p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells
Abstract
:1. Introduction
2. Results
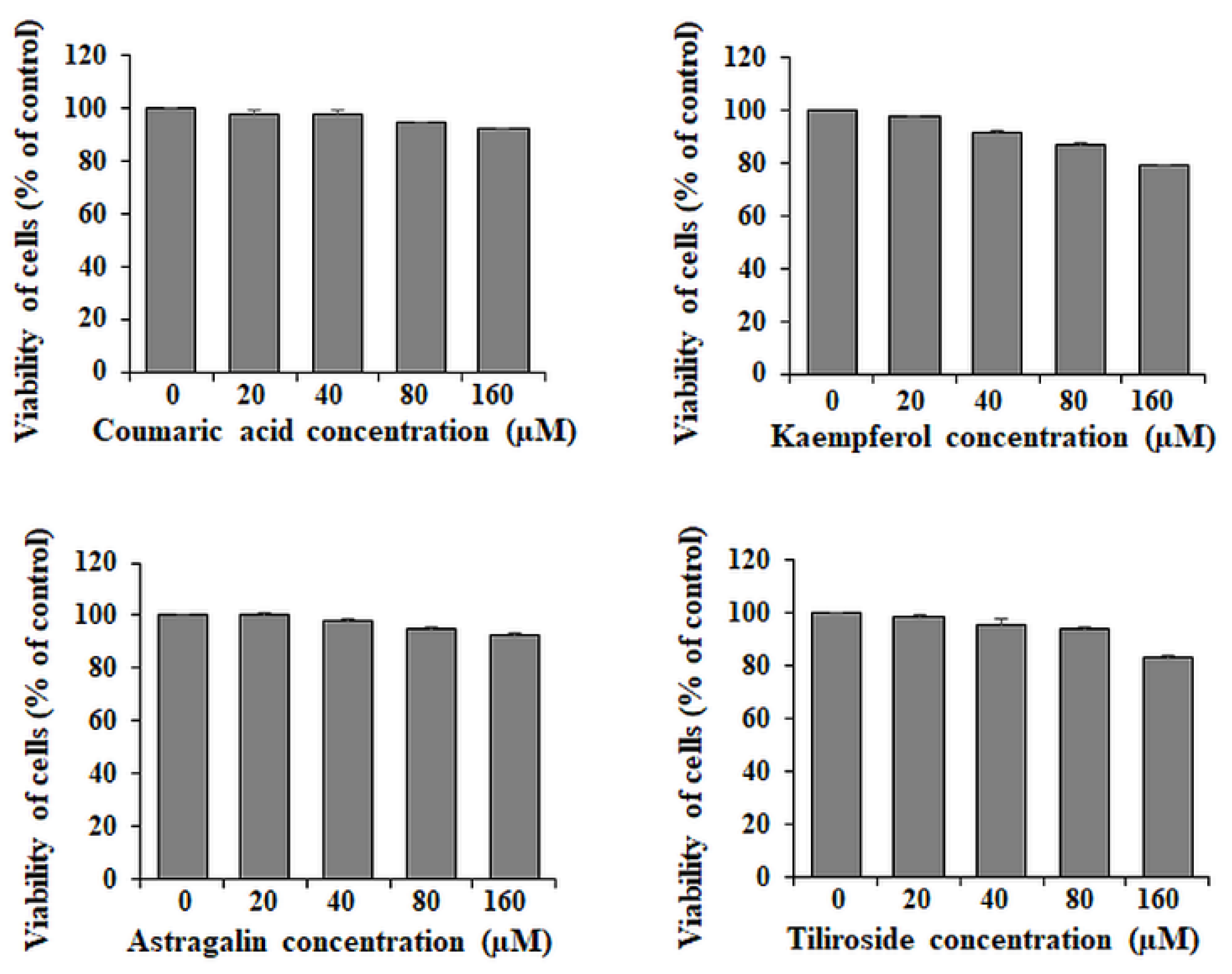
2.1. Viability of AGS Gastric Cancer Cells
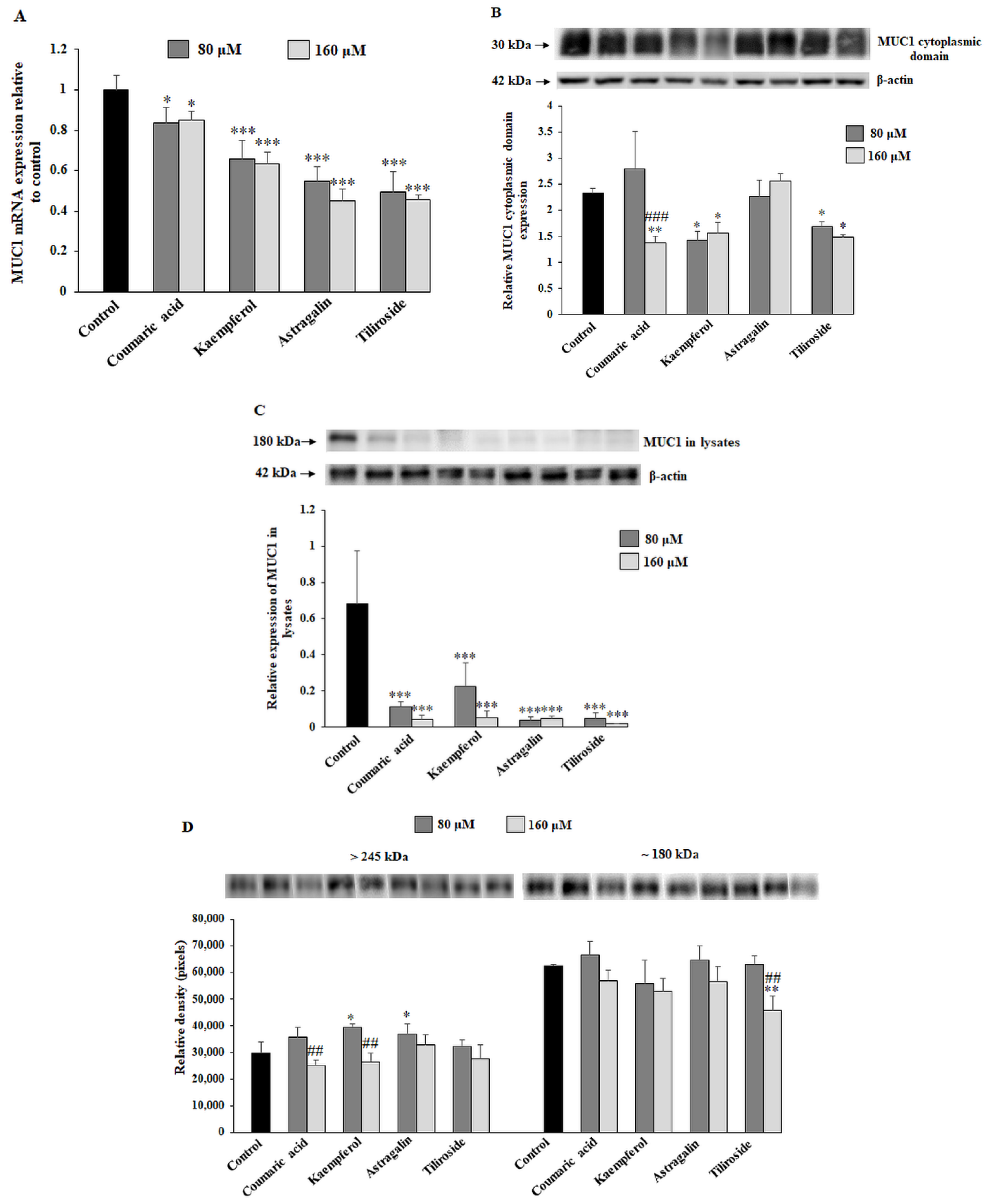
2.2. The Effect of the Compounds on MUC1 Expression
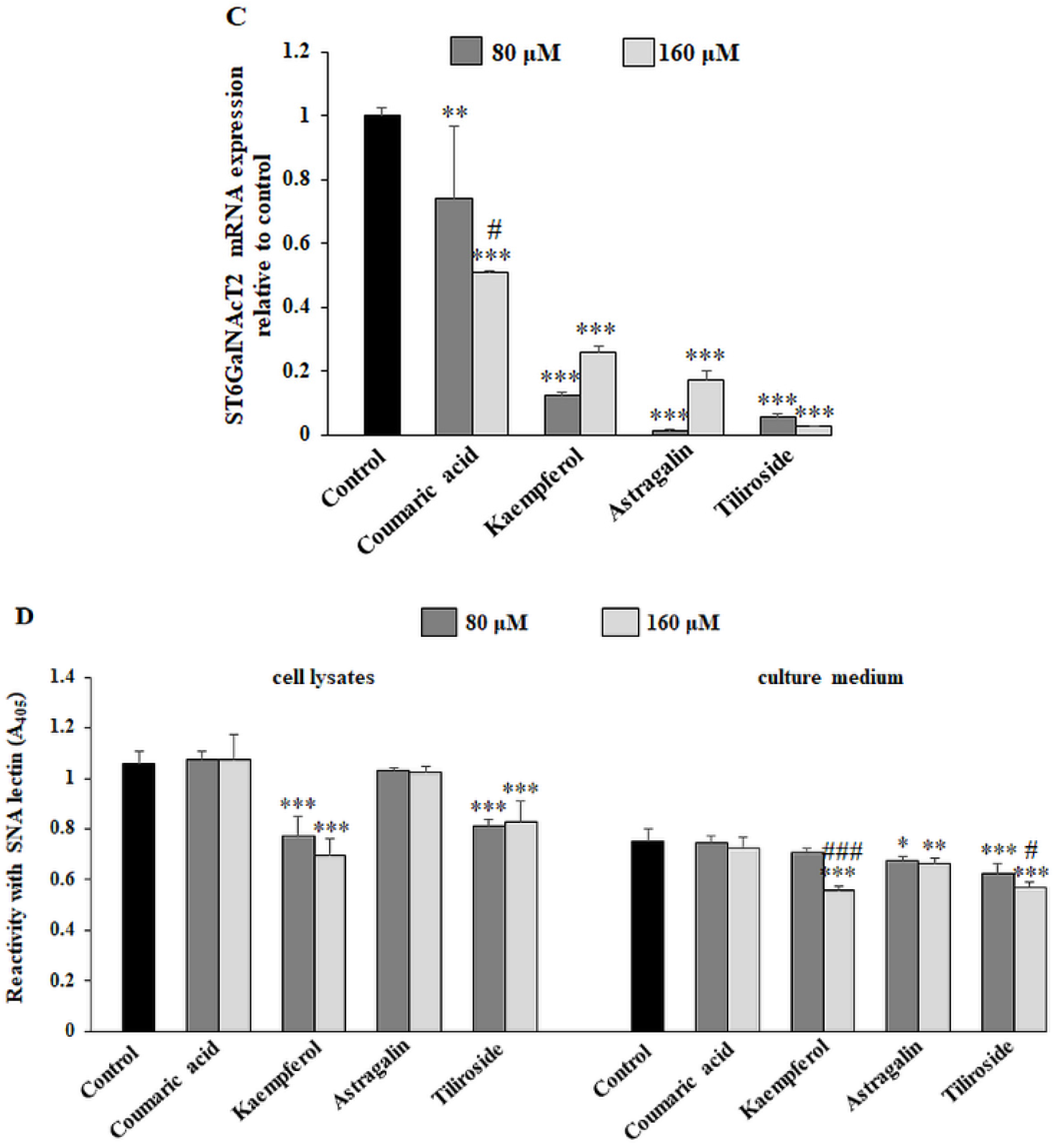
2.3. The Effect of the Compounds on Tn and sialyl Tn Antigen Expression
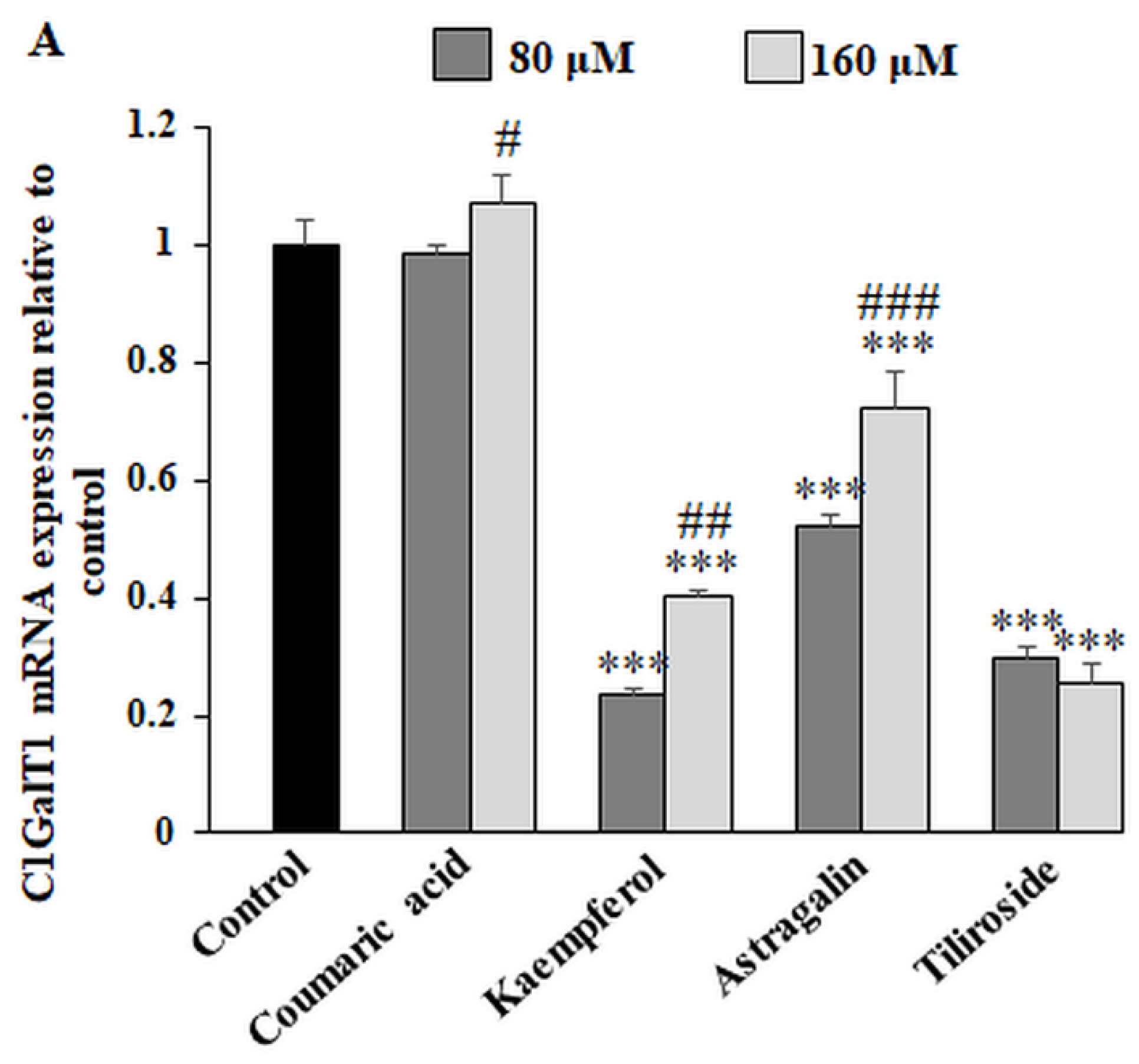
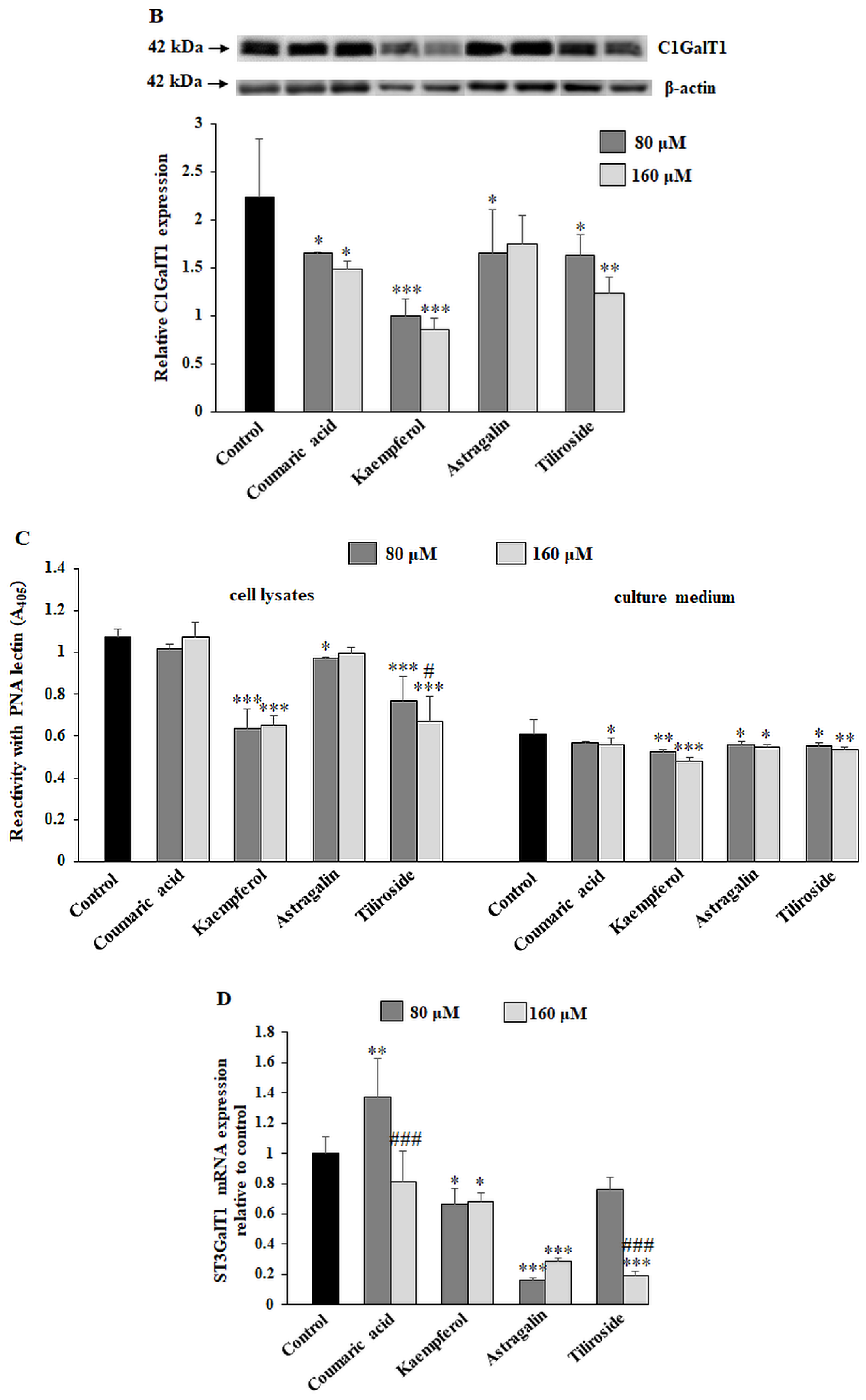
2.4. The Effect of the Polyphenolic Compounds on T and sialyl T Antigen Expression
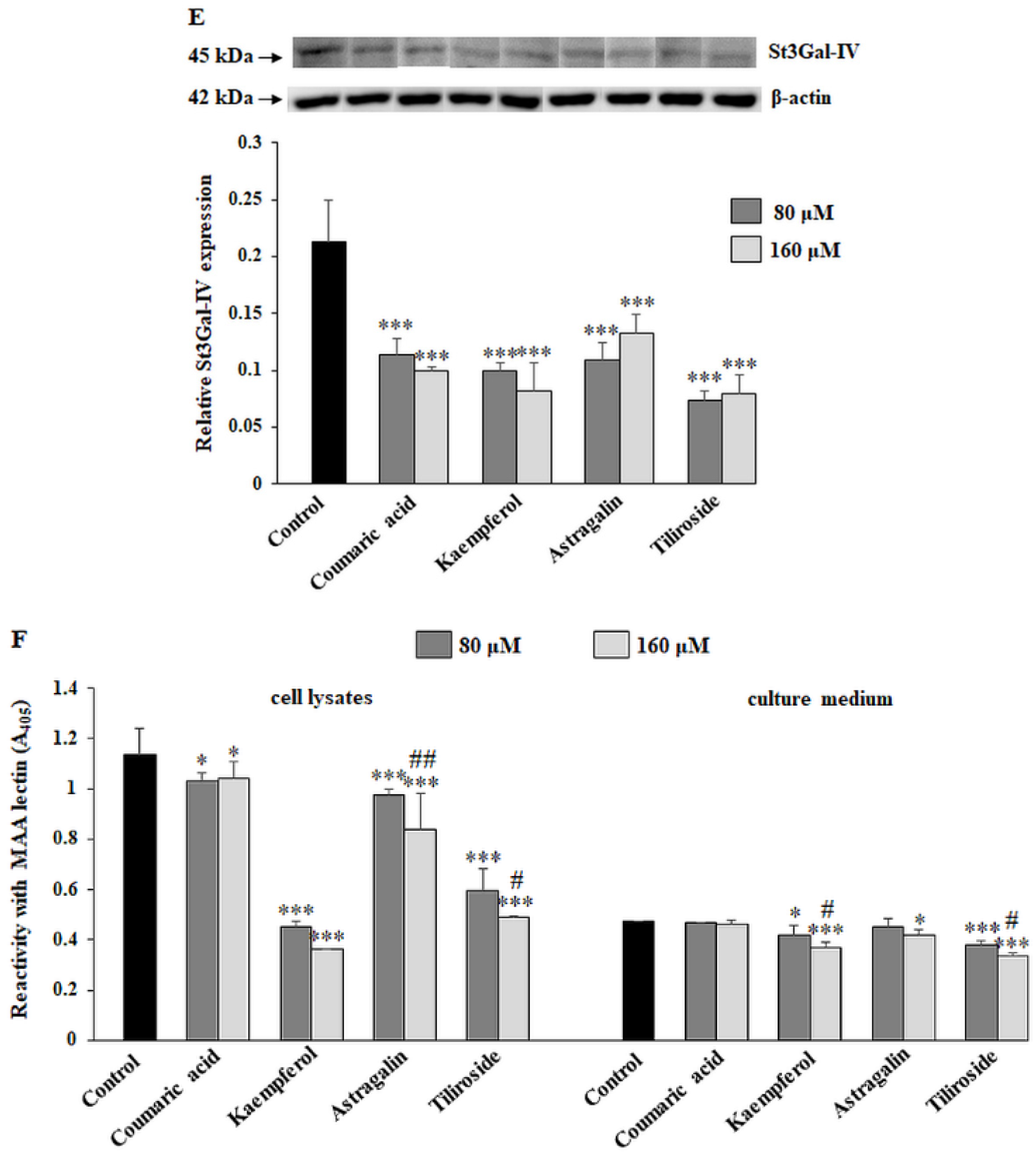
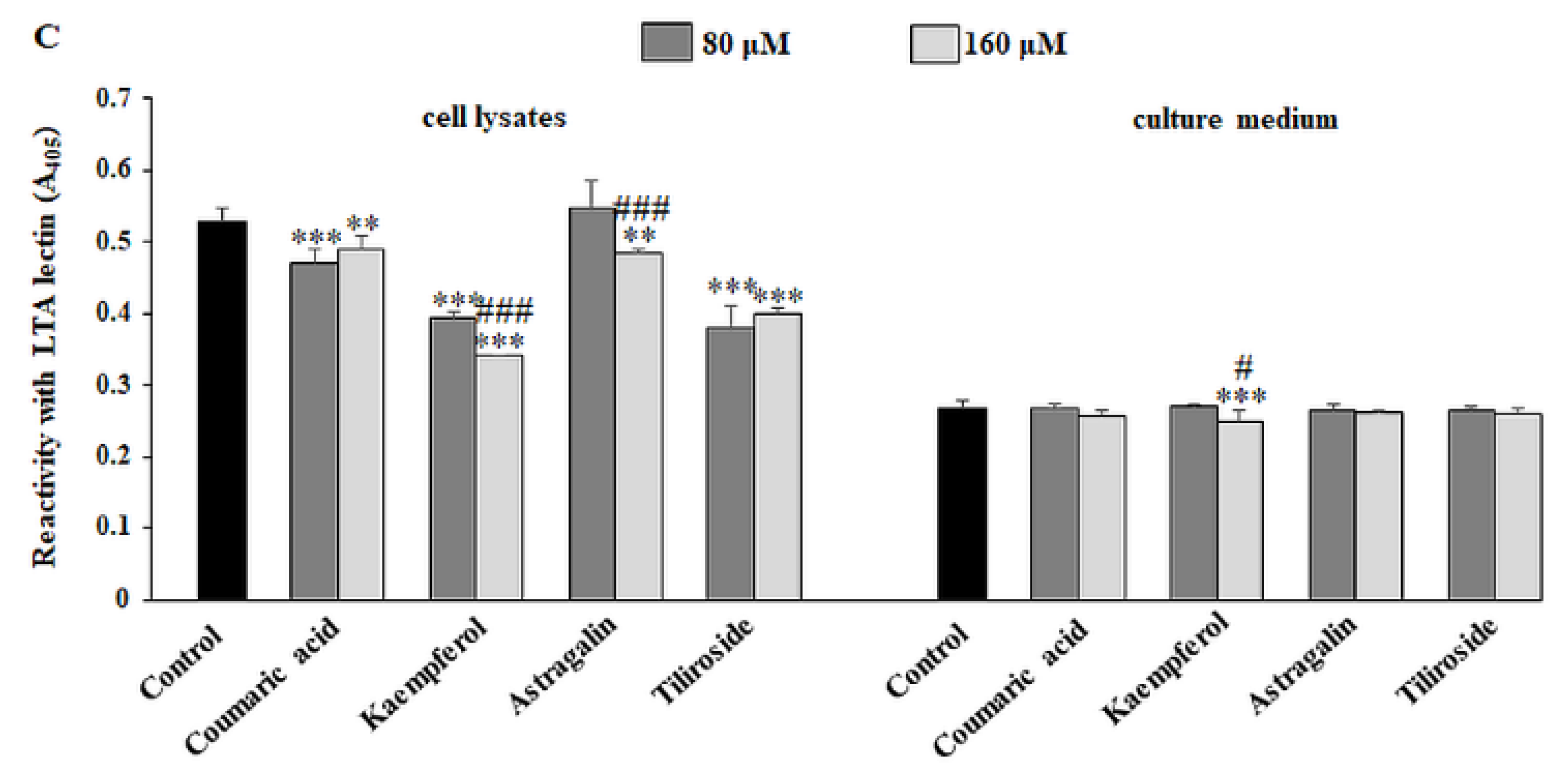
2.5. The Effect of Compounds on Fucosylated Antigen Expression
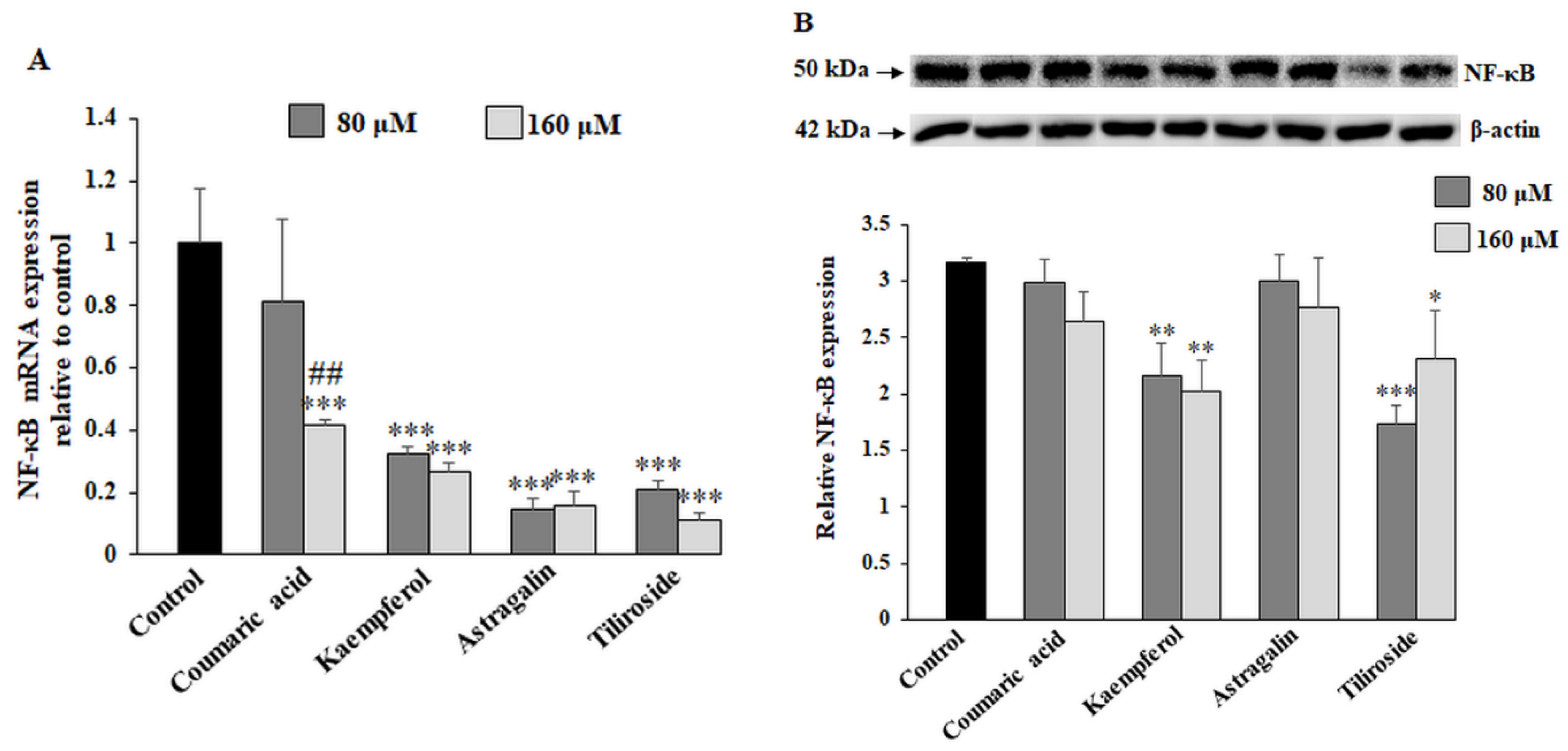
2.6. The Effect of the Compounds on NF-κB Expression
3. Discussion
4. Materials and Methods
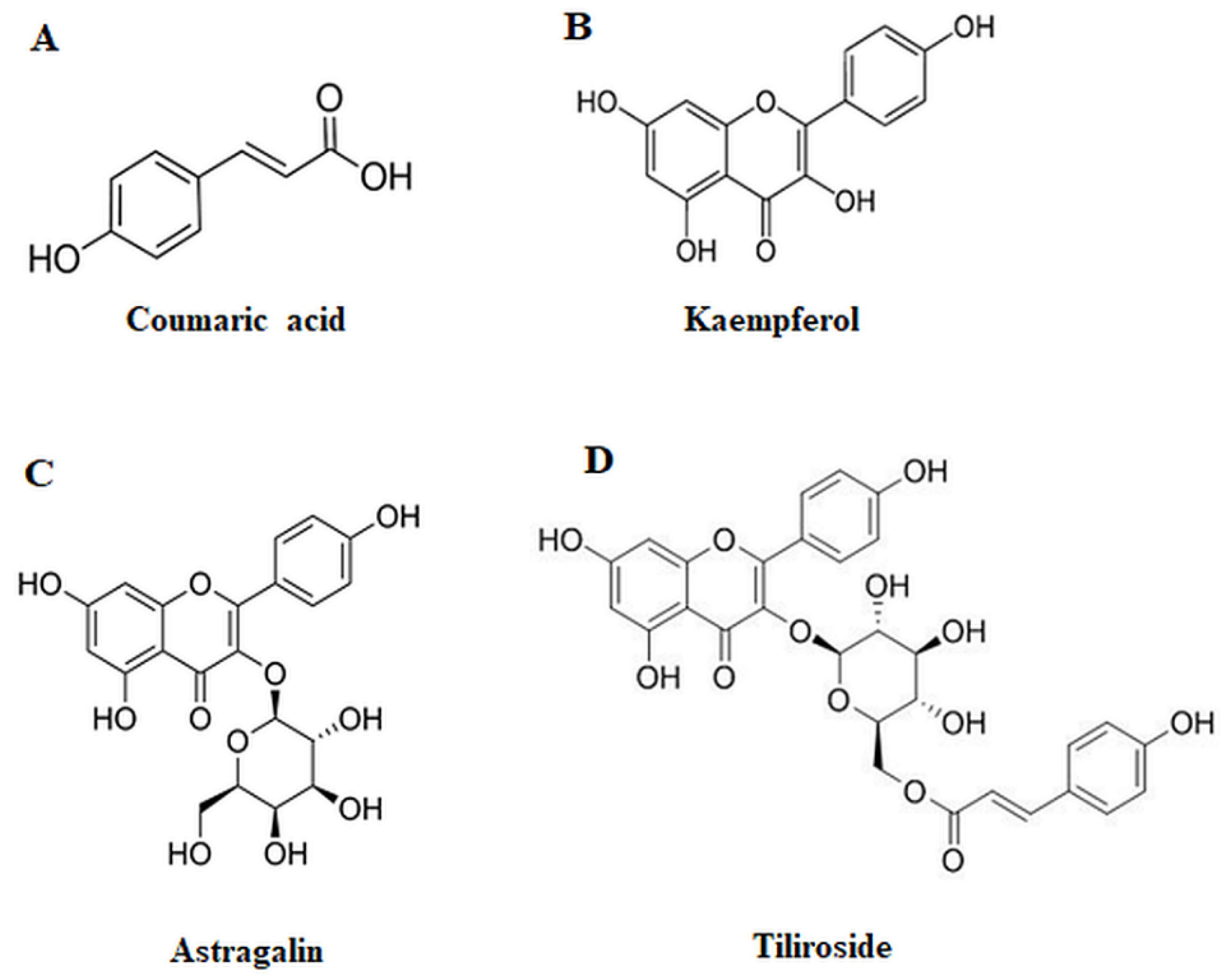
4.1. Polyphenolic compounds
4.2. Cell Culture
4.3. Cell Viability Test
4.4. RNA Isolation and RT-PCR
4.5. Western Blotting
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, F.M.; Beckman, M. Updates on management of gastric cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Correra, P. Gastric cancer: Overview. Gastroenterol. Clin. N. Am. 2013, 42, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuolo, J.; Musolino, V.; Gliozzi, M.; Carresi, C.; Oppedisano, F.; Nucera, S.; Scarano, F.; Scicchitano, M.; Guarnieri, L.; Bosco, F.; et al. The employment of genera Vaccinium, Citrus, Olea, and Cynara polyphenols for the reduction of selected anti-cancer drug side effects. Nutrients 2022, 14, 1574. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, S.S.; Carvalho, S.; Marcos-Pinto, R.; Magalhaes, A.; Oliveira, C.; Gu, J.; Dinis-Ribeiro, M.; Carneiro, F.; Seruca, R.; Reis, C.A. Gastric cancer: Adding glycosylation to the equation. Trends Mol. Med. 2013, 19, 664–676. [Google Scholar] [CrossRef]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.A.; Matos, R.; Mereiter, S.; Pinto, M.T.; Polonia, A.; Gartner, F.; et al. O-glycans modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine 2019, 40, 349–362. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Fu, J.; Bergstrom, K.; Shan, X.; McDaniel, J.M.; McGee, S.; Bai, X.; Chen, W.; Xia, L. Core 1-derived mucin-type O-glycosylation protects against spontaneous gastritis and gastric cancer. J. Exp. Med. 2020, 17, e20182325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mereiter, S.; Balmana, M.; Gomes, J.; Magalhaes, A.; Reis, C.A. Glycomic approaches for the discovery of targets in gastrointestinal cancer. Front. Oncol. 2016, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.A.; Magalhaes, A.; Gomes, J.; Peixoto, A.; Gaiteiro, C.; Fernandes, E.; Santos, L.L.; Reis, C.A. Protein glycosylation in gastric and colorectal cancers: Toward cancer detection and targeted therapeutics. Cancer Lett. 2017, 387, 32–45. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Nazaruk, J.; Karna, E.; Popławska, B.; Galicka, A. Rosmarinic acid influences collagen, MMPs, TIMPs, glycosylation and MUC1 in CRL-1739 gastric cancer cell line. Biomed. Pharmacol. 2018, 107, 397–407. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-cancer effect of combined action of anti-MUC1 and rosmarinic acid in AGS gastric cancer cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, C. Kaempferol suppresses human gastric cancer SNU-216 cell proliferation, promotes cell autophagy, but has no influence on cell apoptosis. Braz. J. Med. Biol. Res. 2019, 52, e7843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, H.O.; Freitas, D.; Gomes, C.; Gomes, J.; Magalhaes, A.; Reis, C.A. Mucin-type O-glycosylation in gastric carcinogenesis. Biomolecules 2016, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; He, Y.; Li, Y.; Gu, M.; Wu, M.; Li, L. Eriodicytol suppresses the malignant progression of colorectal cancer by downregulating tissue specific transplantation antigen P35B (ASTA3) expression to restrain fucosylation. Bioengineered 2022, 13, 5551–5563. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Ma, L.L.; Cao, B.; Lin, J.Z.; Han, L.; Li, C.Y.; Xu, R.C.; Zhang, D.K. Progress in research into the role of abnormal glycosylation modification in tumor immunity. Immunol. Lett. 2021, 229, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rankin, G.Y.; Liu, L.; Daddysman, M.K.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Le, S.Y.; Kim, M.; Cheon, C.; Ko, S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef]
- Yang, M.; Li, W.Y.; Xie, J.; Wang, Z.L.; Wen, Y.L.; Zhao, C.C.; Tao, L.; Li, L.F.; Tian, Y.; Sheng, J. Astragalin inhibits the proliferation and migration of human colon cancer HTC116 cells by regulating the NF-κB signaling pathway. Front. Pharmacol. 2021, 12, 639256. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Lu, L.; Lin, L. Tiliroside as a CAXII inhibitor suppresses liver cancer development and modulates F2Fs/caspase-3 axis. Sci. Rep. 2021, 11, 8626. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A review on the dietary flavonoid tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascio, S.; Finn, O.J. Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, invasion, and metastasis. Biomolecules 2016, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bafna, S.; Kaur, S.; Batra, S.K. Membrane-bound mucins: The mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010, 29, 2893–2904. [Google Scholar] [CrossRef] [Green Version]
- Kufe, D. MUC1-C oncoprotein as a target in breast cancer; activation of signaling pathways and therapeutic approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecgt, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckwith, D.M.; Cudic, M. Tumor-associated of O-glycans of MUC1: Carriers of the glyco-code and targets for cancer vaccine design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef]
- Nardy, A.F.F.R.; Freire-de-Lima, L.; Morrot, A. The sweet side of immune evasion: Role of glycans in the mechanisms of cancer progression. Front. Oncol. 2016, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Cagnoni, A.J.; Perez Saez, J.M.; Rabinovich, G.A.; Marino, K.V. Turning-off signaling by siglecs, selectins, and galectins: Chemical inhibition of glycan-dependent interactions in cancer. Front. Oncol. 2016, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kufe, D. Oncogenic function of the MUC1 receptor submit in gene regulation. Oncogene 2010, 29, 5663–5666. [Google Scholar] [CrossRef]
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1A1 (ALDH1A1), chemokine receptor type 4 (CXCR4), mucin 1 (MUC1), and epithelial cell adhesion molecule (EpCAM). Med. Sci. Monit. 2018, 24, 412–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Rajabi, H.; Kufe, D.M. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Mol. Pharmacol. 2011, 78, 886–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radziejewska, I.; Borzym-Kluczyk, K.; Leszczyńska, L. Luteolin alters MUC1 extracellular domain, sT antygen, ADAM-17, IL-8, IL-10 and NF-κB expression in Helicobacter pylori-infected gastric cancer CRL-1739 cells: A preliminary study. Biomed. Rep. 2021, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent advance in tumor-associated carbohydrate antigens (TACAs)-based antitumor vaccines. ASC Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Hosain, F.; Andreana, P.R. Developments in carbohydrate-based cancer therapeutics. Pharmaceuticals 2019, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanas, S.; Sahasrabudhe, N.M.; Rodriguez, E.; Kooyk, Y.; Vliet, S.J. Fucosylated antigens in cancer: An alliance toward tumor progression, metastasis, and resistance to chemotherapy. Front. Oncol. 2018, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cui, Y.; Yang, F.; Xu, Z.; Da, L.T.; Zhang, Y. Inhibition of polypeptide N-acetyl-α-galactosaminyltransferases in an underlying mechanism of dietary polyphenols preventing colorectal tumorigenesis. Bioorg. Med. Chem. 2019, 27, 3372–3382. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Czarnomysy, R.; Buzun, K.; Bielawska, A. Anti-cancer potential of afzelin towards gastric cancer cells. Pharmaceuticals 2021, 14, 973. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.X.; Li, L.L.; Cao, Y.Z.; Geng, Y.D.; Feng, Y.J.; Wang, A.Y.; Chen, Z.L.; Lu, Y.; Shen, A.Z. Paeonol suppresses proliferation and motility of non-small-cell lung cancer cells by disrupting STAT3/NF-κB signaling. Front. Pharmacol. 2020, 11, 572616. [Google Scholar] [CrossRef]
- Chen, M.; Cai, F.; Zha, D.; Wang, X.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z.C. Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-κB pathway. Oncotarget 2017, 8, 26941–26958. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Ullah, H.; Castiulho, P.C.M.F.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Navabi, S.M.; Daglia, M.; Silva, A.S.; et al. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2020, 60, 2790–2800. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Naumann, M. NF-κB signaling in gastric cancer. Toxins 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.W.; Tsai, H.E.; Chen, W.S.; Chang, T.T.; Chen, C.L.; Hsiao, P.W.; Li, W.S. Sialyltransferase inhibitors suppress breast cancer metastasis. J. Med. Chem. 2021, 64, 527–542. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Wang, T.; Lin, Q.; Feng, X.; Jiang, Q.; Liu, Y.; Chen, D. Role of the p-coumaroyl moiety in the antioxidant and cytoprotective effects of flavonoid glycosides: Comparison of astragalin ant tiliroside. Molecules 2017, 22, 1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grochowski, D.M.; Strawa, J.W.; Granica, S.; Tomczyk, M. Secondary metabolites of Rubus caesius (Rosaceae). Biochem. Syst. Ecol. 2020, 92, 104111. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Polyphenolic compounds from the flowers of Ficaria verna Huds. Acta Pol. Pharm. 1999, 56, 475–476. [Google Scholar]
- Carmichael, J.; Degraff, W.; Gazdar, A.; Minna, J.; Mitchell, J. Evaluation of tetrazolium-based semi-automated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Towbin, T.; Stachelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [Green Version]



| Origin and Abbreviations of Lectins | Binding Preference |
|---|---|
| Arachis hypogaea (peanut) (PNA) | Galβ1,3-GalNAcα1-O-Ser/Thr (T antigen) |
| Lotus tetragonolobus (LTA) | Fucα1,3-GlcNAc |
| Maackia amurensis (MAAII) | NeuAcα2,3-Gal (sialyl T antigen) |
| Sambucus nigra (SNA) | NeuAcα2,6-Gal/GalNAc (sialyl Tn antigen) |
| Vicia villosa (VVA) | GalNAcα1-O-Ser/Thr (Tn antigen) |
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| MUC1 | TGCCTTGGCTGTCTGTCAGT | GTAGGTATCCCGGGCTGGAA |
| C1GalT1 | AAGCAGGGCTACATGAGTGG | GCATCTCCCCAGTGCTAAGT |
| ppGalNAcT2 | AAGAAAGACCTTCATCACAGCAATGGAGAA | ATCAAAACCGCCCTTCAAGTCAGCA |
| ST6GalNAcT2 | CCTTCTGAACGGCTCAGAGAGT | GCACACCGGATACACTTTGGA |
| ST3GalT1 | TCGGCCTGGTTCGATGA | CGCGTTCTGGGCAGTCA |
| FUT4 | AAGCCGTTGAGGCGGTTT | ACAGTTGTGTATGAGATTTGGAAGCT |
| NF-κB | CTGAACCAGGGCATACCTGT | GAGAAGTCCATGTCCGCAAT |
| GAPDH | GTGAACCATGAGAAGTATGACAA | CATGAGTCCTTCCACGATAC |
| Antibody | Clone | Source |
|---|---|---|
| Anti-MUC1; extracellular domain (mouse IgG) Anti-MUC1; cytoplasmic tail (Armenian hamster IgG) Anti-NF-κB (mouse IgG) Anti-C1GalT1 (mouse IgG) Anti-FUT4 (mouse IgG) Anti-St3Gal-IV (mouse IgG) Anti-β-actin (rabbit IgG) Anti-mouse IgG peroxidase conjugated Anti-rabbit IgG peroxidase conjugated Anti-Armenian hamster IgG peroxidase conjugated | BC2 CT2 5D10D11 F-31 A-10 1F4 | Abcam Abcam Cell Sign Tech Santa Cruz Santa Cruz Santa Cruz Sigma Sigma Sigma Abcam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radziejewska, I.; Supruniuk, K.; Tomczyk, M.; Izdebska, W.; Borzym-Kluczyk, M.; Bielawska, A.; Bielawski, K.; Galicka, A. p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells. Int. J. Mol. Sci. 2022, 23, 8602. https://doi.org/10.3390/ijms23158602
Radziejewska I, Supruniuk K, Tomczyk M, Izdebska W, Borzym-Kluczyk M, Bielawska A, Bielawski K, Galicka A. p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells. International Journal of Molecular Sciences. 2022; 23(15):8602. https://doi.org/10.3390/ijms23158602
Chicago/Turabian StyleRadziejewska, Iwona, Katarzyna Supruniuk, Michał Tomczyk, Wiktoria Izdebska, Małgorzata Borzym-Kluczyk, Anna Bielawska, Krzysztof Bielawski, and Anna Galicka. 2022. "p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells" International Journal of Molecular Sciences 23, no. 15: 8602. https://doi.org/10.3390/ijms23158602
APA StyleRadziejewska, I., Supruniuk, K., Tomczyk, M., Izdebska, W., Borzym-Kluczyk, M., Bielawska, A., Bielawski, K., & Galicka, A. (2022). p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells. International Journal of Molecular Sciences, 23(15), 8602. https://doi.org/10.3390/ijms23158602








