Effects of MICU1-Mediated Mitochondrial Calcium Uptake on Energy Metabolism and Quality of Vitrified-Thawed Mouse Metaphase II Oocytes
Abstract
:1. Introduction
2. Results
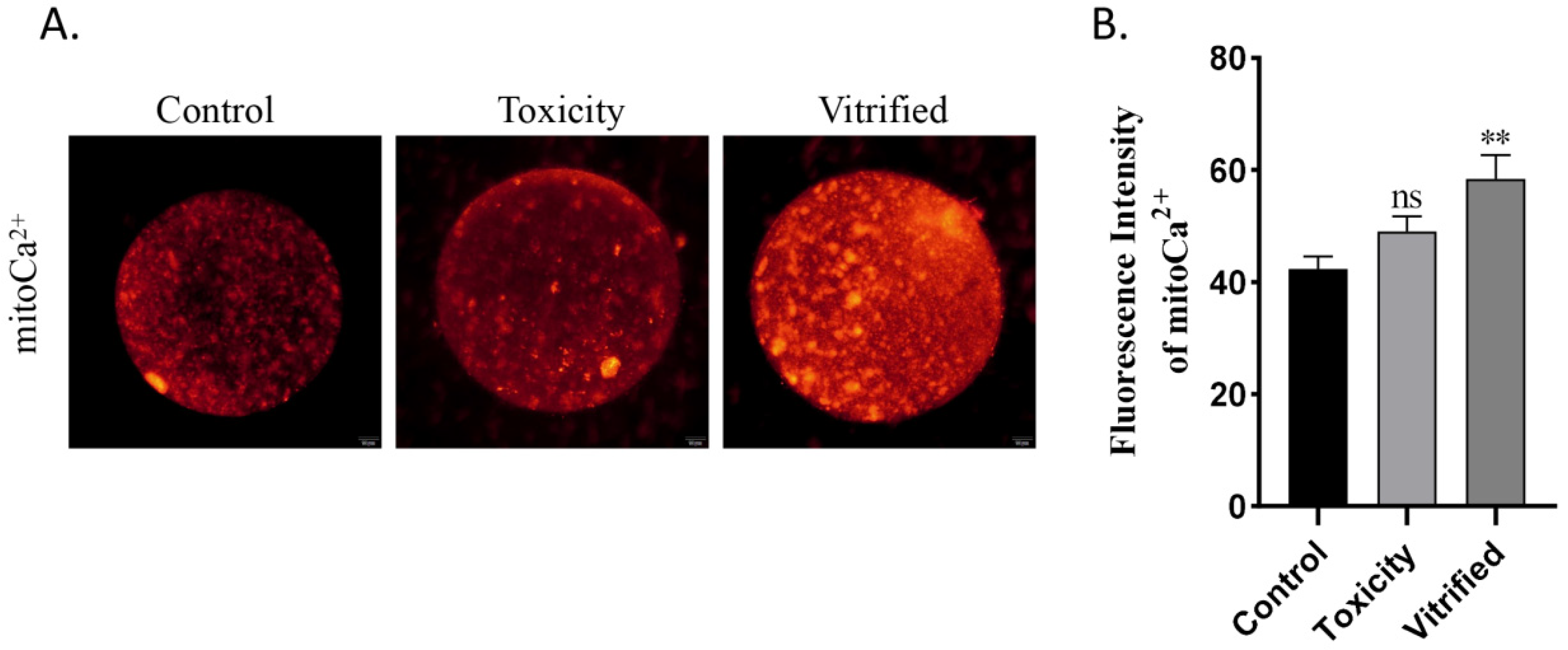
2.1. Effects of Oocyte Vitrification on Mitochondrial Ca2+ Uptake
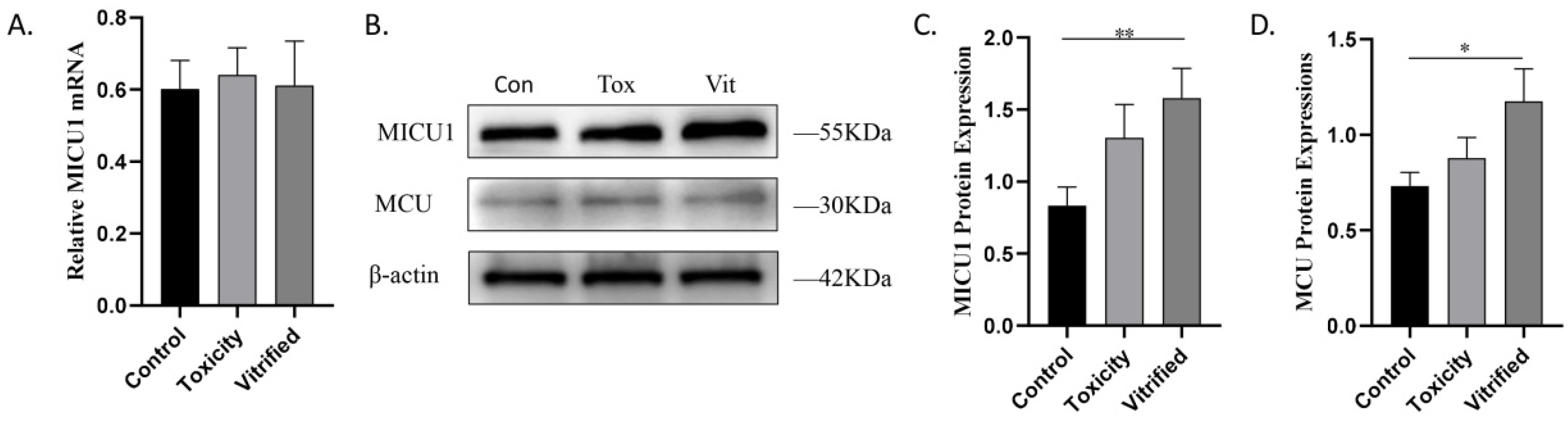
2.2. MICU1 Is Upregulated in Vitrified Oocytes
2.3. MICU1 Regulates Mitochondrial Ca2+ Uptake
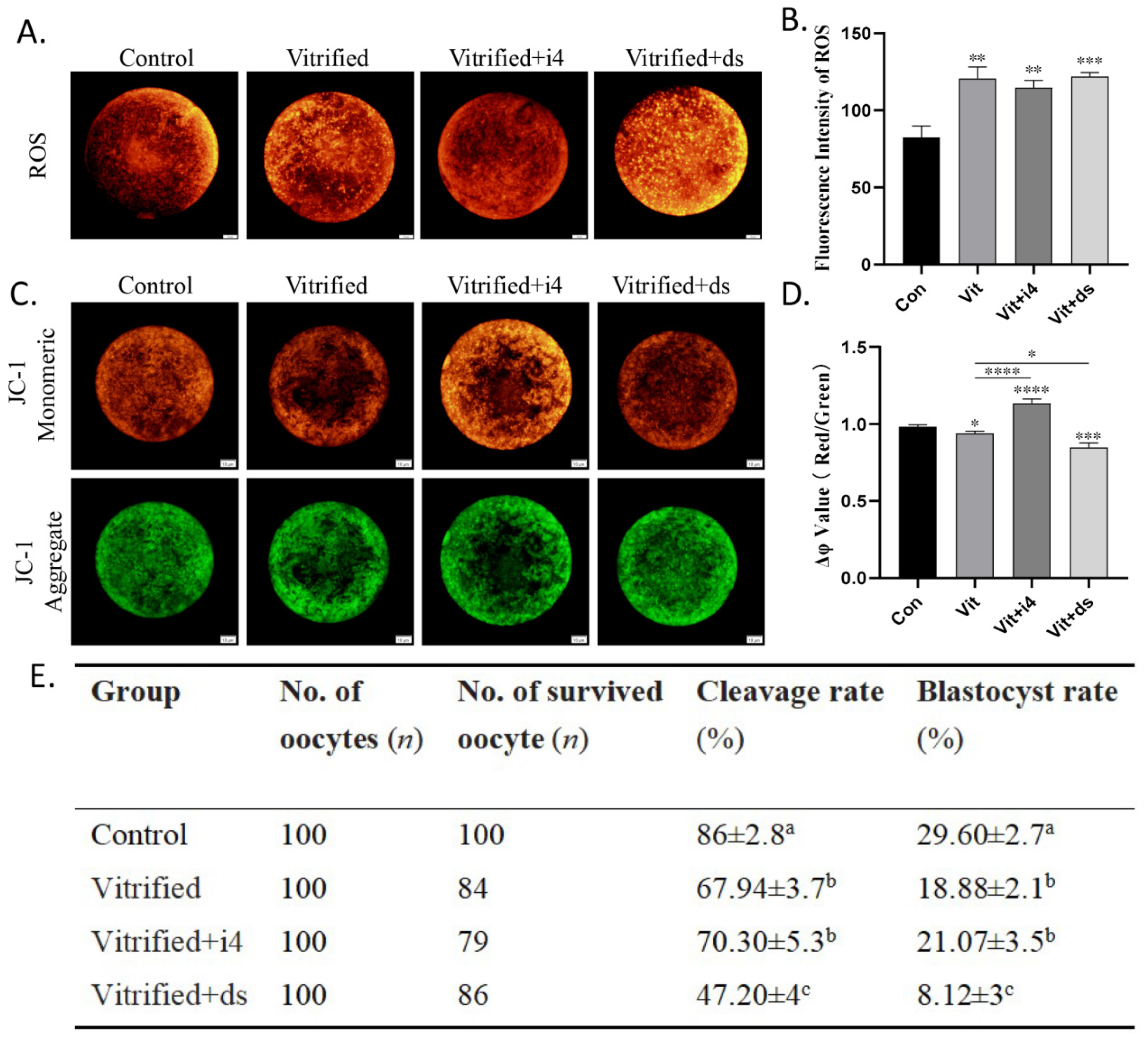
2.4. MICU1 Affects Mitochondrial Membrane Potential and Embryonic Development of Vitrified Oocytes
2.5. MICU1 Regulates Pyruvate Metabolism in Vitrified Oocytes
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Groups
4.2. Mature Oocytes Collection
4.3. Oocyte Vitrification and Thawing
4.4. Quantitative RT-PCR
4.5. Western Blot
4.6. Determination of Mitochondrial Calcium Concentration
4.7. Determination of Reactive Oxygen Species Levels
4.8. Determination of Mitochondrial Membrane Potential
4.9. Determination of ATP Levels
4.10. Parthenogenetic Activation and In Vitro Culture of Embryos
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasai, M. Advances in the cryopreservation of mammalian oocytes and embryos: Development of ultrarapid vitrification. Reprod. Med. Biol. 2002, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Cobo, A.; Paffoni, A.; Scarduelli, C.; Capalbo, A.; Vajta, G.; Remohí, J.; Ragni, G.; Ubaldi, F.M. Consistent and predictable delivery rates after oocyte vitrification: An observational longitudinal cohort multicentric study. Hum. Reprod. 2012, 27, 1606–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.L.; Fu, X.W.; Zhou, G.B.; Zhao, X.M.; Suo, L.; Zhu, S.E. Mitochondrial behaviors in the vitrified mouse oocyte and its parthenogenetic embryo: Effect of Taxol pretreatment and relationship to competence. Fertil. Steril. 2010, 93, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, C.; Muneri, C.W.; Niu, Y.; Zhang, S.; Rui, R.; Zhang, D. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 2015, 71, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Pathak, T.; Trebak, M. Mitochondrial Ca2+ signaling. Pharmacol. Ther. 2018, 192, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Balaban, R.S. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 2012, 51, 2959–2973. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, N.E.; Chandramoorthy, H.C.; Shamugapriya, S.; Zhang, X.; Rajan, S.; Mallilankaraman, K.; Gandhirajan, R.K.; Vagnozzi, R.J.; Ferrer, L.M.; Sreekrishnanilayam, K.; et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep. 2013, 5, 1576–1588. [Google Scholar] [CrossRef] [Green Version]
- Payne, R.; Hoff, H.; Roskowski, A.; Foskett, J.K. MICU2 Restricts Spatial Crosstalk between InsP3R and MCU Channels by Regulating Threshold and Gain of MICU1-Mediated Inhibition and Activation of MCU. Cell Rep. 2017, 21, 3141–3154. [Google Scholar] [CrossRef] [Green Version]
- Csordás, G.; Golenár, T.; Seifert, E.L.; Kamer, K.J.; Sancak, Y.; Perocchi, F.; Moffat, C.; Weaver, D.; Perez, S.F.; Bogorad, R.; et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013, 17, 976–987. [Google Scholar] [CrossRef] [Green Version]
- Mallilankaraman, K.; Doonan, P.; Cárdenas, C.; Chandramoorthy, H.C.; Müller, M.; Miller, R.; Hoffman, N.E.; Gandhirajan, R.K.; Molgó, J.; Birnbaum, M.J.; et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 2012, 151, 630–644. [Google Scholar] [CrossRef] [Green Version]
- Patron, M.; Checchetto, V.; Raffaello, A.; Teardo, E.; Vecellio Reane, D.; Mantoan, M.; Granatiero, V.; Szabò, I.; De Stefani, D.; Rizzuto, R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell 2014, 53, 726–737. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.K.; Mustafi, S.B.; Xiong, X.; Dwivedi, S.K.D.; Nesin, V.; Saha, S.; Zhang, M.; Dhanasekaran, D.; Jayaraman, M.; Mannel, R.; et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat. Commun. 2017, 8, 14634. [Google Scholar] [CrossRef]
- Kamer, K.J.; Mootha, V.K. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015, 16, 545–553. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Rutter, G.A.; Burnett, P.; Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T.; Tavaré, J.M.; Denton, R.M. Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: Significance for the regulation of pyruvate dehydrogenase activity. Proc. Natl. Acad. Sci. USA 1996, 93, 5489–5494. [Google Scholar] [CrossRef] [Green Version]
- Hansford, R.G.; Zorov, D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol. Cell. Biochem. 1998, 184, 359–369. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Kharitonova, E.K.; Russ, A.N.; Das, S.; Fan, Z.; Muzikansky, A.; Garcia-Alloza, M.; Serrano-Pozo, A.; et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 2146. [Google Scholar] [CrossRef]
- Seidlmayer, L.K.; Juettner, V.V.; Kettlewell, S.; Pavlov, E.V.; Blatter, L.A.; Dedkova, E.N. Distinct mPTP activation mechanisms in ischaemia-reperfusion: Contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc. Res. 2015, 106, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Morley, P.; Whitfield, J.F. The differentiation inducer, dimethyl sulfoxide, transiently increases the intracellular calcium ion concentration in various cell types. J. Cell. Physiol. 1993, 156, 219–225. [Google Scholar] [CrossRef]
- Takahashi, T.; Igarashi, H.; Doshida, M.; Takahashi, K.; Nakahara, K.; Tezuka, N.; Kurachi, H. Lowering intracellular and extracellular calcium contents prevents cytotoxic effects of ethylene glycol-based vitrification solution in unfertilized mouse oocytes. Mol. Reprod. Dev. 2004, 68, 250–258. [Google Scholar] [CrossRef]
- Nikiforaki, D.; Vanden Meerschaut, F.; Qian, C.; De Croo, I.; Lu, Y.; Deroo, T.; Van den Abbeel, E.; Heindryckx, B.; De Sutter, P. Oocyte cryopreservation and in vitro culture affect calcium signalling during human fertilization. Hum. Reprod. 2014, 29, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, K.; Sano, D.; Seita, Y.; Inomata, T.; Ito, J.; Kashiwazaki, N. Ethylene glycol-supplemented calcium-free media improve zona penetration of vitrified rat oocytes by sperm cells. J. Reprod. Dev. 2010, 56, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larman, M.G.; Katz-Jaffe, M.G.; Sheehan, C.B.; Gardner, D.K. 1,2-propanediol and the type of cryopreservation procedure adversely affect mouse oocyte physiology. Hum. Reprod. 2007, 22, 250–259. [Google Scholar] [CrossRef]
- Larman, M.G.; Sheehan, C.B.; Gardner, D.K. Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction 2006, 131, 53–61. [Google Scholar] [CrossRef]
- Wang, N. Effects of Vitrification on Ca~(2+) and Its Regulatory Genes in Bovine Oocytes. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2017. [Google Scholar]
- Zhang, L.Y.; Lin, M.; Qingrui, Z.; Zichuan, W.; Junjin, L.; Kexiong, L.; Xiangwei, F.; Yunpeng, H. Mitochondrial Calcium uniporters are essential for meiotic progression in mouse oocytes by controlling Ca2+ entry. Cell Prolif. 2021, 54, e13127. [Google Scholar] [CrossRef]
- Biggers, J.D.; Whittingham, D.G.; Donahue, R.P. The pattern of energy metabolism in the mouse oöcyte and zygote. Proc. Natl. Acad. Sci. USA 1967, 58, 560–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Liu, H.; Gu, X.; Boots, C.; Moley, K.H.; Wang, Q. Metabolic control of oocyte development: Linking maternal nutrition and reproductive outcomes. Cell. Mol. Life Sci. CMLS 2015, 72, 251–271. [Google Scholar] [CrossRef]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef] [Green Version]
- Manipalviratn, S.; Tong, Z.B.; Stegmann, B.; Widra, E.; Carter, J.; DeCherney, A. Effect of vitrification and thawing on human oocyte ATP concentration. Fertil. Steril. 2011, 95, 1839–1841. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, H.; He, Q.; Xue, Y.; Zhang, K.; Kang, J.; Wang, Y.; Xu, Z.; Zhang, Y.; Quan, F. Effect of oocyte vitrification on glucose transport in mouse metaphase II oocytes. Reproduction 2021, 161, 549–559. [Google Scholar] [CrossRef]
- Rossini, M.; Filadi, R. Sarcoplasmic Reticulum-Mitochondria Kissing in Cardiomyocytes: Ca(2+), ATP, and Undisclosed Secrets. Front. Cell Dev. Biol. 2020, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Regulation of Mitochondrial Ca2+ Uptake. Annu. Rev. Physiol. 2021, 83, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.; Freeman, E.A.; Gardner, D.K.; Hunt, P.A. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol. Reprod. 2007, 77, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.M.; Silva, C.A.S.E.; Acevedo, N.; Baracat, E.; Serafini, P.; Smith, G.D. Influence of vitrification on mouse metaphase II oocyte spindle dynamics and chromatin alignment. Fertil. Steril. 2008, 90 (Suppl. 4), 1396–1404. [Google Scholar] [CrossRef]
- Chang, H.; Chen, H.; Zhang, L.; Wang, Y.; Xie, X.; Zhang, Y.; Quan, F. Effect of oocyte vitrification on DNA damage in metaphase II oocytes and the resulting preimplantation embryos. Mol. Reprod. Dev. 2019, 86, 1603–1614. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Deng, T.; Zou, P.; Wang, Y.; Quan, F.; Zhang, Y. Effects of oocyte vitrification on epigenetic status in early bovine embryos. Theriogenology 2016, 86, 868–878. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, L.; Wang, Z.; Chang, H.; Xie, X.; Fu, L.; Zhang, Y.; Quan, F. Resveratrol improved the developmental potential of oocytes after vitrification by modifying the epigenetics. Mol. Reprod. Dev. 2019, 86, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Pang, Y.; Zhao, S.; Hao, H.; Zhao, X.; Du, W.; Wang, Y.; Zhu, H. Thioredoxin-interacting protein regulates glucose metabolism and improves the intracellular redox state in bovine oocytes during in vitro maturation. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E405–E416. [Google Scholar] [CrossRef]
- Park, S.K.; Won, C.; Choi, Y.J.; Kang, H.; Roh, S. The leading blastomere of the 2-cell stage parthenogenetic porcine embryo contributes to the abembryonic part first. J. Vet. Med. Sci. 2009, 71, 569–576. [Google Scholar] [CrossRef] [Green Version]



| Gene | Sequence (5′–3′) | ||
|---|---|---|---|
| Forward | Reverse | AT (°C) | |
| MICU1 | ACCTGGTGAAACCGAAGTGT | TCCAAGGCTGTAGAAGATGC | 60 |
| β-actin | TGCTGTCCCTGTATGCCTCT | TTTGATGTCACGCACGATTT | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, T.; Zhang, K.; Lin, F.; He, Q.; Wu, S.; Xu, Z.; Zhang, Y.; Quan, F. Effects of MICU1-Mediated Mitochondrial Calcium Uptake on Energy Metabolism and Quality of Vitrified-Thawed Mouse Metaphase II Oocytes. Int. J. Mol. Sci. 2022, 23, 8629. https://doi.org/10.3390/ijms23158629
Lan T, Zhang K, Lin F, He Q, Wu S, Xu Z, Zhang Y, Quan F. Effects of MICU1-Mediated Mitochondrial Calcium Uptake on Energy Metabolism and Quality of Vitrified-Thawed Mouse Metaphase II Oocytes. International Journal of Molecular Sciences. 2022; 23(15):8629. https://doi.org/10.3390/ijms23158629
Chicago/Turabian StyleLan, Tianyang, Kang Zhang, Feifei Lin, Qifu He, Shenghui Wu, Zhiming Xu, Yong Zhang, and Fusheng Quan. 2022. "Effects of MICU1-Mediated Mitochondrial Calcium Uptake on Energy Metabolism and Quality of Vitrified-Thawed Mouse Metaphase II Oocytes" International Journal of Molecular Sciences 23, no. 15: 8629. https://doi.org/10.3390/ijms23158629





