston, W.J.; Johnston, L.C.; Di Monte, D.A. Alpha-synuclein expression in the substantia nigra of MPTP-lesioned non-human primates. Neurobiol Dis. 2005, 20, 898–906. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.L.; Mak, S.K.; Shenasa, M.; Langston, W.J.; Forno, L.S.; Di Monte, D.A. Pathologic modifications of α-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J. Neuropathol. Exp. Neurol. 2008, 67, 793–802. [Google Scholar] [CrossRef]
- Deffains, M.; Canron, M.H.; Teil, M.; Li, Q.; Dehay, B.; Bezard, E.; Fernagut, P.O. L-DOPA regulates α-synuclein accumulation in experimental parkinsonism. Neuropathol. Appl. Neurobiol. 2021, 47, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Vukosavic, S.; Jackson-Lewis, V.; Neystat, M.; Jakowec, M.; Przedborski, S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J. Neurochem. 2000, 74, 721–729. [Google Scholar] [CrossRef]
- Vermilyea, S.C.; Guthrie, S.; Hernandez, I.; Bondarenko, V.; Emborg, M.E. α-Synuclein Expression Is Preserved in Substantia Nigra GABAergic Fibers of Young and Aged Neurotoxin-Treated Rhesus Monkeys. Cell Transplant. 2019, 28, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Connor-Robson, N.; Peters, O.P.; Millership, S.; Ninkina, N.; Buchman, V.L. Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behavior and dopamine metabolism. Neurobiol. Aging 2016, 46, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokhan, V.S.; Van’kin, G.I.; Bachurin, S.O.; Shamakina, I.Y. Differential involvement of the gamma-synuclein in cognitive abilities on the model of knockout mice. BMC Neurosci. 2013, 14, 53. [Google Scholar] [CrossRef] [Green Version]
- Kokhan, V.S.; Kokhan, T.Y.G.; Samsonova, A.N.; Fisenko, V.P.; Ustyugov, A.A.; Aliev, G. The Dopaminergic Dysfunction and Altered Working Memory Performance of Aging Mice Lacking Gamma-synuclein Gene. CNS Neurol. Disord. Drug Targets 2018, 17, 604–607. [Google Scholar] [CrossRef]
- Gagnon, D.; Gregoire, L.; Di Paolo, T.; Parent, M. Serotonin hyperinnervation of the striatum with high synaptic incidence in parkinsonian monkeys. Brain Struct. Funct. 2016, 221, 3675–3691. [Google Scholar] [CrossRef]
- Masilamoni, G.J.; Smith, Y. Chronic MPTP administration regimen in monkeys: A model of dopaminergic and non-dopaminergic cell loss in Parkinson’s disease. J. Neural Transm. 2018, 125, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Deusser, J.; Schmidt, S.; Ettle, B.; Plötz, S.; Huber, S.; Müller, C.P.; Masliah, E.; Winkler, J.; Kohl, Z. Serotonergic dysfunction in the A53T α-synuclein mouse model of Parkinson’s disease. J. Neurochem. 2015, 135, 589–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miquel-Rio, L.; Alarcón-Arís, D.; Torres-López, M.; Cóppola-Segovia, V.; Pavia-Collado, R.; Paz, V.; Ruiz-Bronchal, E.; Campa, L.; Casal, C.; Montefeltro, A.; et al. Human α-synuclein overexpression in mouse serotonin neurons triggers a depressive-like phenotype. Rescue by oligonucleotide therapy. Transl. Psychiatry 2022, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, M.; Michel, P.P.; Clark, S.D.; Hirsch, E.C.; François, C. Role of pedunculopontine cholinergic neurons in the vulnerability of nigral dopaminergic neurons in Parkinson’s disease. Exp. Neurol. 2016, 275 Pt 1, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, N.E.; Lanza, K.; Bishop, C. Pedunculopontine Nucleus Degeneration Contributes to Both Motor and Non-Motor Symptoms of Parkinson’s Disease. Front. Pharmacol. 2020, 10, 1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan-Palay, V.; Asan, E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J. Comp. Neurol. 1989, 287, 373–392. [Google Scholar] [CrossRef]
- Karachi, C.; Grabli, D.; Bernard, F.A.; Tandé, D.; Wattiez, N.; Belaid, H.; Bardinet, E.; Prigent, A.; Nothacker, H.P.; Hunot, S.; et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Investig. 2010, 120, 2745–2754. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, P.; Gray, F. Dementia in idiopathic Parkinson’s disease. A neuropathological study of 32 cases. Acta Neuropathol. 1984, 64, 43–52. [Google Scholar] [CrossRef]
- German, D.C.; Manaye, K.F.; White, C.L., 3rd; Woodward, D.J.; McIntire, D.D.; Smith, W.K.; Kalaria, R.N.; Mann, D.M. Disease-specific patterns of locus coeruleus cell loss. Ann. Neurol. 1992, 32, 667–676. [Google Scholar] [CrossRef]
- Patt, S.; Gerhard, L. A Golgi study of human locus coeruleus in normal brains and in Parkinson’s disease. Neuropathol. Appl. Neurobiol. 1993, 19, 519–523. [Google Scholar] [CrossRef]
- Zarow, C.; Lyness, S.A.; Mortimer, J.A.; Chui, H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 2003, 60, 337–341. [Google Scholar] [CrossRef]
- McMillan, P.J.; White, S.S.; Franklin, A.; Greenup, J.L.; Leverenz, J.B.; Raskind, M.A.; Szot, P. Differential response of the central noradrenergic nervous system to the loss of locus coeruleus neurons in Parkinson’s disease and Alzheimer’s disease. Brain Res. 2011, 1373, 240–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Tredici, K.; Braak, H. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J. Neurol. Neurosurg. Psychiatry 2013, 84, 774–783. [Google Scholar] [CrossRef]
- Forno, L.S.; DeLanney, L.E.; Irwin, I.; Langston, J.W. Similarities and differences between MPTP-induced parkinsonsim and Parkinson’s disease. Neuropathol. Consid. Adv. Neurol. 1993, 60, 600–608. [Google Scholar]
- Gibb, W.R.; Terruli, M.; Lees, A.J.; Jenner, P.; Marsden, C.D. The evolution and distribution of morphological changes in the nervous system of the common marmoset following the acute administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Mov. Disord. 1989, 4, 53–74. [Google Scholar] [CrossRef]
- Herrero, M.T.; Hirsch, E.C.; Javoy-Agid, F.; Obeso, J.A.; Agid, Y. Differential vulnerability to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine of dopaminergic and cholinergic neurons in the monkey mesopontine tegmentum. Brain Res. 1993, 624, 281–285. [Google Scholar] [CrossRef]
- Masilamoni, G.J.; Bogenpohl, J.; Alagille, D.; Delevich, K.; Tamagnan, G.; Votaw, J.R.; Wichmann, T.; Smith, Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain 2011, 134, 2057–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddhala, C.; Loftin, S.K.; Kuley, B.M.; Cairns, N.J.; Campbell, M.C.; Perlmutter, J.S.; Kotzbauer, P.T. Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann. Clin. Transl. Neurol. 2015, 2, 949–959. [Google Scholar] [CrossRef]
- Masilamoni, G.J.; Groover, O.; Smith, Y. Reduced noradrenergic innervation of ventral midbrain dopaminergic cell groups and the subthalamic nucleus in MPTP-treated parkinsonian monkeys. Neurobiol. Dis. 2016, 100, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Nayyar, T.; Bubser, M.; Ferguson, M.C.; Neely, M.D.; Goodwin, J.S.; Montine, T.J.; Deutch, A.Y.; Ansah, T.A. Cortical serotonin and norepinephrine denervation in parkinsonism: Preferential loss of the beaded serotonin innervation. Eur. J. Neurosci. 2009, 30, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Beaudoin-Gobert, M.; Météreau, E.; Duperrier, S.; Thobois, S.; Tremblay, L.; Sgambato, V. Pathophysiology of levodopa-induced dyskinesia: Insights from multimodal imaging and immunohistochemistry in non-human primates. Neuroimage 2018, 183, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Météreau, E.; Beaudoin-Gobert, M.; Duperrier, S.; Thobois, S.; Tremblay, L.; Sgambato-Faure, V. Diffusion tensor imaging marks dopaminergic and serotonergic lesions in the Parkinsonian monkey. Mov. Disord. 2018, 33, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Millot, M.; Saga, Y.; Duperrier, S.; Météreau, E.; Beaudoin-Gobert, M.; Sgambato, V. Prior MDMA administration aggravates MPTP-induced Parkinsonism in macaque monkeys. Neurobiol. Dis. 2020, 134, 104643. [Google Scholar] [CrossRef]
| MPTP-Intoxicated Monkeys | Maximal Motor Score (on 29) | TH Cell Loss in A9 (%) |
|---|---|---|
| MF1 | 21 | 77.5 |
| MF4 | 16 | 75.4 |
| MF11 | 12 | 67.8 |
| MF16 | 25 | 86 |
| MF17 | 25 | 81.1 |
| MF18 | 24 | 85.6 |
| MF25 | 20 | 79.9 |
| MI 81 | 12 | 75 |
| MI 93 | 10 | 73 |
| Brain Regions | Cell Loss | Alpha-Synuclein Levels | Correlation Motor Score | Correlation Cell Loss |
|---|---|---|---|---|
| SNc | DA p < 0.001 | p < 0.05 | Yes p = 0.033 | Yes p = 0.0303 |
| DR | 5-HT p < 0.05 | p < 0.01 | Yes p = 0.0002 | Yes p = 0.0003 |
| PPN | ChaT p < 0.05 | unchanged | NA | NA |
| LC | unchanged | unchanged | NA | NA |
| Cell Loss | Beta-Synuclein Levels | Correlation Motor Score | Correlation Cell Loss | |
| SNc | DA p < 0.001 | p < 0.05 | Yes p = 0.036 | Yes p = 0.0441 |
| DR | 5-HT p < 0.05 | p < 0.05 | No correlation | No correlation |
| PPN | ChaT p < 0.05 | unchanged | NA | NA |
| LC | unchanged | unchanged | NA | NA |
| Cell Loss | Gamma-Synuclein Levels | Correlation Motor Score | Correlation Cell Loss | |
| SNc | DA p < 0.001 | p < 0.01 | Yes p = 0.0219 | Yes p = 0.05 |
| DR | 5-HT p < 0.05 | p < 0.01 | Yes p = 0.007 | Yes p = 0.0011 |
| PPN | ChaT p < 0.05 | p < 0.05 | Yes p = 0.0014 | Yes p = 0.001 |
| LC | unchanged | unchanged | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duperrier, S.; Bortolozzi, A.; Sgambato, V. Increased Expression of Alpha-, Beta-, and Gamma-Synucleins in Brainstem Regions of a Non-Human Primate Model of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 8586. https://doi.org/10.3390/ijms23158586
Duperrier S, Bortolozzi A, Sgambato V. Increased Expression of Alpha-, Beta-, and Gamma-Synucleins in Brainstem Regions of a Non-Human Primate Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2022; 23(15):8586. https://doi.org/10.3390/ijms23158586
Chicago/Turabian StyleDuperrier, Sandra, Analia Bortolozzi, and Véronique Sgambato. 2022. "Increased Expression of Alpha-, Beta-, and Gamma-Synucleins in Brainstem Regions of a Non-Human Primate Model of Parkinson’s Disease" International Journal of Molecular Sciences 23, no. 15: 8586. https://doi.org/10.3390/ijms23158586







